Compensatory Neural Recruitment for Error-Related Cerebral Activity in Patients with Moderate-To-Severe Obstructive Sleep Apnea
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Modified Flanker Task and Assessments of Error-Related ERPs
2.3. Polysomnography
2.4. fMRI Scan Protocol
2.5. fMRI Data Analysis
2.6. Statistical Analysis
3. Results
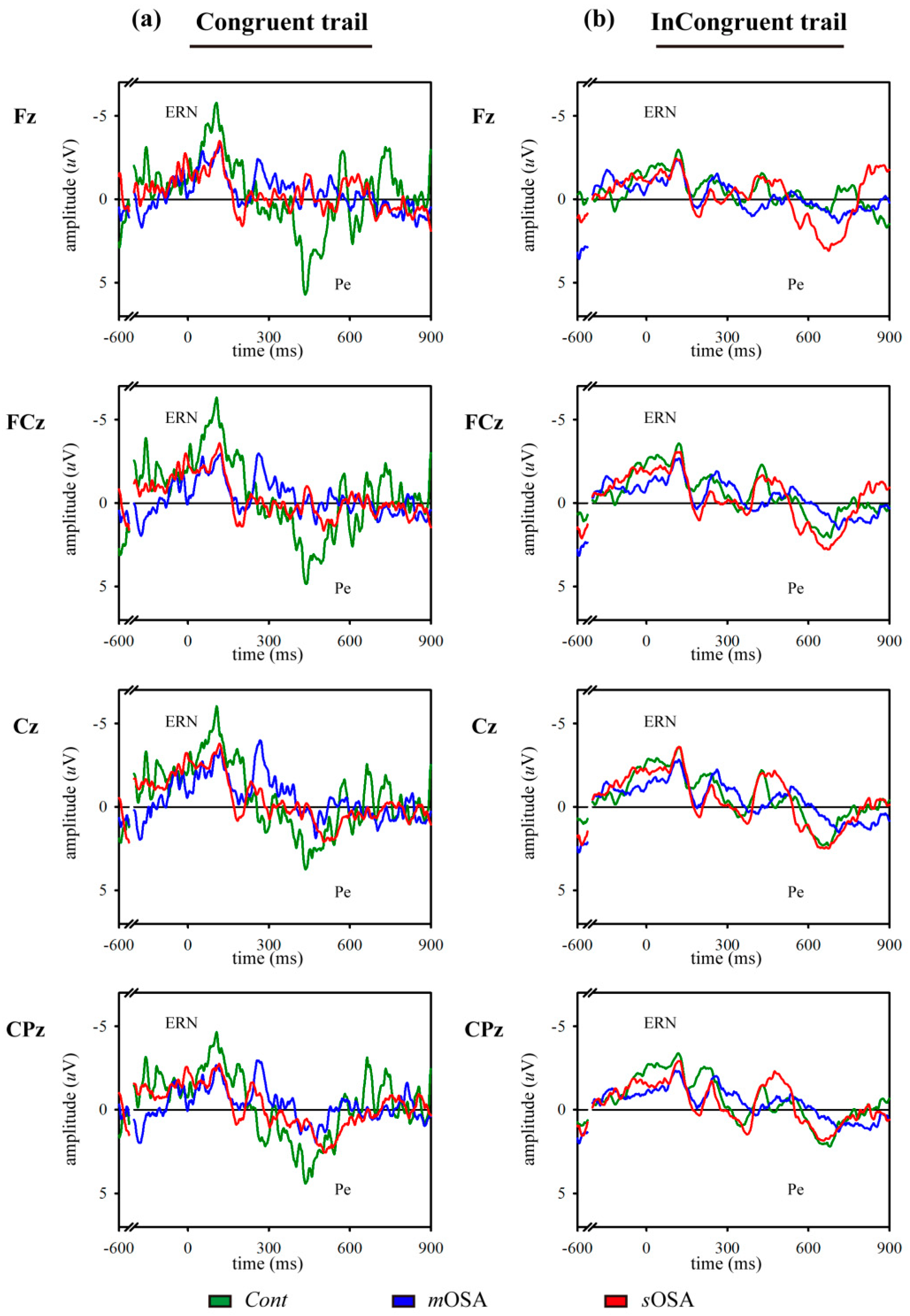
3.1. Behavioral Response and Error-Related ERP Analysis in the Modified Flanker Task
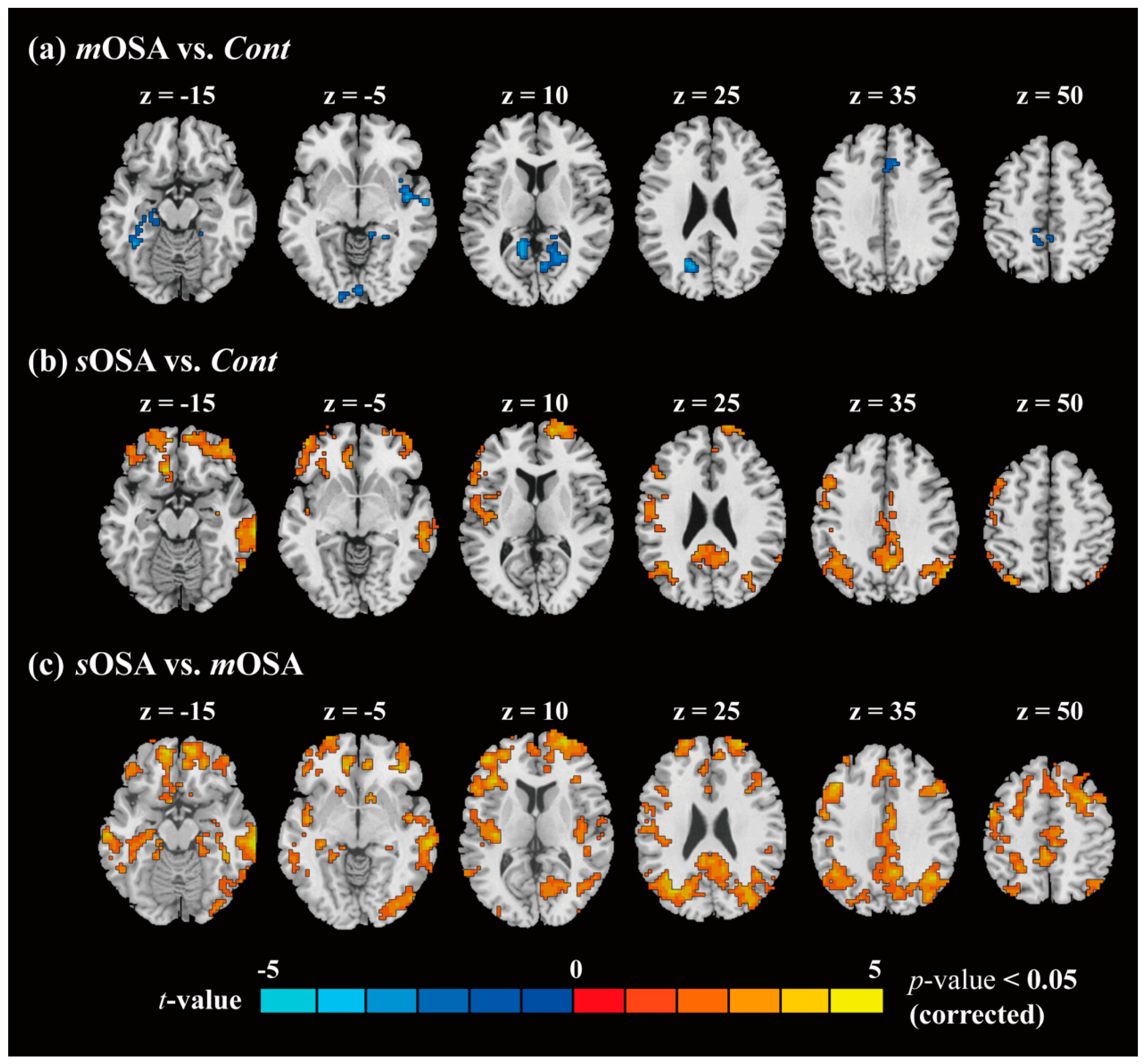
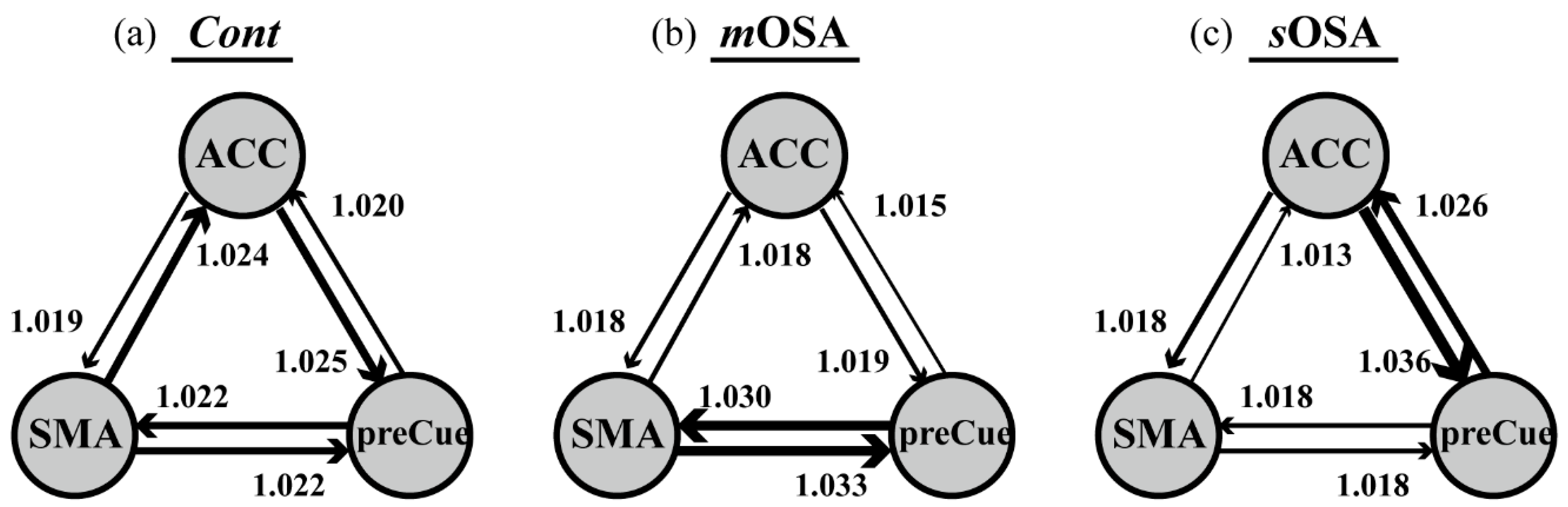
3.2. fMRI Response and Functional Connectivity
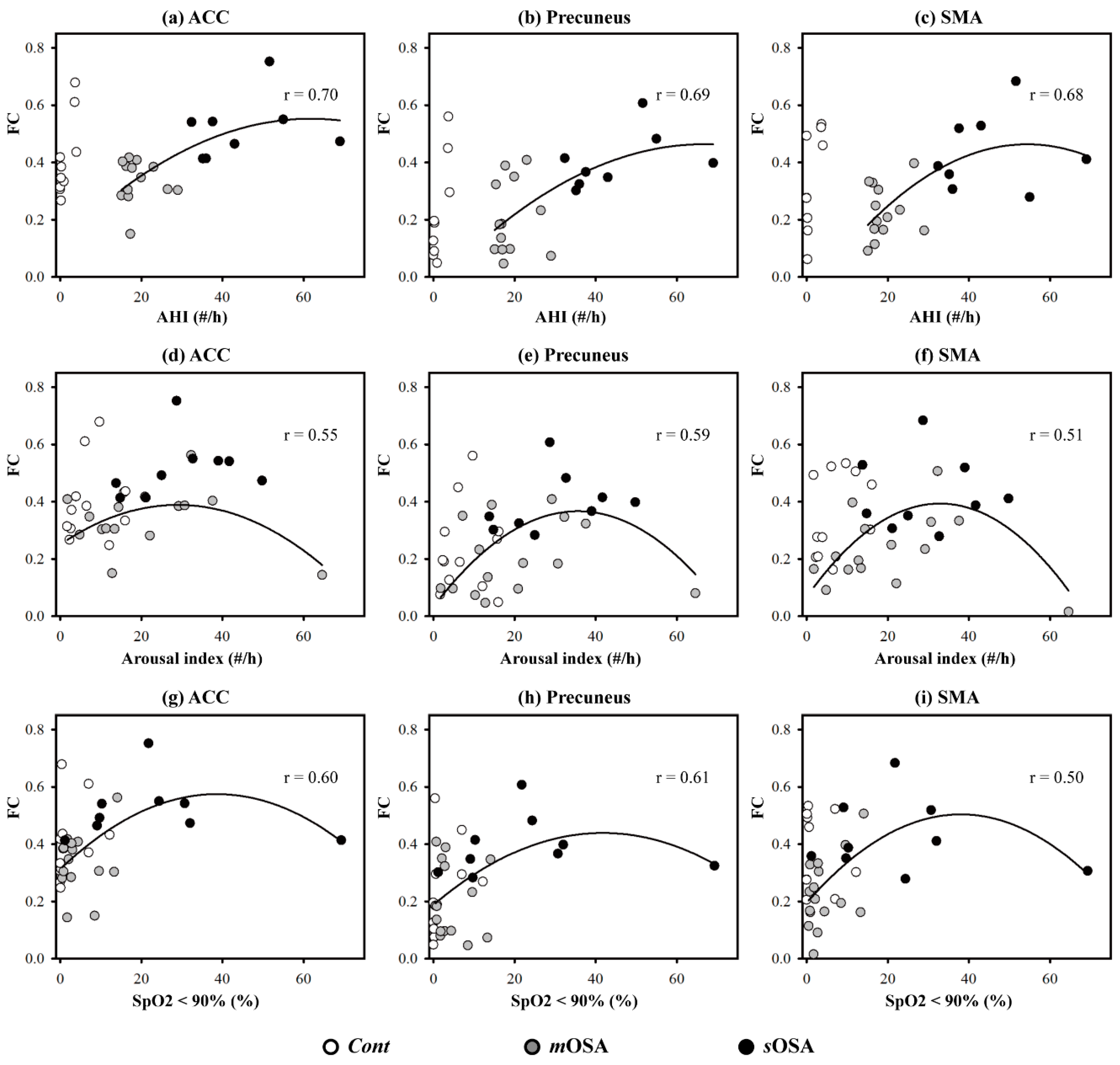
3.3. The Relationship between Functional Performance and PSG Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mannarino, M.R.; Di Filippo, F.; Pirro, M. Obstructive sleep apnea syndrome. Eur. J. Int. Med. 2012, 23, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.W.; Gozal, D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J. Sleep Res. 2002, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive impairment in obstructive sleep apnea. Chest 2012, 141, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Pack, A.I. Obstructive sleep apnea and cognitive impairment: Addressing the blood-brain barrier. Sleep Med. Rev. 2014, 18, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Bucks, R.S.; Olaithe, M.; Eastwood, P. Neurocognitive function in obstructive sleep apnoea: A meta-review. Respirology 2013, 18, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Rabbitt, P.; Rodgers, B. What does a man do after he makes an error? An analysis of response programming. Q. J. Exp. Psychol. 1977, 29, 727–743. [Google Scholar] [CrossRef]
- Rabbitt, P.M. Errors and error correction in choice-response tasks. J. Exp. Psychol. 1966, 71, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.J.; Knight, R.T. Prefrontal-cingulate interactions in action monitoring. Nat. Neurosci. 2000, 3, 516–520. [Google Scholar] [CrossRef]
- Chou, P.S.; Hsu, C.Y.; Wu, M.N.; Liou, L.M.; Lu, S.R.; Liu, C.K.; Lai, C.L. Action-Monitoring Dysfunction in Obstructive Sleep Apnea—A Pilot Study. PLoS ONE 2016, 11, e0157575. [Google Scholar] [CrossRef]
- Falkenstein, M.; Hohnsbein, J.; Hoormann, J.; Blanke, L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr. Clin. Neurophysiol. 1991, 78, 447–455. [Google Scholar] [CrossRef]
- Van Veen, V.; Carter, C.S. The timing of action-monitoring processes in the anterior cingulate cortex. J. Cogn. Neurosci. 2002, 14, 593–602. [Google Scholar] [CrossRef]
- Falkenstein, M.; Hoormann, J.; Christ, S.; Hohnsbein, J. ERP components on reaction errors and their functional significance: A tutorial. Biol. Psychol. 2000, 51, 87–107. [Google Scholar] [CrossRef]
- Yeung, N.; Botvinick, M.M.; Cohen, J.D. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol. Rev. 2004, 111, 931–959. [Google Scholar] [CrossRef]
- Stahl, J.; Gibbons, H. Dynamics of response-conflict monitoring and individual differences in response control and behavioral control: An electrophysiological investigation using a stop-signal task. Clin. Neurophysiol. 2007, 118, 581–596. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Ridderinkhof, K.R.; Blom, J.; Band, G.P.; Kok, A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology 2001, 38, 752–760. [Google Scholar] [CrossRef]
- Endrass, T.; Reuter, B.; Kathmann, N. ERP correlates of conscious error recognition: Aware and unaware errors in an antisaccade task. Eur. J. Neurosci. 2007, 26, 1714–1720. [Google Scholar] [CrossRef]
- Vocat, R.; Pourtois, G.; Vuilleumier, P. Unavoidable errors: A spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia 2008, 46, 2545–2555. [Google Scholar] [CrossRef]
- Asaoka, S.; Fukuda, K.; Murphy, T.I.; Abe, T.; Inoue, Y. The effects of a nighttime nap on the error-monitoring functions during extended wakefulness. Sleep 2012, 35, 871–878. [Google Scholar] [CrossRef]
- Hsieh, S.; Cheng, I.C.; Tsai, L.L. Immediate error correction process following sleep deprivation. J. Sleep Res. 2007, 16, 137–147. [Google Scholar] [CrossRef]
- Murphy, T.I.; Richard, M.; Masaki, H.; Segalowitz, S.J. The effect of sleepiness on performance monitoring: I know what I am doing, but do I care? J. Sleep Res. 2006, 15, 15–21. [Google Scholar] [CrossRef]
- Tsai, L.L.; Young, H.Y.; Hsieh, S.; Lee, C.S. Impairment of error monitoring following sleep deprivation. Sleep 2005, 28, 707–713. [Google Scholar] [CrossRef]
- Ayalon, L.; Ancoli-Israel, S.; Aka, A.A.; McKenna, B.S.; Drummond, S.P. Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. Sleep 2009, 32, 373–381. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Ma, L.; Li, S.; Wang, Y.; Wang, L. A functional MRI evaluation of frontal dysfunction in patients with severe obstructive sleep apnea. Sleep Med. 2011, 12, 335–340. [Google Scholar] [CrossRef]
- Ayalon, L.; Ancoli-Israel, S.; Drummond, S.P. Altered brain activation during response inhibition in obstructive sleep apnea. J. Sleep Res. 2009, 18, 204–208. [Google Scholar] [CrossRef]
- Biswal, B.B. Resting state fMRI: A personal history. NeuroImage 2012, 62, 938–944. [Google Scholar] [CrossRef]
- Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999, 22, 667–689.
- Teng, E.L.; Hasegawa, K.; Homma, A.; Imai, Y.; Larson, E.; Graves, A.; Sugimoto, K.; Yamaguchi, T.; Sasaki, H.; Chiu, D.; et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr. 1994, 6, 45–58. [Google Scholar] [CrossRef]
- Liu, H.C.; Teng, E.L.; Lin, K.N.; Chuang, Y.Y.; Wang, P.N.; Fuh, J.L.; Liu, C.Y. Performance on the cognitive abilities screening instrument at different stages of Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2002, 13, 244–248. [Google Scholar] [CrossRef]
- Hsiao, K.-Y.; Yeh, S.-A.; Chang, C.-C.; Tsai, P.-C.; Wu, J.-M.; Gau, J.-S. Cognitive function before and after intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 722–726. [Google Scholar] [CrossRef]
- Ullsperger, M.; Von Cramon, D.Y. Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage 2001, 14, 1387–1401. [Google Scholar] [CrossRef]
- Iannaccone, R.; Hauser, T.U.; Staempfli, P.; Walitza, S.; Brandeis, D.; Brem, S. Conflict monitoring and error processing: New insights from simultaneous EEG–fMRI. NeuroImage 2015, 105, 395–407. [Google Scholar] [CrossRef]
- Pourtois, G. Early error detection predicted by reduced pre-response control process: An ERP topographic mapping study. Brain Topogr. 2011, 23, 403–422. [Google Scholar] [CrossRef][Green Version]
- Von der Gablentz, J.; Tempelmann, C.; Münte, T.; Heldmann, M. Performance monitoring and behavioral adaptation during task switching: An fMRI study. Neuroscience 2015, 285, 227–235. [Google Scholar] [CrossRef]
- Zang, Z.-X.; Yan, C.-G.; Dong, Z.-Y.; Huang, J.; Zang, Y.-F. Granger causality analysis implementation on MATLAB: A graphic user interface toolkit for fMRI data processing. J. Neurosci. Methods 2012, 203, 418–426. [Google Scholar] [CrossRef]
- Levine, T.R.; Hullett, C.R. Eta squared, partial eta squared, and misreporting of effect size in communication research. Hum. Commun. Res. 2002, 28, 612–625. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NY, USA, 1988; p. 567. [Google Scholar]
- Renn, R.P.; Cote, K.A. Performance monitoring following total sleep deprivation: Effects of task type and error rate. Int. J. Psychophysiol. 2013, 88, 64–73. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, P.; Peng, Y.; Ouyang, R. Role of Oxidative Stress in the Neurocognitive Dysfunction of Obstructive Sleep Apnea Syndrome. Oxid. Med. Cell. Longev. 2016, 2016, 9626831. [Google Scholar] [CrossRef]
- Chen, H.L.; Lin, H.C.; Lu, C.H.; Chen, P.C.; Huang, C.C.; Chou, K.H.; Su, M.C.; Friedman, M.; Chen, Y.W.; Lin, W.C. Systemic inflammation and alterations to cerebral blood flow in obstructive sleep apnea. J. Sleep Res. 2017, 26, 789–798. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; He, X.; Li, Q.; Chen, B.; Zhang, Y.; Yu, C. Functional disconnection of the right anterior insula in obstructive sleep apnea. Sleep Med. 2015, 16, 1062–1070. [Google Scholar] [CrossRef]
- Joo, E.Y.; Tae, W.S.; Lee, M.J.; Kang, J.W.; Park, H.S.; Lee, J.Y.; Suh, M.; Hong, S.B. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep 2010, 33, 235–241. [Google Scholar] [CrossRef]
- Macey, P.M.; Kumar, R.; Woo, M.A.; Valladares, E.M.; Yan-Go, F.L.; Harper, R.M. Brain structural changes in obstructive sleep apnea. Sleep 2008, 31, 967–977. [Google Scholar]
- Logan, J.M.; Sanders, A.L.; Snyder, A.Z.; Morris, J.C.; Buckner, R.L. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron 2002, 33, 827–840. [Google Scholar] [CrossRef]
- Drummond, S.P.; Meloy, M.J.; Yanagi, M.A.; Orff, H.J.; Brown, G.G. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. Neuroimaging 2005, 140, 140–211. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Qin, W.; Li, Q.; Chen, B.; Zhang, Y.; Yu, C. Altered resting-state brain activity in obstructive sleep apnea. Sleep 2013, 36, 651–659. [Google Scholar] [CrossRef]
- Ayalon, L.; Ancoli-Israel, S.; Klemfuss, Z.; Shalauta, M.D.; Drummond, S.P. Increased brain activation during verbal learning in obstructive sleep apnea. NeuroImage 2006, 31, 1817–1825. [Google Scholar] [CrossRef]
- Archbold, K.H.; Borghesani, P.R.; Mahurin, R.K.; Kapur, V.K.; Landis, C.A. Neural activation patterns during working memory tasks and OSA disease severity: Preliminary findings. J. Clin. Sleep Med. 2009, 5, 21–27. [Google Scholar]
- Park, B.; Palomares, J.A.; Woo, M.A.; Kang, D.W.; Macey, P.M.; Yan-Go, F.L.; Harper, R.M.; Kumar, R. Disrupted functional brain network organization in patients with obstructive sleep apnea. Brain Behav. 2016, 6, e00441. [Google Scholar] [CrossRef]
- Bonini, F.; Burle, B.; Liégeois-Chauvel, C.; Régis, J.; Chauvel, P.; Vidal, F. Action monitoring and medial frontal cortex: Leading role of supplementary motor area. Science 2014, 343, 888–891. [Google Scholar] [CrossRef]
- Nagahama, Y.; Okada, T.; Katsumi, Y.; Hayashi, T.; Yamauchi, H.; Sawamoto, N.; Toma, K.; Nakamura, K.; Hanakawa, T.; Konishi, J. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. NeuroImage 1999, 10, 193–199. [Google Scholar] [CrossRef]
- Redline, S.; Strauss, M.E.; Adams, N.; Winters, M.; Roebuck, T.; Spry, K.; Rosenberg, C.; Adams, K. Neuropsychological function in mild sleep-disordered breathing. Sleep 1997, 20, 160–167. [Google Scholar] [CrossRef]
- Kim, H.; Thomas, R.J.; Yun, C.H.; Au, R.; Lee, S.K.; Lee, S.; Shin, C. Association of Mild Obstructive Sleep Apnea with Cognitive Performance, Excessive Daytime Sleepiness, and Quality of Life in the General Population: The Korean Genome and Epidemiology Study (KoGES). Sleep 2017, 40, zsx012. [Google Scholar] [CrossRef][Green Version]

| Cont (n = 14) | mOSA (n = 17) | sOSA (n = 10) | p value | ||
|---|---|---|---|---|---|
| Gender (males, %) † | 6 (42.9) | 13 (76.5) | 7 (70.0) | 0.136 | - |
| Age (year) | 42.5 (2.4) | 49.2 (1.8) | 50.7 (3.7) | 0.183 | 0.095 |
| Education (years) | 14.5 (0.7) | 13.6 (0.6) | 12.9 (0.8) | 0.640 | 0.026 |
| CASI (total scores) | 96.3 (1.2) | 93.6 (1.0) | 93.7 (1.4) | 0.183 | 0.095 |
| AHI (#/h) | 1.2 (0.4) | 19.8 (1.1) | 45.6 (3.8) | <0.001 ** | 0.878 |
| Arousal index (#/h) | 8.2 (1.4) | 20.6 (3.7) | 29.6 (3.7) | <0.001 ** | 0.347 |
| SpO2 < 90% (%) | 4.1 (2.0) | 4.7 (1.1) | 21.6 (6.3) | 0.001 * | 0.326 |
| ESS | 7 (0.7) | 10.4 (1.1) | 9.1 (1.0) | 0.036 * | 0.183 |
| Error response rate (%) | |||||
| Congruent | 1.6 (0.9) | 13.9 (6.3) | 18.7 (8.3) | 0.122 | 0.105 |
| Incongruent | 4.9 (1.0) | 21.7 (7.7) | 25.2 (8.8) | 0.091 | 0.118 |
| pError correction rate (%) | |||||
| Congruent | 86.3 (11.8) | 70.4 (9.1) | 53.2 (13.4) | 0.195 | 0.110 |
| Incongruent | 89.8 (6.6) | 74.2 (6.0) | 64.1 (7.9) | 0.048 * | 0.148 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, P.-S.; Chen, S.C.-J.; Hsu, C.-Y.; Liou, L.-M.; Wu, M.-N.; Liu, C.-K.; Lai, C.-L. Compensatory Neural Recruitment for Error-Related Cerebral Activity in Patients with Moderate-To-Severe Obstructive Sleep Apnea. J. Clin. Med. 2019, 8, 1077. https://doi.org/10.3390/jcm8071077
Chou P-S, Chen SC-J, Hsu C-Y, Liou L-M, Wu M-N, Liu C-K, Lai C-L. Compensatory Neural Recruitment for Error-Related Cerebral Activity in Patients with Moderate-To-Severe Obstructive Sleep Apnea. Journal of Clinical Medicine. 2019; 8(7):1077. https://doi.org/10.3390/jcm8071077
Chicago/Turabian StyleChou, Ping-Song, Sharon Chia-Ju Chen, Chung-Yao Hsu, Li-Min Liou, Meng-Ni Wu, Ching-Kuan Liu, and Chiou-Lian Lai. 2019. "Compensatory Neural Recruitment for Error-Related Cerebral Activity in Patients with Moderate-To-Severe Obstructive Sleep Apnea" Journal of Clinical Medicine 8, no. 7: 1077. https://doi.org/10.3390/jcm8071077
APA StyleChou, P.-S., Chen, S. C.-J., Hsu, C.-Y., Liou, L.-M., Wu, M.-N., Liu, C.-K., & Lai, C.-L. (2019). Compensatory Neural Recruitment for Error-Related Cerebral Activity in Patients with Moderate-To-Severe Obstructive Sleep Apnea. Journal of Clinical Medicine, 8(7), 1077. https://doi.org/10.3390/jcm8071077