Effect of F-18 Fluorodeoxyglucose Uptake by Bone Marrow on the Prognosis of Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
- Patients with HNSCC who had undergone FDG PET/CT for initial staging work-up
- Patients who had no evidence of distant metastasis on staging imaging examinations
- Patients who had received any type of curative or palliative treatment after staging work-up
- Patients who had distant lymph node or organ metastasis on staging imaging studies
- Patients who had received any treatment before PET/CT scan
- Patients who underwent only supportive care without any palliative treatment
- Patients who had coexisting acute inflammatory disease at the time of staging work-up
- Patients who were diagnosed with chronic liver disease
- Patients who had a previous history of another malignancy or BM disease
- Patients who were not followed up for 12 months after the initial treatment without disease progression
2.2. Staging Work-Up and Follow-Up
2.3. FDG PET/CT Imaging
2.4. Imaging Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
3.2. FDG Uptake of BM
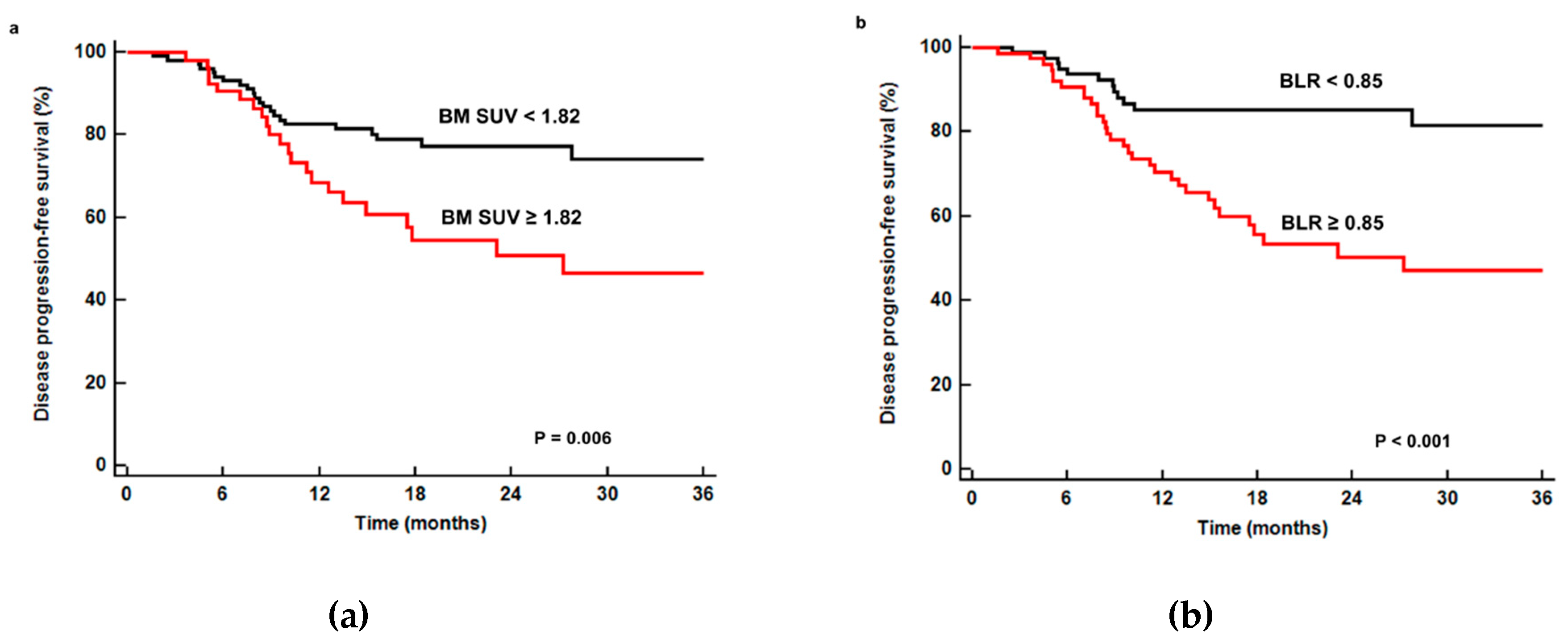
3.3. Disease Progression-Free Survival Analysis
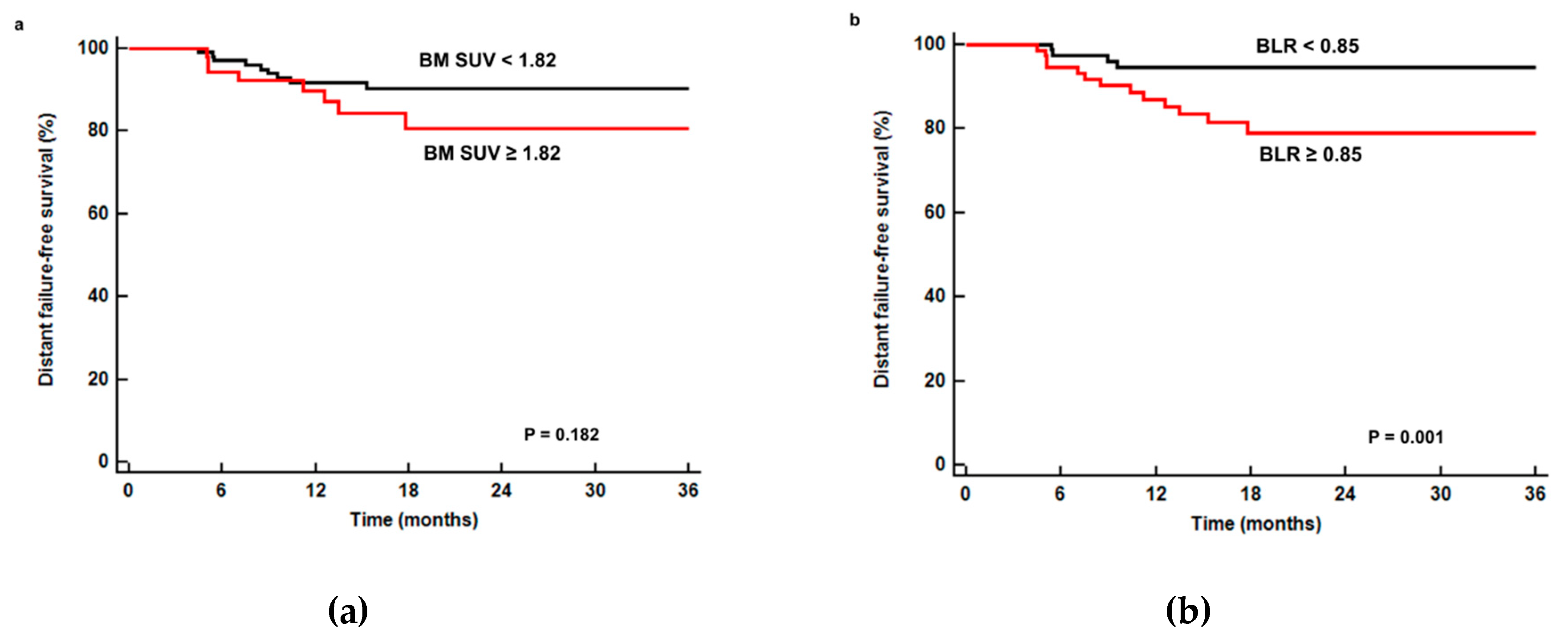
3.4. Distant Failure-Free Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Tang, Y.; Hua, S. Immunological Approaches Towards Cancer and Inflammation: A Cross Talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ahbap, E.; Sakaci, T.; Kara, E.; Sahutoglu, T.; Koc, Y.; Basturk, T.; Sevinc, M.; Akgöl, C.; Kayalar, A.O.; Ucar, Z.A.; et al. Neutrophil-to-lymphocyte ratio and platelet-tolymphocyte ratio in evaluation of inflammation in end-stage renal disease. Clin. Nephrol. 2016, 85, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; McSorley, S.T.; Horgan, P.G.; Laird, B.; McMillan, D.C. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta -analysis. Crit. Rev. Oncol. 2017, 116, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Mascarella, M.A.; Mannard, E.; Silva, S.D.; Zeitouni, A.; Wurzba, S.D.S. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: A systematic review and meta-analysis. Head Neck 2018, 40, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Li, H.; Zheng, Y.; Tang, M.; Li, J.; Wu, H.; Zhong, W.; Gao, J.; Ou, N.; Cai, Y. Prognostic significance of neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, and platelet to lymphocyte ratio in patients with nasopharyngeal carcinoma. Biomed. Res. Int. 2017, 2017, 3047802. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xu, C.; Wu, P.; Zhang, L.H.; Li, D.W.; Sun, J.H.; Li, W.F.; Liao, Z.S. Prognostic role of C-reactive protein in patients with nasopharyngeal carcinoma: A meta-analysis and literature review. Medicine 2017, 96, 8463. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, J.S.; Lyu, J.; Lee, S.M. Prognostic significance of 18 F-fluorodeoxyglucose uptake of bone marrow measured on positron emission tomography in patients with small cell lung cancer. Lung Cancer 2018, 118, 41–47. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, M.S.; Chung, I.K.; Son, M.W.; Cho, Y.S.; Lee, S.M. Clinical implication of FDG uptake of bone marrow on PET/CT in gastric cancer patients with surgical resection. World J. Gastroenterol. 2017, 23, 2385–2395. [Google Scholar] [CrossRef]
- Lee, J.W.; Seo, K.H.; Kim, E.S.; Lee, S.M. The role of (18) F-fluorodeoxyglucose uptake of bone marrow on PET/CT in predicting clinical outcomes in non-small cell lung cancer patients treated with chemoradiotherapy. Eur. Radiol. 2017, 27, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.W.; Becker, A.K.; Goo, J.M.; Cheon, G.J. FDG whole-body PET/MRI in oncology: A systematic review. Nucl. Med. Mol. Imaging 2017, 51, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Helsen, N.; Wyngaert, T.V.D.; Carp, L.; Stroobants, S. FDG-PET/CT for treatment response assessment in head and neck squamous cell carcinoma: A systematic review and meta-analysis of diagnostic performance. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, P.; Merlotti, A.; Olmetto, E.; Bianchi, A.; Desideri, I.; Bacigalupo, A.; Franco, P.; Franzese, C.; Orlandi, E.; Livi, L.; et al. What is the prognostic impact of FDG PET in locally advanced head and neck squamous cell carcinoma treated with concomitant chemo-radiotherapy? A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2122–2138. [Google Scholar] [CrossRef] [PubMed]
- Allegra, E.; Saita, V.; De Natale, M.; Marino, N.; Trapasso, S.; Tamburrini, S.; Alessio, C.; Ippolito, M. Use of PET/CT to detect local and regional laryngeal cancer recurrence after surgery. Rep. Med. Imaging 2017, 10, 31–36. [Google Scholar] [CrossRef]
- Inoue, K.; Goto, R.; Okada, K.; Kinomura, S.; Fukuda, H. A bone marrow F-18 FDG uptake exceeding the liver uptake may indicate bone marrow hyperactivity. Ann. Nucl. Med. 2009, 23, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Bural, G.G.; Torigian, A.D.; Chen, W.; Houseni, M.; Basu, S.; Alavi, A. Increased 18F-FDG uptake within the reticuloendothelial system in patients with active lung cancer on PET imaging may indicate activation of the systemic immune response. Hell. J. Nucl. Med. 2010, 13, 23–25. [Google Scholar]
- Cicone, F.; Loose, D.; Deron, P.; Vermeersch, H.; Signore, A.; Van De Vyvere, F.; Scopinaro, F.; Van De Wiele, C. Prognostic value of FDG uptake by the bone marrow in squamous cell carcinoma of the head and neck. Nucl. Med. Commun. 2008, 29, 431–435. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.C.; Kim, H.J.; Lee, S.M. Prognostic value of bone marrow (18) F-FDG uptake on PET/CT in lymphoma patients with negative bone marrow involvement. Hell. J. Nucl. Med. 2017, 20, 17–25. [Google Scholar]
- Lee, J.W.; Na, J.O.; Kang, D.Y.; Lee, S.Y.; Lee, S.M. Prognostic significance of FDG uptake of bone marrow on PET/CT in patients with non-small-cell lung cancer after curative surgical resection. Clin. Lung Cancer 2017, 18, 198–206. [Google Scholar] [CrossRef]
- Lee, J.W.; Ahn, T.S.; Baek, M.J. Fluorine-18-fluorodeoxyglucose uptake of bone marrow on PET/CT can predict prognosis in patients with colorectal cancer after curative surgical resection. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1–194. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeon, S.; Mun, S.T.; Lee, S.M. Prognostic Value of Fluorine-18 Fluorodeoxyglucose Uptake of Bone Marrow on Positron Emission Tomography/Computed Tomography for Prediction of Disease Progression in Cervical Cancer. Int. J. Gynecol. Cancer 2017, 27, 776–783. [Google Scholar] [CrossRef]
- Bang, J.I.; Yoon, H.J.; Kim, B.S. Clinical utility of FDG uptake within reticuloendothelial system on F-18 FDG PET/CT for prediction of tumor recurrence in breast cancer. PLoS ONE 2018, 13, e0208861. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, S.J.; Pak, K.; Lee, S.W.; Ahn, B.C.; Lee, J. Prognostic value of 18F-fluorodeoxyglucose bone marrow uptake in patients with solid tumors. Medicine (Baltimore) 2018, 97, e12859. [Google Scholar] [CrossRef]
- Murata, Y.; Kubota, K.; Yukihiro, M.; Ito, K.; Watanabe, H.; Shibuya, H. Correlations between 18F-FDG uptake by bone marrow and hematological parameters: Measurements by PET/CT. Nucl. Med. Biol. 2006, 33, 999–1004. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Tsai, D.E.; Vergilio, J.A.; Downs, L.H.; Alavi, A.; Schuster, S.J. Enhanced marrow [18F]fluorodeoxyglucose uptake related to myeloid hyperplasia in Hodgkin’s lymphoma can simulate lymphoma involvement in marrow. Clin. Lymphoma 2004, 5, 62–64. [Google Scholar] [CrossRef]
- Elboğa, U.; Şahin, E. Relationship between reticuloendothelial systems’ FDG uptake level and clinicopathological features in patient with invasive ductal breast cancer. La Radiol. Med. 2017, 122, 785–792. [Google Scholar]
- Katano, A.; Takahashi, W.; Yamashita, H.; Yamamoto, K.; Ando, M.; Yoshida, M.; Saito, Y.; Abe, O.; Nakagawa, K. The impact of elevated C-reactive protein level on the prognosis for oro-hypopharynx cancer patients treated with radiotherapy. Sci. Rep. 2017, 7, 17805. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, I.S.; Park, S.G.; Kim, H.; Choi, Y.J.; Seol, Y.M. Prognostic value of posttreatment neutrophil–lymphocyte ratio in head and neck squamous cell carcinoma treated by chemoradiotherapy. Auris Nasus Larynx 2017, 44, 199–204. [Google Scholar] [CrossRef]
- Seban, R.D.; Robert, C.; Dercle, L.; Yeh, R.; Dunant, A.; Reuze, S.; Schernberg, A.; Sun, R.; Mignot, F.; Terroir, M.; et al. Increased bone marrow SUVmax on 18F-FDG PET is associated with higher pelvic treatment failure in patients with cervical cancer treated by chemoradiotherapy and brachytherapy. OncoImmunology 2019, 8, e1574197. [Google Scholar] [CrossRef]
- Ozmen, O.; Koyuncu, A.; Koksal, D.; Tatci, E.; Alagoz, E.; Demirag, F.; Gokcek, A.; Arslan, N. The potential value of volume-based quantitative PET parameters and increased bone marrow uptake for the prediction of survival in patients with malignant pleural mesothelioma. Nucl. Med. Commun. 2016, 37, 43–49. [Google Scholar] [CrossRef]
- Urbanska, A.M.; Zhang, X.; Prakash, S. Bioengineered Colorectal Cancer Drugs: Orally Delivered Anti-Inflammatory Agents. Cell Biophys. 2015, 72, 757–769. [Google Scholar] [CrossRef]
- Verdoodt, F.; Dehlendorff, C.; Friis, S.; Kjaer, S.K. Non-aspirin NSAID use and ovarian cancer mortality. Gynecol. Oncol. 2018, 150, 331–337. [Google Scholar] [CrossRef]
- Wiegand, S.; Zimmermann, A.; Wilhelm, T.; Werner, A.J. Survival after Distant Metastasis in Head and Neck Cancer. Anticancer. Res. 2015, 35, 5499–5502. [Google Scholar]
- Bonomi, M.; Patsias, A.; Posner, M.; Sikora, A. The Role of Inflammation in Head and Neck Cancer. Results Probl. Cell Differ. 2014, 816, 107–127. [Google Scholar]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Panwar, A.; Interval, E.; Lydiatt, W.M. Emergence of a novel staging system for oropharyngeal squamous cell carcinoma based on HPV status. Oncology 2017, 31, 33–40. [Google Scholar]
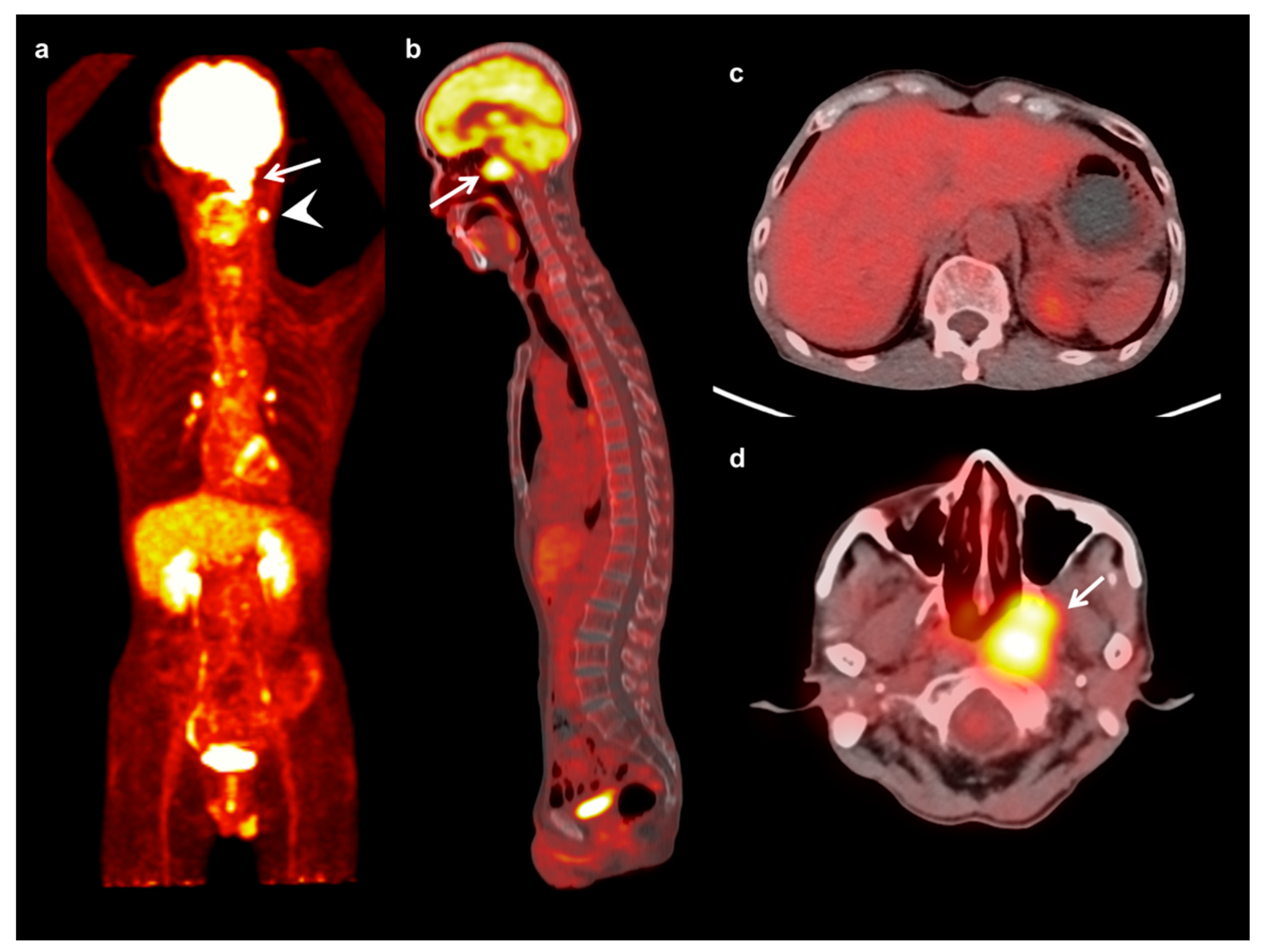
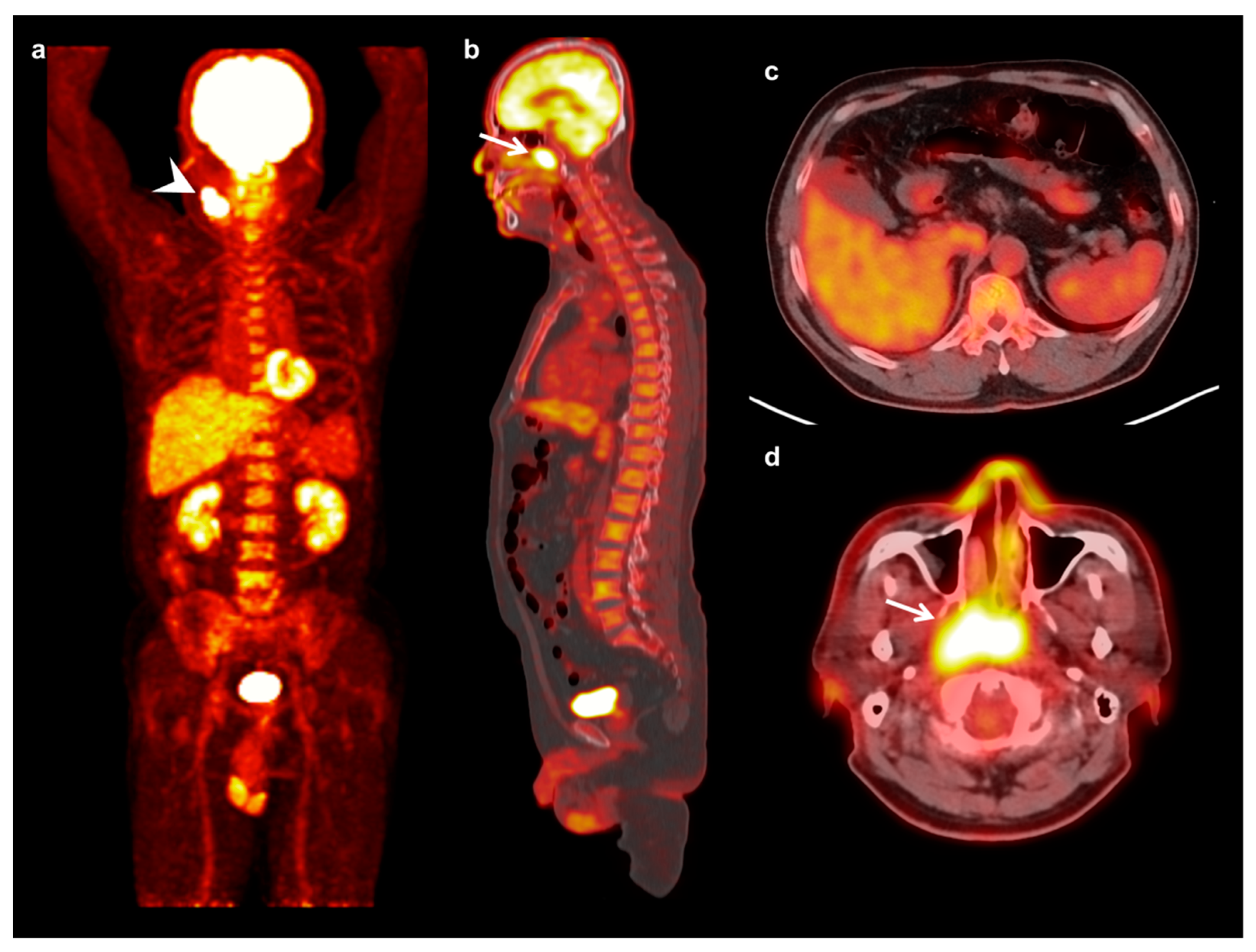
| Characteristics | Mean ± SD | Number (%) | |
|---|---|---|---|
| Age (years) | 61 ± 11 | ||
| Sex | Men | 131 (83.4%) | |
| Women | 26 (16.6%) | ||
| Smoking | Current or former | 87 (55.4%) | |
| Never | 70 (44.6%) | ||
| Tumor location | Nasopharynx | 23 (14.6%) | |
| Oropharynx and oral cavity | 72 (45.9%) | ||
| Hypopharynx | 62 (39.5%) | ||
| T stage | T1 | 57 (36.3%) | |
| T2 | 45 (28.7%) | ||
| T3 | 26 (16.6%) | ||
| T4 | 29 (18.5%) | ||
| N stage | N0 | 83 (52.9%) | |
| N1 | 27 (17.2%) | ||
| N2 | 41 (26.1%) | ||
| N3 | 6 (3.8%) | ||
| TNM stage | Stage I | 44 (28.0%) | |
| Stage II | 23 (14.6%) | ||
| Stage III | 35 (22.3%) | ||
| Stage IV | 55 (35.0%) | ||
| Blood tests | White blood cell count (×1012 cells/L) | 7.48 ± 3.04 | |
| Absolute neutrophil count (×1012 cells/L) | 4.63 ± 2.69 | ||
| Hemoglobin (g/dL) | 13.1 ± 2.2 | ||
| CRP (mg/dL) | 14.35 ± 33.52 | ||
| NLR | 3.21 ± 3.72 | ||
| PLR | 161.88 ± 92.30 | ||
| Tumor size (cm) | 2.5 ± 1.5 | ||
| PET/CT parameters of primary tumor | Maximum SUV | 9.34 ± 5.36 | |
| MTV (cm3) | 14.23 ± 21.71 | ||
| TLG (g) | 80.92 ± 159.51 | ||
| Mean FDG uptake of normal liver tissue | 2.06 ± 0.32 | ||
| PET/CT parameters of BM | BM SUV | 1.73 ± 0.36 | |
| BLR | 0.85 ± 0.18 | ||
| Treatment | Surgery | 50 (31.8%) | |
| Surgery + chemotherapy, radiotherapy, or chemoradiotherapy | 45 (28.7%) | ||
| Concurrent chemoradiotherapy | 53 (33.8%) | ||
| Radiotherapy alone | 8 (5.1%) | ||
| Chemotherapy alone | 1 (0.6%) |
| BM SUV | BLR | ||||
|---|---|---|---|---|---|
| Value | p-Value | Value | p-Value | ||
| T stage | T1T2 | 1.67 ± 0.33 | 0.013 | 0.81 ± 0.15 | 0.002 |
| T3T4 | 1.83 ± 0.38 | 0.91 ± 0.20 | |||
| N stage | N0 | 1.67 ± 0.34 | 0.032 | 0.82 ± 0.17 | 0.023 |
| N1–N3 | 1.80 ± 0.38 | 0.88 ± 0.17 | |||
| TNM stage | Stage I–II | 1.63 ± 0.31 | 0.006 | 0.80 ± 0.16 | <0.001 |
| Stage III–IV | 1.80 ± 0.38 | 0.89 ± 0.18 | |||
| Variables | Univariate Analysis | Multivariate Model with BM SUV | Multivariate Model with BLR | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (<60 years versus ≥60 years) | 0.97 (0.55–1.73) | 0.929 | ||||
| Sex (woman versus. man) | 1.32 (0.59–2.95) | 0.502 | ||||
| Smoking (no versus yes) | 1.03 (0.58–1.81) | 0.932 | ||||
| T stage (T1T2 versus T3T4) | 1.77 (1.00–3.13) | 0.048 | ||||
| N stage (N0 versus N1–N3) | 2.01 (1.13–3.58) | 0.018 | ||||
| TNM stage (stage I–II versus stage III–IV) | 4.12 (2.05–8.30) | <0.001 | 2.98 (1.30–6.89) | 0.010 | 2.87 (1.23–6.69) | 0.014 |
| Tumor size (<3.0 cm versus ≥3.0 cm) | 2.23 (1.26–3.93) | 0.006 | 1.29 (0.67–2.51) | 0.447 | 1.17 (0.61–2.26) | 0.632 |
| CRP (<5.0 mg/dL versus ≥5.0 mg/dL) | 1.70 (1.01–3.01) | 0.047 | 1.32 (0.73–2.41) | 0.357 | 1.34 (0.73–2.43) | 0.342 |
| NLR (<2.75 versus ≥2.75) | 1.41 (0.80–2.50) | 0.239 | ||||
| PLR (<201.00 versus ≥201.00) | 2.33 (1.30–4.20) | 0.005 | 1.85 (1.02–3.37) | 0.045 | 1.69 (0.92–3.11) | 0.091 |
| Maximum SUV (<6.60 versus ≥6.60) | 3.98 (1.86–8.52) | <0.0011 | 2.65 (1.02–6.87) | 0.045 | 2.38 (1.01–6.19) | 0.046 |
| MTV (<8.50 cm3 versus ≥8.50 cm3) | 2.49 (1.39–4.48) | 0.002 | ||||
| TLG (<32.50 g versus ≥32.50 g) | 2.26 (1.26–4.06) | 0.007 | 1.52 (0.85–3.20) | 0.124 | 1.59 (0.87–3.28) | 0.179 |
| BM SUV (<1.82 versus ≥1.82) | 2.17 (1.23–3.83) | 0.007 | 1.77 (0.95–3.28) | 0.072 | ||
| BLR (<0.85 versus ≥0.85) | 2.77 (1.50–5.12) | 0.001 | 1.96 (1.01–3.80) | 0.044 | ||
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (<60 yr versus ≥60 yr) | 1.27 (0.48–3.35) | 0.623 | ||
| Sex (woman versus man) | 3.43 (0.46–25.88) | 0.232 | ||
| Smoking (no versus yes) | 1.06 (0.41–2.74) | 0.911 | ||
| T stage (T1T2 versus T3T4) | 2.34 (0.90–6.07) | 0.081 | ||
| N stage (N0 versus N1–N3) | 3.33 (1.17–9.47) | 0.024 | ||
| TNM stage (stage I–II versus stage III–IV) | 7.23 (1.65–31.72) | 0.009 | 3.59 (0.61–21.33) | 0.159 |
| Tumor size (<3.0 cm versus ≥3.0 cm) | 4.33 (1.60–11.72) | 0.004 | 2.06 (0.62–6.83) | 0.237 |
| CRP (<5.0 mg/dL versus ≥5.0 mg/dL) | 1.54 (0.94–2.70) | 0.078 | ||
| NLR (<2.75 versus ≥2.75) | 1.92 (0.74–4.99) | 0.179 | ||
| PLR (<201.00 versus ≥201.00) | 0.60 (0.17–2.08) | 0417 | ||
| Maximum SUV (<6.60 versus ≥6.60) | 5.45 (1.24–23.86) | 0.024 | 1.39 (0.20–9.63) | 0.740 |
| MTV (<8.50 cm3 versus ≥8.50 cm3) | 3.50 (1.23–9.95) | 0.019 | ||
| TLG (<32.50 g versus ≥32.50 g) | 4.29 (1.40–13.17) | 0.011 | 0.92 (0.20–4.18) | 0.916 |
| BM SUV (<1.82 versus ≥1.82) | 1.89 (0.73–4.91) | 0.189 | ||
| BLR (<0.85 versus ≥0.85) | 8.28 (1.89–36.24) | 0.005 | 2.35 (0.73–7.61) | 0.154 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; Ban, M.J.; Park, J.H.; Lee, S.M. Effect of F-18 Fluorodeoxyglucose Uptake by Bone Marrow on the Prognosis of Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2019, 8, 1169. https://doi.org/10.3390/jcm8081169
Lee JW, Ban MJ, Park JH, Lee SM. Effect of F-18 Fluorodeoxyglucose Uptake by Bone Marrow on the Prognosis of Head and Neck Squamous Cell Carcinoma. Journal of Clinical Medicine. 2019; 8(8):1169. https://doi.org/10.3390/jcm8081169
Chicago/Turabian StyleLee, Jeong Won, Myung Jin Ban, Jae Hong Park, and Sang Mi Lee. 2019. "Effect of F-18 Fluorodeoxyglucose Uptake by Bone Marrow on the Prognosis of Head and Neck Squamous Cell Carcinoma" Journal of Clinical Medicine 8, no. 8: 1169. https://doi.org/10.3390/jcm8081169
APA StyleLee, J. W., Ban, M. J., Park, J. H., & Lee, S. M. (2019). Effect of F-18 Fluorodeoxyglucose Uptake by Bone Marrow on the Prognosis of Head and Neck Squamous Cell Carcinoma. Journal of Clinical Medicine, 8(8), 1169. https://doi.org/10.3390/jcm8081169





