Atrial Fibrillation: A New Indicator for Advanced Colorectal Neoplasia in Screening Colonoscopy
Abstract
1. Introduction
2. Methods
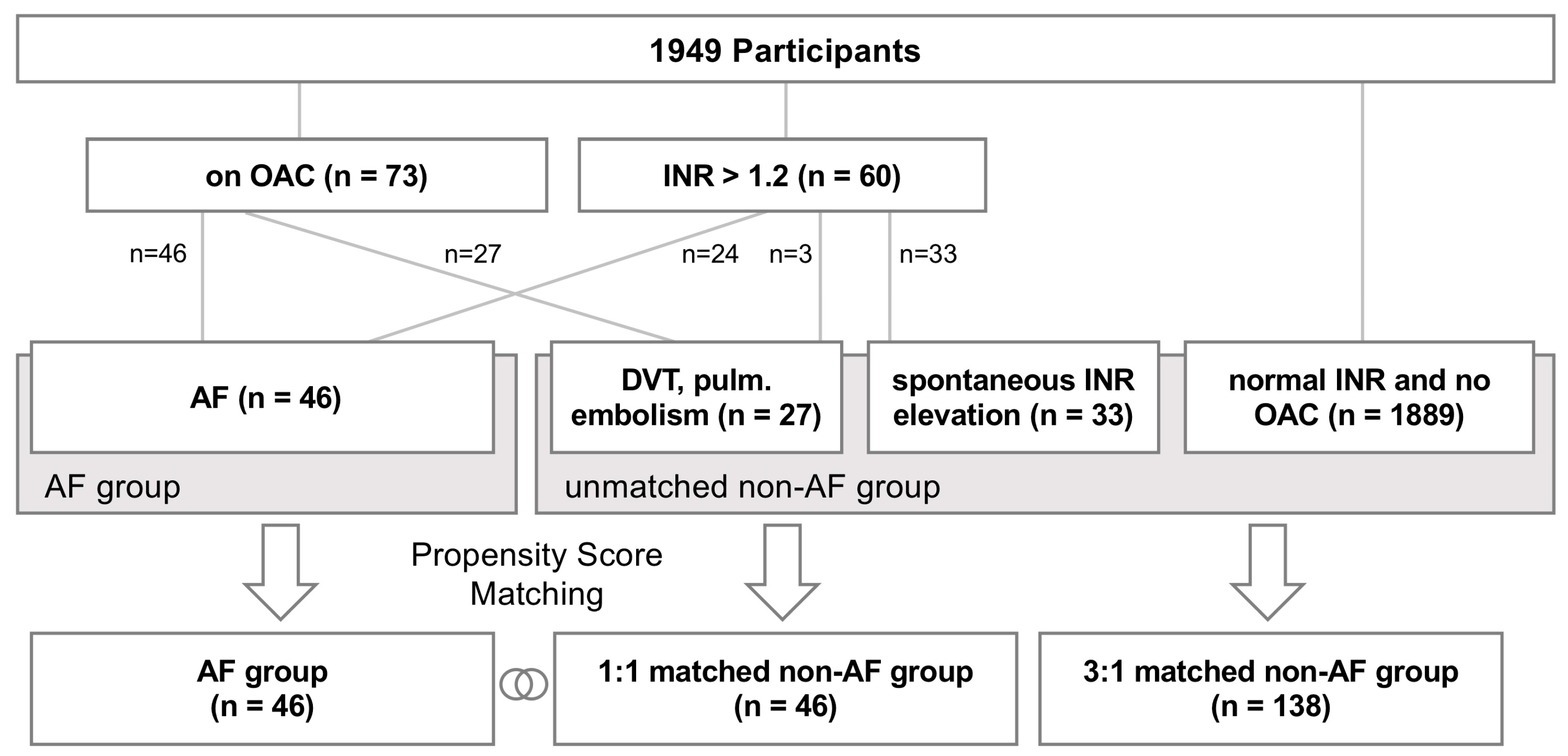
2.1. Subjects
2.2. Patient Assessment
2.3. Propensity Score Matching and Statistical Analysis
3. Results
3.1. Study Population and Cardiovascular Risk
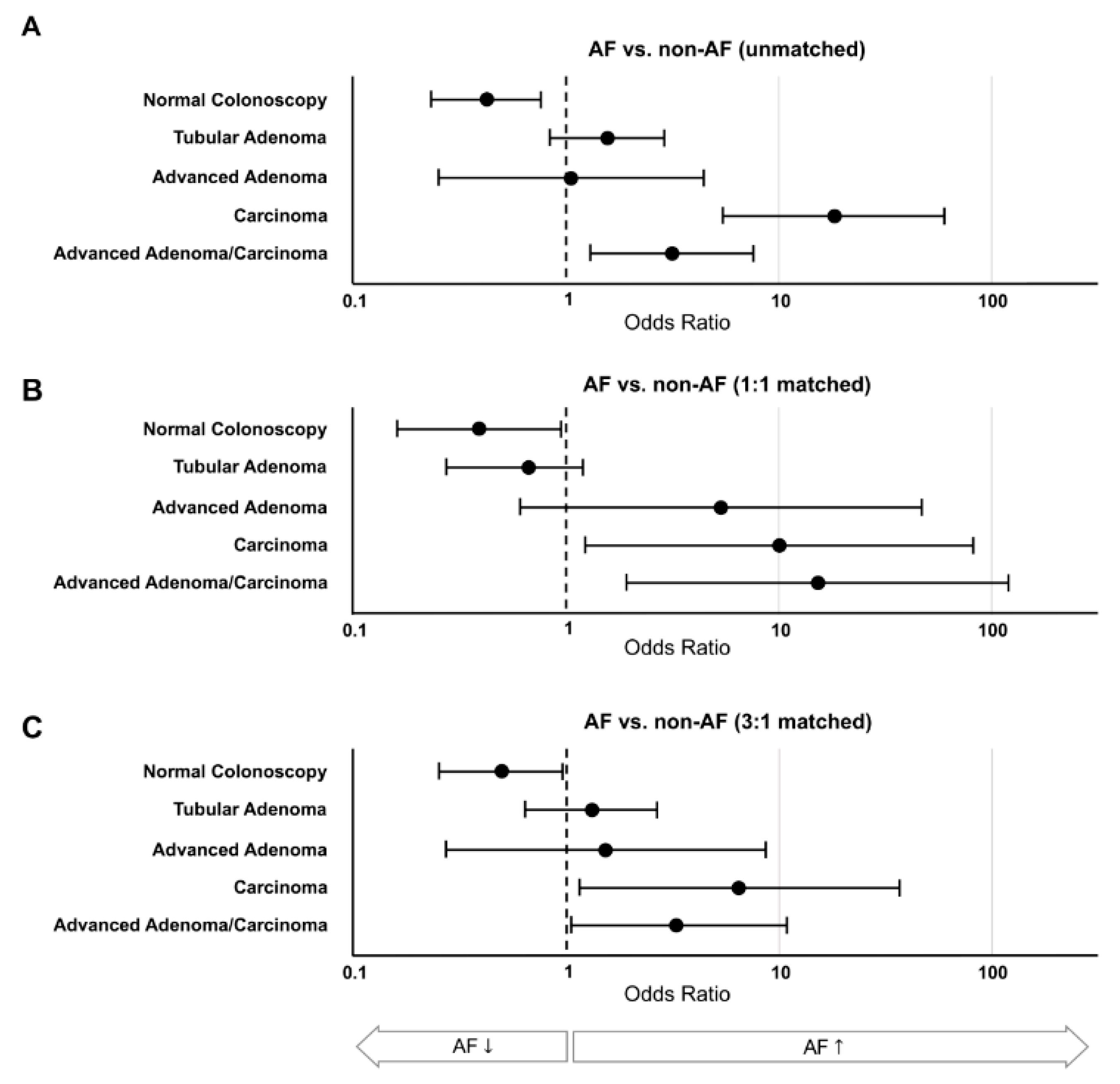
3.2. CRC Screening Results
4. Discussion
4.1. Study Population and Matching
4.2. Obstacles to CRC Screening in AF Patients
4.3. Causality
4.4. AF—an Indicator of Lifestyle Associated Disease
5. Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Presentation at Conference
References
- Eurostat. Cancer Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Cancer_statistics (accessed on 19 July 2019).
- Torre, L.A.; Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Bosetti, C.; Levi, F.; Rosato, V.; Bertuccio, P.; Lucchini, F.; Negri, E.; La Vecchia, C. Recent trends in colorectal cancer mortality in Europe. Int. J. Cancer 2011, 129, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.K.; Kohler, B.A.; Zauberet, A.G.; Anderson, R.N.; Lynn, A.G.; Laura, C.; Maria, J.S.; Elizabeth, W.; Christie, E.; Iris, L.-V.; et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 153, 307–323. [Google Scholar] [CrossRef]
- Von Karsa, L.; Patnick, J.; Segnan, N.; Atkin, W.; Halloran, S.; Vignatelli, L.; Malila, N.; Minozzi, S.; Moss, S.; Quirke, P.L.; et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: Overview and introduction to the full supplement publication. Endoscopy 2013, 45, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; Forouzanfar, M.H.; Naghavi, M.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef]
- Fabritz, L.; Guasch, E.; Antoniades, C.; Bardinet, I.; Benninger, G.; Betts, T.R.; Brand, E.; Breithardt, G.; Camm, A.J.; Cartlidge, D.; et al. Expert consensus document: Defining the major health modifiers causing atrial fibrillation: A roadmap to underpin personalized prevention and treatment. Nat. Rev. Cardiol. 2016, 13, 230–237. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Niederseer, D.; Stadlmayr, A.; Huber-Schönauer, U.; Plöderl, M.; Schmied, C.; Lederer, D.; Patsch, W.; Aigner, E.; Datz, C. Cardiovascular Risk and Known Coronary Artery Disease Are Associated with Colorectal Adenoma and Advanced Neoplasia. J. Am. Coll. Cardiol. 2017, 69, 2348–2350. [Google Scholar] [CrossRef]
- Ferlitsch, M.; Salzl, P.; Weiss, W.; Müller, C.; Bannert, C.; Knoflach, P.; Häfner, M.; Peck-Radosavljevic, M.; Trauner, M.; Gschwantler, M. Empfehlungen der ÖGGH zur Darmkrebsvorsorge und Nachsorge nach koloskopischer Polypektomie. J. für Gastroenterologische und Hepatologische Erkrankungen 2012, 10, 29–30. [Google Scholar]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Winawer, S.J.; Zauber, A.G. The advanced adenoma as the primary target of screening. Gastrointest. Endosc. Clin. North Am. 2002, 12, 1–9. [Google Scholar] [CrossRef]
- Bond, J.H. Polyp guideline: Diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am. J. Gastroenterol. 2000, 95, 3053–3063. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Wilde, S.; Wild, P.S.; Munzel, T.; Blankenberg, S. Atrial fibrillation: Its prevalence and risk factor profile in the German general population. Deutsches Arzteblatt Int. 2012, 109, 293–299. [Google Scholar] [CrossRef]
- Hjern, F.; Johansson, C.; Mellgren, A.; Baxter, N.N.; Hjern, A. Diverticular disease and migration--the influence of acculturation to a Western lifestyle on diverticular disease. Aliment. Pharmacol. Ther. 2006, 23, 797–805. [Google Scholar] [CrossRef]
- Warren, J.L.; Klabunde, C.N.; Mariotto, A.B.; Meekins, A.; Topor, M.; Brown, M.L.; Ransohoff, D.F. Adverse events after outpatient colonoscopy in the Medicare population. Ann. Intern. Med. 2009, 150, 849–857. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Baron, T.H.; Kamath, P.S.; McBane, R.D. Management of Antithrombotic Therapy in Patients Undergoing Invasive Procedures. N. Engl. J. Med. 2013, 368, 2113–2124. [Google Scholar] [CrossRef]
- Slivnick, J.A.; Yeow, R.Y.; McMahon, C.; Paje, D.G.; Kurlander, J.E.; Barnes, G.D. Current Trends in Anticoagulation Bridging for Patients with Chronic Atrial Fibrillation on Warfarin Undergoing Endoscopy. Am. J. Cardiol. 2018, 121, 1548–1551. [Google Scholar] [CrossRef]
- Dunn, M.D.; David, A.; Garcia, M.D.; Jacobson, A.; Amir, K.; Jaffer, M.D.; David, F.; Kong, M.D.; Thomas, L.; Ortel, M.D.; et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N. Engl. J. Med. 2015, 373, 823–833. [Google Scholar] [CrossRef]
- Bujanda, L.; Lanas, A.; Quintero, E.; Castells, A.; Sarasqueta, C.; Cubiella, J.; Hernandez, V.; Salas, D.; Jover, R.; Cosme, A.; et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. Mayo Clin. Proc. 2013, 88, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Bujanda, L.; Mandelli, G.; Radaelli, F.; Paggi, S.; Terreni, N.; Gola, G.; Gramegna, M.; Bonaffini, A.; Terruzzi, V.; Jover, R.; et al. Effect of oral anticoagulants on the outcome of faecal immunochemical test. Br. J. Cancer 2014, 110, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, M.S.; McDougall, H.; Nelson, D.B.; Bond, J.H. Fecal occult blood test in patients on low-dose aspirin, warfarin, clopidogrel, or non-steroidal anti-inflammatory drugs. Dig. Dis. Sci. 2010, 55, 1637–1642. [Google Scholar] [CrossRef]
- Mandelli, G.; Radaelli, F.; Paggi, S.; Terreni, N.; Gola, G.; Gramegna, M.; Bonaffini, A.; Terruzzi, V. Anticoagulant or aspirin treatment does not affect the positive predictive value of an immunological fecal occult blood test in patients undergoing colorectal cancer screening: Results from a nested in a cohort case-control study. Eur. J. Gastroenterol. Hepatol. 2011, 23, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, R.; Christiansen, C.F.; Mehnert, F.; Weiss, N.S.; Baron, J.A.; Sørensen, H.T. Colorectal cancer and risk of atrial fibrillation and flutter: A population-based case-control study. Intern. Emerg. Med. 2012, 7, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef]
- Ostenfeld, E.B.; Erichsen, R.; Pedersen, L.; Farkas, D.K.; Weiss, N.S.; Sørensen, H.T. Atrial fibrillation as a marker of occult cancer. PloS ONE 2014, 9, e102861. [Google Scholar] [CrossRef]
- Conen, D.; Wong, J.A.; Sandhu, R.K.; Cook, N.R.; Lee, I.M.; Buring, J.E.; Albert, C.M. Risk of Malignant Cancer Among Women With New-Onset Atrial Fibrillation. JAMA Cardiol. 2016, 1, 389–396. [Google Scholar] [CrossRef]
- Kim, C.H.; Al-Kindi, S.G.; Oliveira, G.H. Atrial Fibrillation and Cancer-Validation in the Real World. JAMA Cardiol. 2017, 2, 343–344. [Google Scholar] [CrossRef]
- Rahman, F.; Ko, D.; Benjamin, E.J. Association of Atrial Fibrillation and Cancer. JAMA Cardiol. 2016, 1, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, J.; Kors, J.A.; Hofman, A.; van Rooij, F.J.; Witteman, J.C. Cigarette smoking and risk of atrial fibrillation: The Rotterdam Study. Am. Heart J. 2008, 156, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, G.; Radaelli, F.; Paggi, S.; Terreni, N.; Gola, G.; Gramegna, M.; Terruzzi, V.; Sanders, P.; Kalman, M.; Abhayaratna, P.; et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J. Am. Coll. Cardiol. 2015, 65, 2159–2169. [Google Scholar] [CrossRef]
- Middeldorp, M.E.; Pathak, R.K.; Meredith, M.; Mehta, A.B.; Elliott, A.D.; Mahajan, R.; Twomey, D.; Gallagher, C.; Hendriks, J.M.L.; Linz, D.; et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: The REVERSE-AF study. Eur. Eur. 2018, 20, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Elliott, A.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Lau, D.H.; Dennis, H.; Walter, P.; Jonathan, M.; et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals with Atrial Fibrillation: The CARDIO-FIT Study. J. Am. Coll. Cardiol. 2015, 66, 985–996. [Google Scholar] [CrossRef]
- Huxley, R.R.; Ansary-Moghaddam, A.; Clifton, P.; Czernichow, S.; Parr, C.L.; Woodward, M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: A quantitative overview of the epidemiological evidence. Int. J. Cancer 2009, 125, 171–180. [Google Scholar] [CrossRef]
| All Participants | Non-AF Group | AF Group | Statistical Comparison AF vs. Non-AF | |||||
|---|---|---|---|---|---|---|---|---|
| Unmatched | 1:1 Matched | 3:1 Matched | Unmatched | 1:1 Matched | 3:1 Matched | |||
| PARTICIPANTS | 1949 | 1903 | 46 | 138 | 46 | |||
| Female | 957 (49.1%) | 948 (49.8%) | 9 (19.6%) | 28 (20.3%) | 9 (19.6%) | p < 0.001 | p = 1 | p = 0.915 |
| Age (years) | 61 [54–67] | 60 [5–67] | 72 [68–75] | 72 [68–75] | 72 [68–75] | p < 0.001 | p = 1 | p = 0.955 |
| Range | 46–79 | 46–79 | 57–79 | 57–79 | 57–79 | |||
| BMI (kg/m2) | 26.8 [24.1–29.8] | 26.7 [24.1–29.8] | 26.4 [24.3–29.4] | 27.1 [24.9–30.3] | 27.8 [24.0–30.4] | p = 0.264 | p = 0.276 | p = 0.984 |
| COMORBIDITIES | ||||||||
| ESC SCORE | 0.02 [0.01–0.05] | 0.02 [0.01–0.05] | 0.07 [0.05–0.11] | 0.07 [0.04–0.10] | 0.05 [0.03–0.09] | p < 0.001 | p = 0.039 | p = 0.047 |
| Coronary Artery Disease | 112 (6%) | 99 (5%) | 5 (11%) | 20 (14.5%) | 13 (28%) | p < 0.001 | p = 0.036 | p = 0.035 |
| Diabetes Mellitus | 303 (16%) | 282 (15%) | 9 (20%) | 26 (21.8%) | 21 (46%) | p < 0.001 | p = 0.008 | p < 0.001 |
| Arterial Hypertension | 1237 (64%) | 1224 (65%) | 34 (74%) | 104 (75.4%) | 29 (63%) | p = 0.864 | p = 0.262 | p = 0.106 |
| Smoking Ever Current | 545 (48%) 192 (17%) | 533 (48%) 189 (17%) | 22 (48%) 5 (11%) | 55 (39.9%) 14 (10.1%) | 14 (31%) 6 (13%) | p = 0.085 p = 0.333 | p = 0.087 p = 0.748 | p = 0.253 p = 0.584 |
| All Participants | Non-AF Group | AF Group | Statistical Comparison AF vs. Non-AF | |||||
|---|---|---|---|---|---|---|---|---|
| Unmatched | 1:1 Matched | 3:1 Matched | Unmatched | 1:1 Matched | 3:1 Matched | |||
| Number of Participants | 1949 | 1903 | 46 | 138 | 46 | - | - | - |
| CRC Screening Results | ||||||||
| Normal Colonoscopy | 1395 (71.6%) | 1371 (72.1%) | 34 (73.9%) | 95 (68.8%) | 24 (52.2%) | 0.003 | 0.031 | 0.041 |
| Tubular Adenoma | 503 (25.8%) | 487 (25.6%) | 12 (26.1%) | 40 (29.0%) | 16 (34.8%) | 0.160 | 0.365 | 0.459 |
| Advanced Adenoma | 81 (4.2%) | 79 (4.2%) | 0 | 4 (2.9%) | 2 (4.3%) | 0.948 | 0.153 | 0.632 |
| Carcinoma | 14 (0.7%) | 10 (0.5%) | 0 | 2 (1:4%) | 4 (8.7%) | <0.001 | 0.041 | 0.017 |
| Adv. Adenoma & Carcinoma combined | 93 (4.8%) | 87 (4.6%) | 0 | 6 (4.3%) | 6 (13.3%) | 0.008 | 0.013 | 0.039 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahr, P.C.; Hammerl, S.; Huber-Schönauer, U.; Schmied, C.M.; Haegeli, L.M.; Obeid, S.; Eder, S.; Bachmayer, S.; Aigner, E.; Datz, C.; et al. Atrial Fibrillation: A New Indicator for Advanced Colorectal Neoplasia in Screening Colonoscopy. J. Clin. Med. 2019, 8, 1083. https://doi.org/10.3390/jcm8071083
Kahr PC, Hammerl S, Huber-Schönauer U, Schmied CM, Haegeli LM, Obeid S, Eder S, Bachmayer S, Aigner E, Datz C, et al. Atrial Fibrillation: A New Indicator for Advanced Colorectal Neoplasia in Screening Colonoscopy. Journal of Clinical Medicine. 2019; 8(7):1083. https://doi.org/10.3390/jcm8071083
Chicago/Turabian StyleKahr, Peter C., Sabrina Hammerl, Ursula Huber-Schönauer, Christian M Schmied, Laurent M. Haegeli, Slayman Obeid, Sarah Eder, Sebastian Bachmayer, Elmar Aigner, Christian Datz, and et al. 2019. "Atrial Fibrillation: A New Indicator for Advanced Colorectal Neoplasia in Screening Colonoscopy" Journal of Clinical Medicine 8, no. 7: 1083. https://doi.org/10.3390/jcm8071083
APA StyleKahr, P. C., Hammerl, S., Huber-Schönauer, U., Schmied, C. M., Haegeli, L. M., Obeid, S., Eder, S., Bachmayer, S., Aigner, E., Datz, C., & Niederseer, D. (2019). Atrial Fibrillation: A New Indicator for Advanced Colorectal Neoplasia in Screening Colonoscopy. Journal of Clinical Medicine, 8(7), 1083. https://doi.org/10.3390/jcm8071083






