The Potential of Cannabidiol as a Treatment for Psychosis and Addiction: Who Benefits Most? A Systematic Review
Abstract
1. Introduction
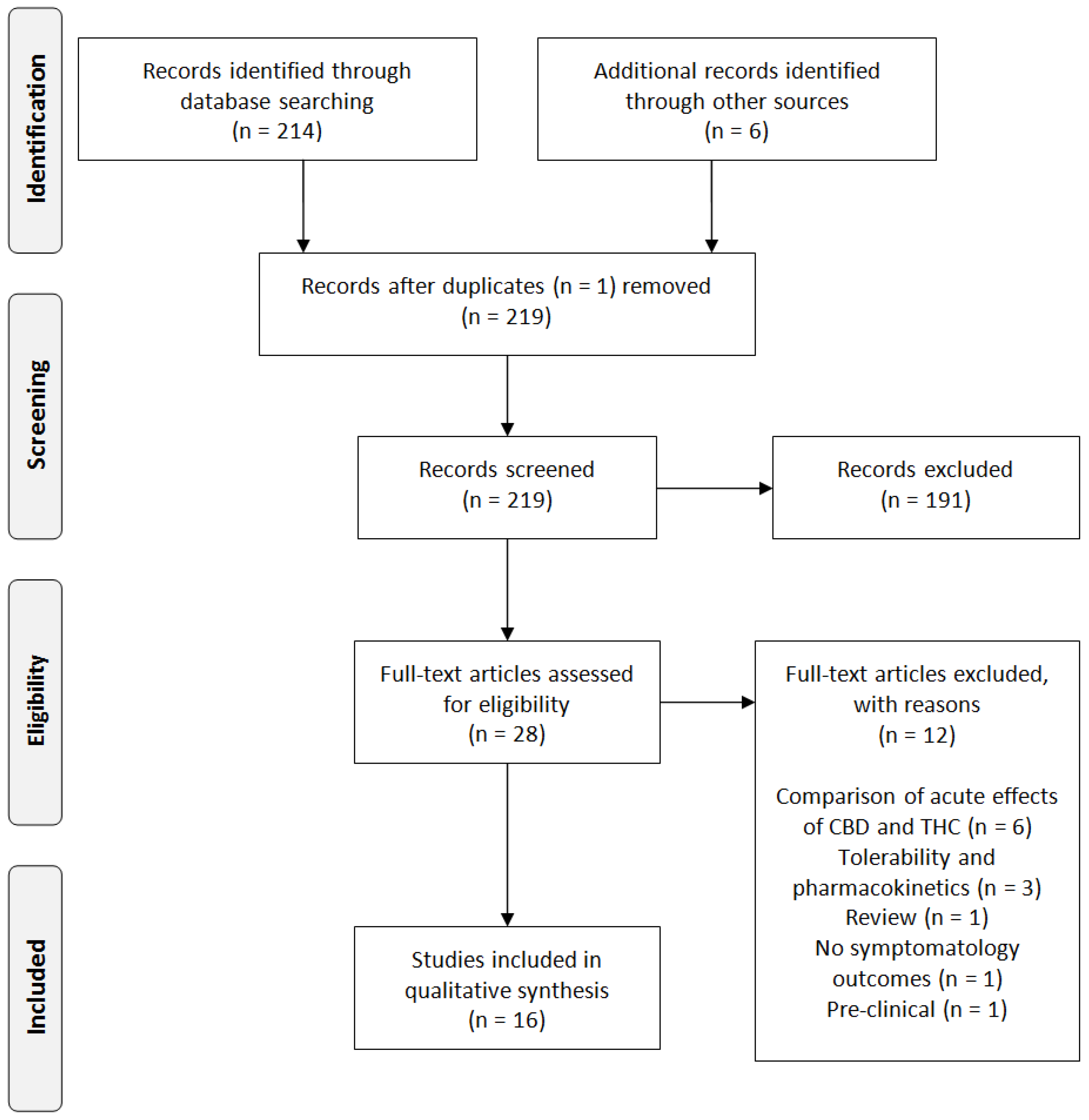
2. Experimental Section
3. Results
3.1. CBD—Psychosis
3.2. CBD—Substance Use Disorders
3.2.1. Cannabis Dependence
3.2.2. Tobacco Dependence
3.3. CBD—Psychosis and SUD
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rössler, W.; Joachim Salize, H.; van Os, J.; Riecher-Rössler, A. Size of burden of schizophrenia and psychotic disorders. Eur. Neuropsychopharmacol. 2005, 15, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Buckley, P.F.; Miller, B.J.; Lehrer, D.S.; Castle, D.J. Psychiatric Comorbidities and Schizophrenia. Schizophr. Bull. 2009, 35, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D. Substance Use Disorders in Schizophrenia—Clinical Implications of Comorbidity. Schizophr. Bull. 2009, 35, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, M.; Carlone, O.; Vitale, B.; Cinti, M.E.; Clare, L. A Meta-Analysis of Cognitive Deficits in Adults with a Diagnosis of Schizophrenia. Neuropsychol. Rev. 2005, 15, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr. Res. 2009, 110, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Van Os, J.; Kenis, G.; Rutten, B.P.F. The environment and schizophrenia. Nature 2010, 468, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R. Antipsychotics in the Treatment of Schizophrenia. J. Clin. Psychiatry 2011, 72. [Google Scholar] [CrossRef]
- Samara, M.T.; Nikolakopoulou, A.; Salanti, G.; Leucht, S. How Many Patients with Schizophrenia Do Not Respond to Antipsychotic Drugs in the Short Term? An Analysis Based on Individual Patient Data from Randomized Controlled Trials. Schizophr. Bull. 2019, 45, 639–646. [Google Scholar] [CrossRef]
- Green, A.I. Schizophrenia and comorbid substance use disorder: Effects of antipsychotics. J. Clin. Psychiatry 2005, 66 (Suppl. 6), 21–26. [Google Scholar]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Katona, I.; Freund, T.F. Multiple Functions of Endocannabinoid Signaling in the Brain. Annu. Rev. Neurosci. 2012, 35, 529–558. [Google Scholar] [CrossRef]
- Wong, D.F.; Kuwabara, H.; Horti, A.G.; Raymont, V.; Brasic, J.; Guevara, M.; Ye, W.; Dannals, R.F.; Ravert, H.T.; Nandi, A.; et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage 2010, 52, 1505–1513. [Google Scholar] [CrossRef]
- Heifets, B.D.; Castillo, P.E. Endocannabinoid Signaling and Long-Term Synaptic Plasticity. Annu. Rev. Physiol. 2009, 71, 283–306. [Google Scholar] [CrossRef]
- Hill, M.N.; Hillard, C.J.; Bambico, F.R.; Patel, S.; Gorzalka, B.B.; Gobbi, G. The Therapeutic Potential of the Endocannabinoid System for the Development of a Novel Class of Antidepressants. Trends Pharmacol. Sci. 2009, 30, 484–493. [Google Scholar] [CrossRef]
- Zanettini, C. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front. Behav. Neurosci. 2011, 5. [Google Scholar] [CrossRef]
- Bossong, M.G.; Jager, G.; Bhattacharyya, S.; Allen, P. Acute and non-acute effects of cannabis on human memory function: A critical review of neuroimaging studies. Curr. Pharm. Des. 2014, 20, 2114–2125. [Google Scholar] [CrossRef]
- Bossong, M.G.; Jansma, J.M.; Bhattacharyya, S.; Ramsey, N.F. Role of the endocannabinoid system in brain functions relevant for schizophrenia: An overview of human challenge studies with cannabis or ∆9-tetrahydrocannabinol (THC). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 52, 53–69. [Google Scholar] [CrossRef]
- Pertwee, R.G. Ligands that target cannabinoid receptors in the brain: From THC to anandamide and beyond. Addict. Biol. 2008, 13, 147–159. [Google Scholar] [CrossRef]
- Cabral, G.A.; Griffin-Thomas, L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef]
- Leweke, F.M.; Koethe, D. Cannabis and psychiatric disorders: It is not only addiction. Addict. Biol. 2008, 13, 264–275. [Google Scholar] [CrossRef]
- Bossong, M.G.; Niesink, R.J.M. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog. Neurobiol. 2010, 92, 370–385. [Google Scholar] [CrossRef]
- Marconi, A.; Di Forti, M.; Lewis, C.M.; Murray, R.M.; Vassos, E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr. Bull. 2016, 42, 1262–1269. [Google Scholar] [CrossRef]
- Large, M.; Sharma, S.; Compton, M.T.; Slade, T.; Nielssen, O. Cannabis Use and Earlier Onset of Psychosis. Arch. Gen. Psychiatry 2011, 68, 555. [Google Scholar] [CrossRef]
- Di Forti, M.; Sallis, H.; Allegri, F.; Trotta, A.; Ferraro, L.; Stilo, S.A.; Marconi, A.; La Cascia, C.; Reis Marques, T.; Pariante, C.; et al. Daily Use, Especially of High-Potency Cannabis, Drives the Earlier Onset of Psychosis in Cannabis Users. Schizophr. Bull. 2014, 40, 1509–1517. [Google Scholar] [CrossRef]
- Schubart, C.D.; van Gastel, W.A.; Breetvelt, E.J.; Beetz, S.L.; Ophoff, R.A.; Sommer, I.E.C.; Kahn, R.S.; Boks, M.P.M. Cannabis use at a young age is associated with psychotic experiences. Psychol. Med. 2011, 41, 1301–1310. [Google Scholar] [CrossRef]
- Di Forti, M.; Marconi, A.; Carra, E.; Fraietta, S.; Trotta, A.; Bonomo, M.; Bianconi, F.; Gardner-Sood, P.; O’Connor, J.; Russo, M.; et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: A case-control study. Lancet Psychiatry 2015, 2, 233–238. [Google Scholar] [CrossRef]
- Di Forti, M.; Quattrone, D.; Freeman, T.P.; Tripoli, G.; Gayer-Anderson, C.; Quigley, H.; Rodriguez, V.; Jongsma, H.E.; Ferraro, L.; La Cascia, C.; et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): A multicentre case-control study. Lancet Psychiatry 2019, 6, 427–436. [Google Scholar] [CrossRef]
- Murray, R.M.; Englund, A.; Abi-Dargham, A.; Lewis, D.A.; Di Forti, M.; Davies, C.; Sherif, M.; McGuire, P.; D’Souza, D.C. Cannabis-associated psychosis: Neural substrate and clinical impact. Neuropharmacology 2017, 124, 89–104. [Google Scholar] [CrossRef]
- Leweke, F.; Giuffrida, A.; Wurster, U.; Emrich, H.M.; Piomelli, D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport 1999, 10, 1665–1669. [Google Scholar] [CrossRef]
- Giuffrida, A.; Leweke, F.M.; Gerth, C.W.; Schreiber, D.; Koethe, D.; Faulhaber, J.; Klosterkötter, J.; Piomelli, D. Cerebrospinal Anandamide Levels are Elevated in Acute Schizophrenia and are Inversely Correlated with Psychotic Symptoms. Neuropsychopharmacology 2004, 29, 2108–2114. [Google Scholar] [CrossRef]
- Leweke, F.M.; Giuffrida, A.; Koethe, D.; Schreiber, D.; Nolden, B.M.; Kranaster, L.; Neatby, M.A.; Schneider, M.; Gerth, C.W.; Hellmich, M.; et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: Impact of cannabis use. Schizophr. Res. 2007, 94, 29–36. [Google Scholar] [CrossRef]
- Ceccarini, J.; De Hert, M.; Van Winkel, R.; Peuskens, J.; Bormans, G.; Kranaster, L.; Enning, F.; Koethe, D.; Leweke, F.M.; Van Laere, K. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage 2013, 79, 304–312. [Google Scholar] [CrossRef]
- Ranganathan, M.; Cortes-Briones, J.; Radhakrishnan, R.; Thurnauer, H.; Planeta, B.; Skosnik, P.; Gao, H.; Labaree, D.; Neumeister, A.; Pittman, B.; et al. Reduced Brain Cannabinoid Receptor Availability in Schizophrenia. Biol. Psychiatry 2016, 79, 997–1005. [Google Scholar] [CrossRef]
- De Vries, T.J.; Schoffelmeer, A.N.M. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol. Sci. 2005, 26, 420–426. [Google Scholar] [CrossRef]
- Maldonado, R.; Valverde, O.; Berrendero, F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006, 29, 225–232. [Google Scholar] [CrossRef]
- Fattore, L.; Fadda, P.; Spano, M.S.; Pistis, M.; Fratta, W. Neurobiological mechanisms of cannabinoid addiction. Mol. Cell. Endocrinol. 2008, 286, S97–S107. [Google Scholar] [CrossRef][Green Version]
- Chye, Y.; Christensen, E.; Solowij, N.; Yücel, M. The Endocannabinoid System and Cannabidiol’s Promise for the Treatment of Substance Use Disorder. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef]
- Hunt, G. Medication compliance and comorbid substance abuse in schizophrenia: Impact on community survival 4 years after a relapse. Schizophr. Res. 2002, 54, 253–264. [Google Scholar] [CrossRef]
- Crockford, D.; Addington, D. Canadian Schizophrenia Guidelines: Schizophrenia and Other Psychotic Disorders with Coexisting Substance Use Disorders. Can. J. Psychiatry 2017, 62, 624–634. [Google Scholar] [CrossRef]
- Schoeler, T.; Petros, N.; Di Forti, M.; Klamerus, E.; Foglia, E.; Murray, R.; Bhattacharyya, S. Poor medication adherence and risk of relapse associated with continued cannabis use in patients with first-episode psychosis: A prospective analysis. Lancet Psychiatry 2017, 4, 627–633. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Abi-Saab, W.M.; Madonick, S.; Forselius-Bielen, K.; Doersch, A.; Braley, G.; Gueorguieva, R.; Cooper, T.B.; Krystal, J.H. Delta-9-tetrahydrocannabinol effects in schizophrenia: Implications for cognition, psychosis, and addiction. Biol. Psychiatry 2005, 57, 594–608. [Google Scholar] [CrossRef]
- Foti, D.J.; Kotov, R.; Guey, L.T.; Bromet, E.J. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am. J. Psychiatry 2010, 167, 987–993. [Google Scholar] [CrossRef]
- Batalla, A.; Bhattacharyya, S.; Yücel, M.; Fusar-Poli, P.; Crippa, J.A.; Nogué, S.; Torrens, M.; Pujol, J.; Farré, M.; Martin-Santos, R. Structural and Functional Imaging Studies in Chronic Cannabis Users: A Systematic Review of Adolescent and Adult Findings. PLoS ONE 2013, 8, e55821. [Google Scholar] [CrossRef]
- Foglia, E.; Schoeler, T.; Klamerus, E.; Morgan, K.; Bhattacharyya, S. Cannabis use and adherence to antipsychotic medication: A systematic review and meta-analysis. Psychol. Med. 2017, 47, 1691–1705. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Shirakawa, I.; Finkelfarb, E.; Karniol, I.G. Action of cannabidiol on the anxiety and other effects produced by δ9-THC in normal subjects. Psychopharmacology (Berl.) 1982, 76, 245–250. [Google Scholar] [CrossRef]
- Englund, A.; Morrison, P.D.; Nottage, J.; Hague, D.; Kane, F.; Bonaccorso, S.; Stone, J.M.; Reichenberg, A.; Brenneisen, R.; Holt, D.; et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 2013, 27, 19–27. [Google Scholar] [CrossRef]
- Iseger, T.A.; Bossong, M.G. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr. Res. 2015, 162, 153–161. [Google Scholar] [CrossRef]
- Leweke, F.M.; Mueller, J.K.; Lange, B.; Rohleder, C. Therapeutic Potential of Cannabinoids in Psychosis. Biol. Psychiatry 2016, 79, 604–612. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Morais, S.L.; Guimarães, F.S.; Mechoulam, R. Antipsychotic effect of cannabidiol. J. Clin. Psychiatry 1995, 56, 485–486. [Google Scholar]
- Zuardi, A.W.; Hallak, J.E.C.; Dursun, S.M.; Morais, S.L.; Sanches, R.F.; Musty, R.E.; Crippa, J.A.S. Cannabidiol monotherapy for treatment-resistant schizophrenia. J. Psychopharmacol. 2006, 20, 683–686. [Google Scholar] [CrossRef]
- Makiol, C.; Kluge, M. Remission of severe, treatment-resistant schizophrenia following adjunctive cannabidiol. Aust. New Zeal. J. Psychiatry 2019, 53, 262. [Google Scholar] [CrossRef]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef]
- Hallak, J.E.C.; Machado-de-Sousa, J.P.; Crippa, J.A.S.; Sanches, R.F.; Trzesniak, C.; Chaves, C.; Bernardo, S.A.; Regalo, S.C.; Zuardi, A.W. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev. Bras. Psiquiatr. 2010, 32, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.L.; Surti, T.; Gupta, A.; Gupta, S.; Niciu, M.; Pittman, B.; Schnakenberg Martin, A.M.; Thurnauer, H.; Davies, A.; D’Souza, D.C.; et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl.) 2018, 235, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Allsop, D.J.; Copeland, J.; Lintzeris, N.; Dunlop, A.J.; Montebello, M.; Sadler, C.; Rivas, G.R.; Holland, R.M.; Muhleisen, P.; Norberg, M.M.; et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal:A randomized clinical trial. JAMA Psychiatry 2014, 71, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.M.; Soliman, A.; Staios, G.; Quilty, L.; Fischer, B.; George, T.P.; Rehm, J.; Selby, P.; Barnes, A.J.; Huestis, M.A.; et al. Sativex associated with behavioral-relapse prevention strategy as treatment for cannabis dependence: A case series. J. Addict. Med. 2016, 10, 274–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trigo, J.M.; Soliman, A.; Quilty, L.C.; Fischer, B.; Rehm, J.; Selby, P.; Barnes, A.J.; Huestis, M.A.; George, T.P.; Streiner, D.L.; et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: A pilot randomized clinical trial. PLoS ONE 2018, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.S.; Hallak, J.E.C.; Machado-De-Sousa, J.P.; Queiroz, R.H.C.; Bergamaschi, M.; Chagas, M.H.N.; Zuardi, A.W. Cannabidiol for the treatment of cannabis withdrawal syndrome: A case report. J. Clin. Pharm. Ther. 2013, 38, 162–164. [Google Scholar] [CrossRef]
- Shannon, S.; Opila-Lehman, J. Cannabidiol Oil for Decreasing Addictive Use of Marijuana: A Case Report. Integr. Med. (Encinitas) 2015, 14, 31–35. [Google Scholar]
- Solowij, N.; Broyd, S.J.; Beale, C.; Prick, J.-A.; Greenwood, L.; van Hell, H.; Suo, C.; Galettis, P.; Pai, N.; Fu, S.; et al. Therapeutic Effects of Prolonged Cannabidiol Treatment on Psychological Symptoms and Cognitive Function in Regular Cannabis Users: A Pragmatic Open-Label Clinical Trial. Cannabis Cannabinoid Res. 2018, 3, 21–34. [Google Scholar] [CrossRef]
- Morgan, C.J.A.; Das, R.K.; Joye, A.; Curran, H.V.; Kamboj, S.K. Cannabidiol reduces cigarette consumption in tobacco smokers: Preliminary findings. Addict. Behav. 2013, 38, 2433–2436. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.P.; Grabski, M.; Stroud, J.B.; Crudgington, H.; Davies, A.C.; Das, R.K.; Lawn, W.; Morgan, C.J.A.; Curran, H.V. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction 2018, 113, 1696–1705. [Google Scholar] [CrossRef]
- Schipper, R.; Dekker, M.; de Haan, L.; van den Brink, W. Medicinal cannabis (Bedrolite) substitution therapy in inpatients with a psychotic disorder and a comorbid cannabis use disorder: A case series. J. Psychopharmacol. 2018, 32, 353–356. [Google Scholar] [CrossRef]
- Monji, A.; Kato, T.; Kanba, S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009, 63, 257–265. [Google Scholar] [CrossRef]
- Gangadin, S.S.; Nasib, L.G.; Sommer, I.E.C.; Mandl, R.C.W. MRI investigation of immune dysregulation in schizophrenia. Curr. Opin. Psychiatry 2019, 32, 164–169. [Google Scholar] [CrossRef]
- Gomes, F.V.; Llorente, R.; Del Bel, E.A.; Viveros, M.-P.; López-Gallardo, M.; Guimarães, F.S. Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr. Res. 2015, 164, 155–163. [Google Scholar] [CrossRef]
- Englund, A.; Freeman, T.P.; Murray, R.M.; McGuire, P. Can we make cannabis safer? Lancet Psychiatry 2017, 4, 643–648. [Google Scholar] [CrossRef]
| Study | Study Design | Participants | Substance Use | Intervention | CBD Administration | Primary Outcomes |
|---|---|---|---|---|---|---|
| Zuardi et al. (1995) [51] | Case report | 19-year-old female schizophrenia inpatient (two years after first hospitalization) | Not reported | Progressive increase of CBD monotherapy over four weeks, followed by haloperidol treatment | Oral; up to 1500 mg/day | Improvement of symptomatology. Improvement did not continue on haloperidol.No side effects. |
| Zuardi et al. (2006) [52] | Case series | Three male inpatients with treatment-resistant schizophrenia | Not reported | Progressive increase of CBD monotherapy over four weeks, followed by olanzapine treatment | Oral; up to 1280 mg/day | Mild improvement of symptomatology of one patient after CBD treatment.No side effects. |
| Makiol and Klunge (2019) [53] | Case report | 57-year old-female treatment-resistant schizophrenia inpatient | Not reported | Treatment with CBD adjunctive to clozapine and lamotrigine | Oral; up to 1500 mg/day | Improvement of symptomatology and the patient fulfilled remission criteria with only mild negative symptoms. |
| Leweke et al. (2012) [54] | Double-blind CBD vs. amisulpride RCT | 39 acutely psychotic inpatients | Not reported, exclusion criteria were SUD or positive urine drug screening for illicit drugs in general and cannabis in particular. | Hospitalization and four-week treatment with CBD or amisulpride | Oral; up to 800 mg/day | Treatment with either CBD or amisulpride is associated with improvement of symptomatology, but CBD has a superior side-effect profile. |
| McGuire et al. (2018) [55] | Double-blind placebo RCT | 88 outpatients with schizophrenia | Not reported, substance use was not an exclusion and not prohibited during the study. | A six-week treatment with CBD adjunctive to antipsychotic medication. | Oral solution; 1000 mg/day | Improvement of symptomatology, no side effects. |
| Hallak et al. (2010) [56] | Single dose double-blind placebo RCT | 28 schizophrenia outpatients | Not reported | Acute treatment with a single dose of CBD | Oral; 300 or 600 mg | CBD 300 mg and placebo both improved cognitive performance as compared to CBD 600 mg. No effects on symptomatology. |
| Boggs et al. (2018) [57] | Double-blind placebo RCT | 36 outpatients with chronic schizophrenia | Not reported, patients with substance abuse in the past three months or dependence in the past six months were excluded. | Six-week treatment with CBD added to a stable dose of antipsychotic medication | Oral; 600 mg/day | Cognitive performance improved after placebo, symptomatology improved in both groups, no differences between groups. |
| Study | Study Design | Participants | Intervention | CBD Administration | Primary Outcomes |
|---|---|---|---|---|---|
| Cannabis Dependence | |||||
| Allsop et al. (2014) [58] | Double-blind placebo RCT | 51 inpatients with cannabis dependence | A six-day treatment with Sativex in combination with CBT | Intranasal; maximum daily: 86.4 mg THC and 80 mg CBD | Sativex reduced cannabis withdrawal and cravings, and improved treatment retention rates. |
| Trigo et al. (2016) [59] | Double-blind placebo RCT | Nine individuals with cannabis dependence | Eight-week treatment with self-titrated or fixed doses of Sativex or placebo. | Intranasal; up to 108 mg THC and 100 mg CBD | During interruption of cannabis use both fixed and titrated doses of Sativex reduced cannabis withdrawal symptoms (but not craving), however the high fixed dose seemed the most effective. |
| Trigo et al. (2018) [60] | Double-blind placebo RCT | 27 individuals with cannabis dependence | Twelve-week treatment with self-titrated dosages of Sativex next to weekly CBT sessions. | Intranasal; up to 113.4 mg THC and 105 mg CBD/day | Cannabis use, cravings, and withdrawal decreased in both groups over time. Sativex reduced cannabis cravings. |
| Crippa et al. (2013) [61] | Case report | 19-year-old female diagnosed with cannabis dependence | Hospitalization and progressive increase of CBD | Oral; 300 to 600 mg | A progressive reduction of cannabis withdrawal, anxiety, and dissociative symptoms. |
| Shannon and Opila-Lehman (2015) [62] | Case report | 27-year-old male diagnosed with bipolar disorder and cannabis dependence | Treatment with CBD oil added to citalopram and lamotrigine | Intranasal; decreasing from 24 to 18 mg | Abstinence from cannabis, better sleep quality, and decrease in anxiety during the use of CBD oil. |
| Solowij et al. (2018) [63] | Open-label clinical trial | 20 ongoing cannabis users | Ten-week treatment with CBD | Oral; 200 mg daily | CBD improved psychological and cognitive symptomatology. Greater benefits were observed in dependent than in nondependent cannabis users. |
| Tobacco Dependence | |||||
| Morgan et al. (2013) [64] | Double-blind placebo RCT | 24 individuals who smoked >10 cigarettes per day and intended to quit | Optional CBD treatment during one week | Inhalation; 400 µg CBD per dose | CBD reduced the total number of cigarettes smoked. Reduction of craving in both groups after one week of treatment, but this did not maintain at follow-up. |
| Hindocha et al. (2018) [65] | Single dose double-blind placebo RCT | 30 individuals with tobacco dependence | Treatment with a single dose of CBD after an overnight of cigarette abstinence | Oral; 800 mg CBD | CBD reduced the salience and peasantness of cigarette cues but had no effect on craving and withdrawal. |
| Study | Study Design | Participants | Intervention | CBD Administration | Primary Outcomes |
|---|---|---|---|---|---|
| Schipper et al. (2018) [66] | Case report | Seven inpatients with a psychotic disorder and a comorbid treatment-resistant cannabis use disorder | Eight-week treatment with medicinal cannabis (Bedrolite: 0.4% THC and 9% CBD) adjunctive to antipsychotic medication. | Inhalation: 11–45 mg/day | No effect on symptomatology or craving. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batalla, A.; Janssen, H.; Gangadin, S.S.; Bossong, M.G. The Potential of Cannabidiol as a Treatment for Psychosis and Addiction: Who Benefits Most? A Systematic Review. J. Clin. Med. 2019, 8, 1058. https://doi.org/10.3390/jcm8071058
Batalla A, Janssen H, Gangadin SS, Bossong MG. The Potential of Cannabidiol as a Treatment for Psychosis and Addiction: Who Benefits Most? A Systematic Review. Journal of Clinical Medicine. 2019; 8(7):1058. https://doi.org/10.3390/jcm8071058
Chicago/Turabian StyleBatalla, Albert, Hella Janssen, Shiral S. Gangadin, and Matthijs G. Bossong. 2019. "The Potential of Cannabidiol as a Treatment for Psychosis and Addiction: Who Benefits Most? A Systematic Review" Journal of Clinical Medicine 8, no. 7: 1058. https://doi.org/10.3390/jcm8071058
APA StyleBatalla, A., Janssen, H., Gangadin, S. S., & Bossong, M. G. (2019). The Potential of Cannabidiol as a Treatment for Psychosis and Addiction: Who Benefits Most? A Systematic Review. Journal of Clinical Medicine, 8(7), 1058. https://doi.org/10.3390/jcm8071058





