Small Cell Lung Cancer Therapeutic Responses Through Fractal Measurements: From Radiology to Mitochondrial Biology
Abstract
1. Introduction
2. Experimental Section
2.1. Cell Culture and Reagents
2.2. Immunoblotting
2.3. Immunofluorescence
2.4. Tissue Microarray
2.5. Immunohistochemistry
2.6. Fractal Dimension and Lacunarity Analysis
2.7. Cell Metabolism
2.8. Cytotoxicity Assays
2.9. Statistical Analysis
3. Results
3.1. Radiological Quantification of SCLC CT Scans
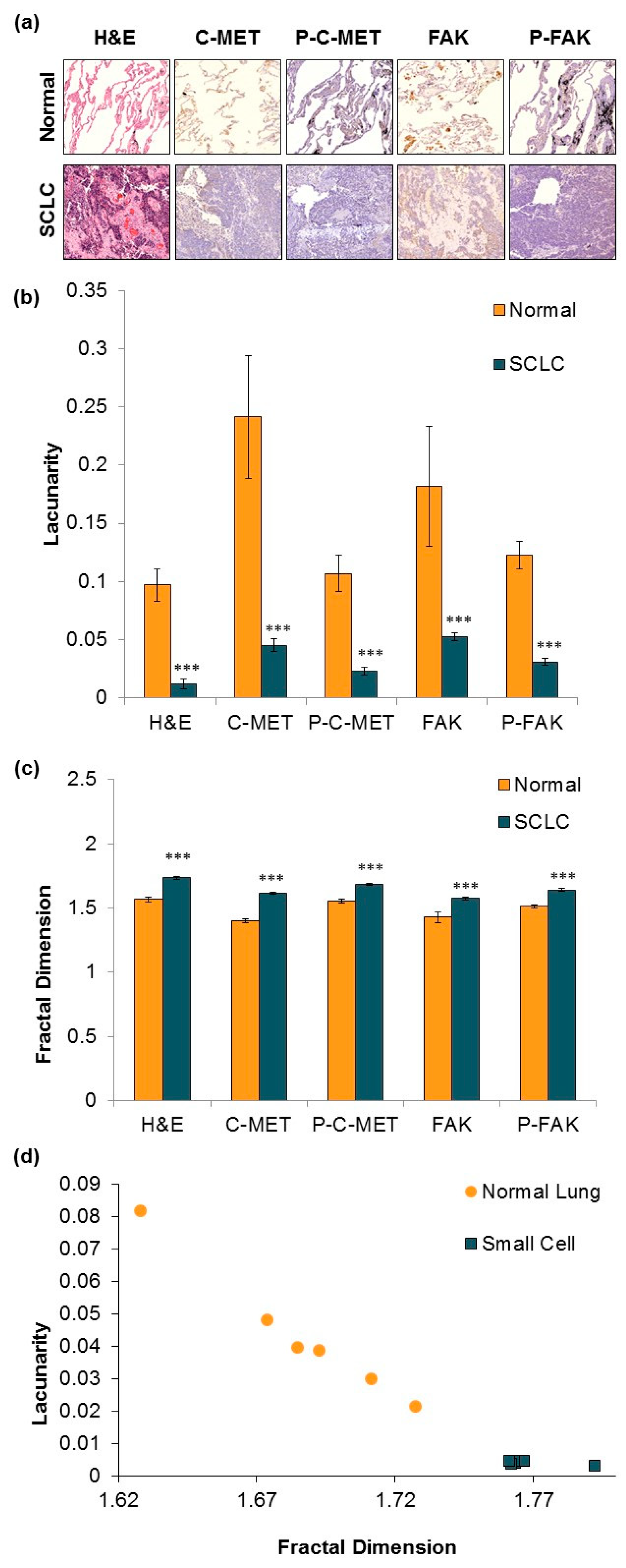
3.2. IHC Staining and Fractal Analysis of Normal and Malignant SCLC Tissue.
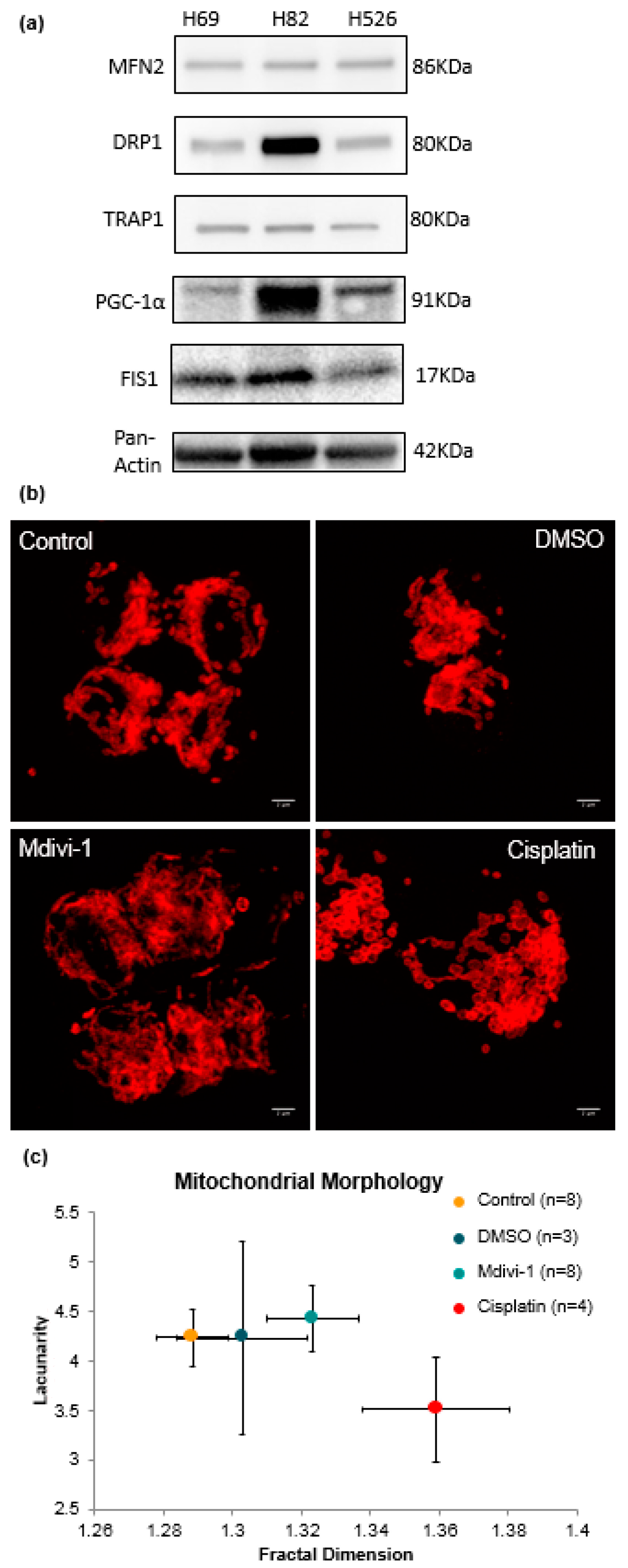
3.3. Fractal Analysis of Mitochondrial Morphology in SCLC
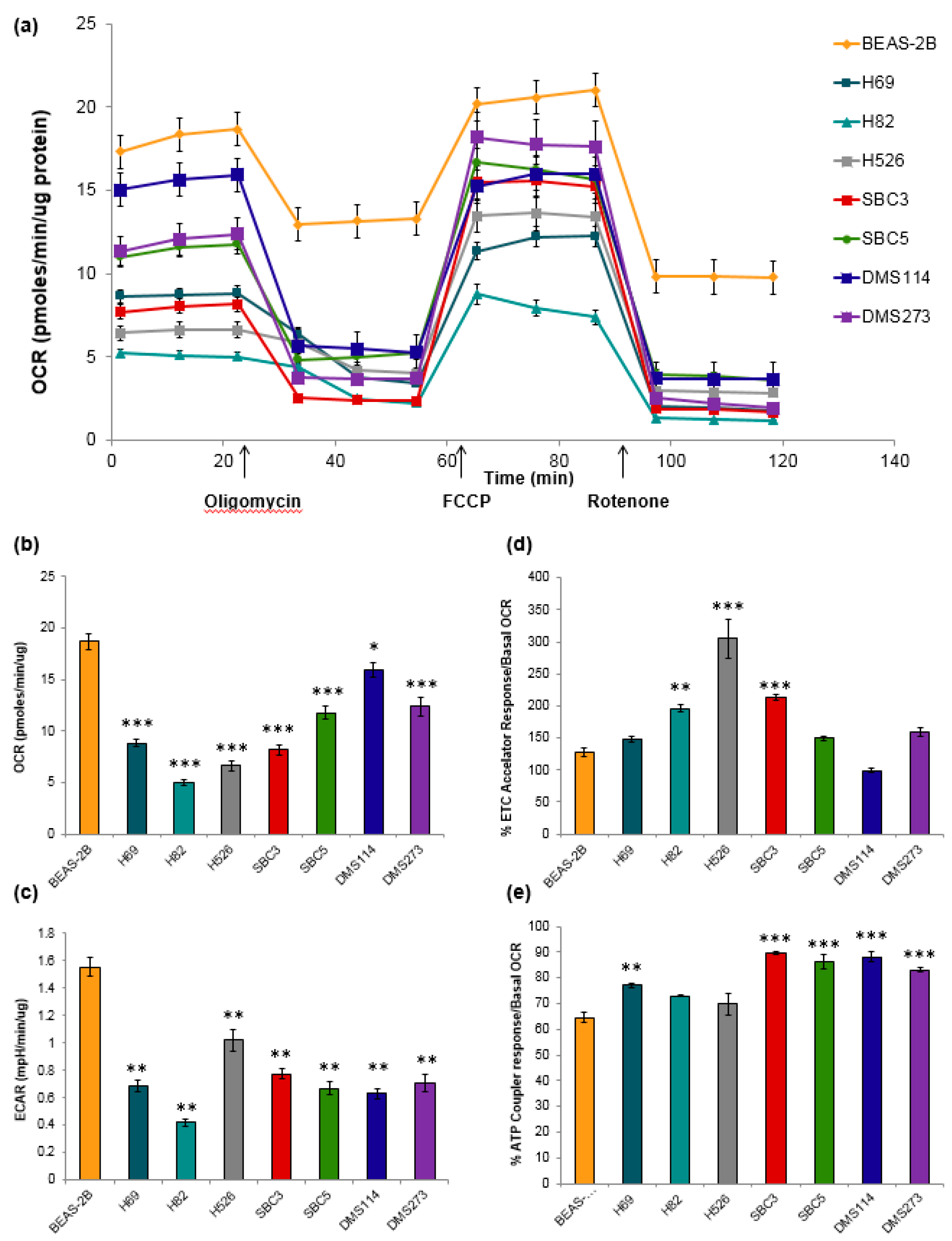
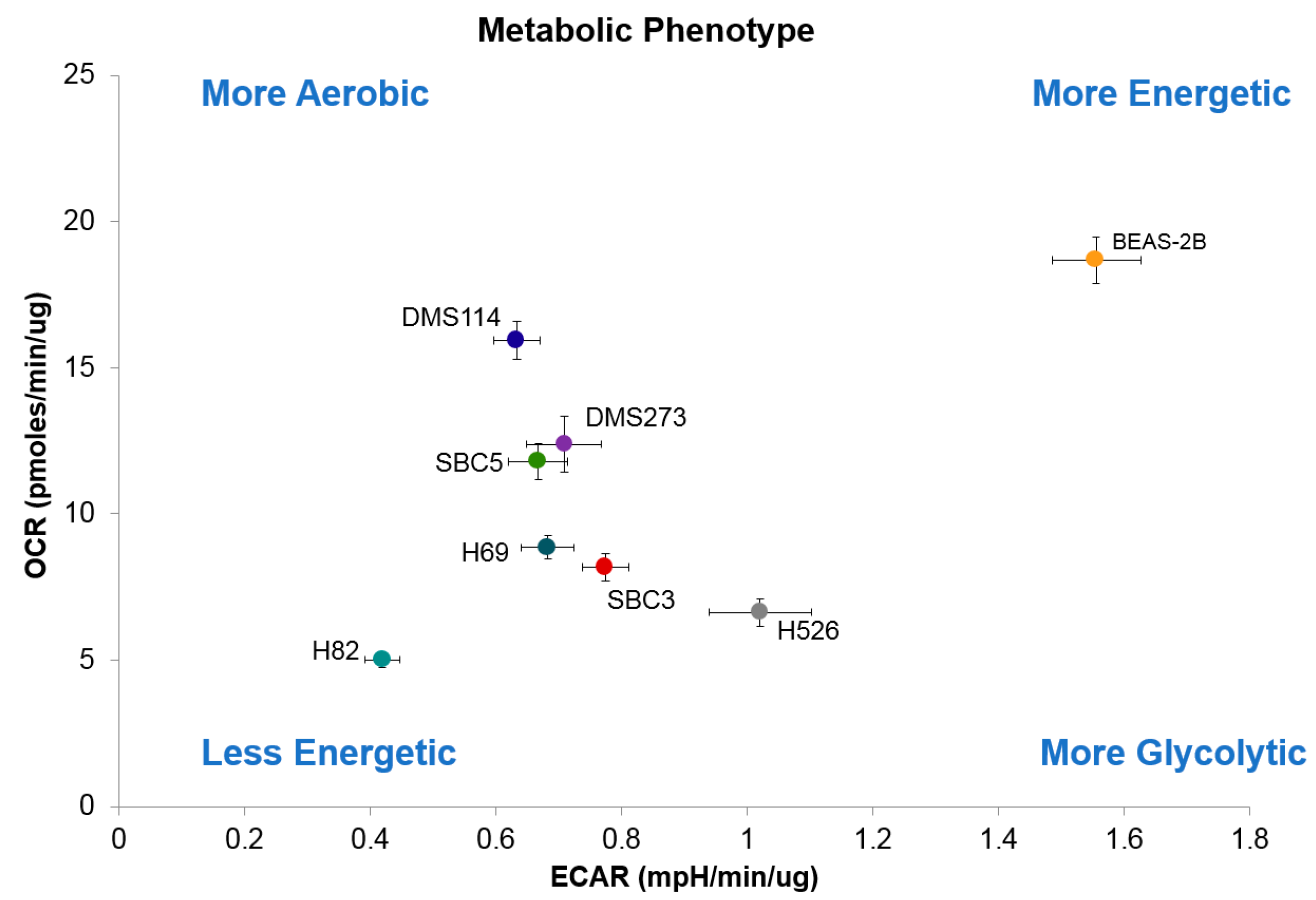
3.4. Metabolic Characteristics of SCLC Cells
3.5. Cytotoxicity of SCLC Cell Lines with Metformin and Mdivi-1
4. Discussion
4.1. The Lung Is a Fractal Pattern
4.2. Radiological Measurements of Fractal Geometry
4.3. Fractal Patterns of Tumor Tissue Differentiate SCLC
4.4. Dysfunction of Mitochondrial Morphology Correlates with Fission and Fusion Dynamics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gazdar, A.F.; Bunn, P.A.; Minna, J.D. Small-cell lung cancer: What we know, what we need to know and the path forward. Nat. Rev. Cancer 2017, 17, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [PubMed]
- Thomas, A.; Pattanayak, P.; Szabo, E.; Pinsky, P. Characteristics and Outcomes of Small Cell Lung Cancer Detected by CT Screening. Chest 2018, 154, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Demello, S.; Berg, C.D.; Black, W.C.; Brewer, B.; Church, T.R.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.F.; et al. Results of the two incidence screenings in the National Lung Screening Trial. New Engl. J. Med. 2013, 369, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Lennon, F.E.; Cianci, G.C.; Cipriani, N.A.; Hensing, T.A.; Zhang, H.J.; Chen, C.T.; Murgu, S.D.; Vokes, E.E.; Vannier, M.W.; Salgia, R. Lung cancer-a fractal viewpoint. Nat. Rev. Clin. Oncol. 2015, 12, 664–675. [Google Scholar] [CrossRef]
- Lennon, F.E.; Cianci, G.C.; Kanteti, R.; Riehm, J.J.; Arif, Q.; Poroyko, V.A.; Lupovitch, E.; Vigneswaran, W.; Husain, A.; Chen, P.; et al. Unique fractal evaluation and therapeutic implications of mitochondrial morphology in malignant mesothelioma. Sci. Rep. 2016, 6, 24578. [Google Scholar] [CrossRef]
- Tolle, C.R.; McJunkin, T.R.; Gorsich, D.J. An efficient implementation of the gliding box lacunarity algorithm. Phys. D Nonlinear Phenm. 2008, 237, 306–315. [Google Scholar] [CrossRef]
- Parmar, C.; Leijenaar, R.T.; Grossmann, P.; Rios, V.E.; Bussink, J.; Rietveld, D.; Rietbergen, M.M.; Haibe-Kains, B.; Lambin, P.; Aerts, H.J. Radiomic feature clusters and prognostic signatures specific for Lung and Head & Neck cancer. Sci. Rep. 2015, 5, 11044. [Google Scholar]
- Kanteti, R.; Dhanasingh, I.; Kawada, I.; Lennon, F.E.; Arif, Q.; Bueno, R.; Hasina, R.; Husain, A.N.; Vigneswaran, W.; Seiwert, T.; et al. MET and PI3K/mTOR as a potential combinatorial therapeutic target in malignant pleural mesothelioma. PLoS ONE 2014, 9, e105919. [Google Scholar] [CrossRef] [PubMed]
- Karperien, A. (1999-2013) FracLac for ImageJ. Available online: https://imagej.nih.gov/ij/plugins/fraclac/FLHelp/FLCitations.htm (accessed on 1 January 2019.).
- Cross, S.S. Fractals in pathology. J. Pathol. 1997, 182, 1–8. [Google Scholar] [CrossRef]
- Karperien, A.; Ahammer, H.; Jelinek, H.F. Quantitating the subtleties of microglial morphology with fractal analysis. Front. Cell. Neurosci. 2013, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.; Yu, M.; Xie, Y.; Huang, Y.; Wolff, D.W.; Abel, P.W.; Tu, Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 2013, 32, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Loson, O.C.; Song, Z.; Chen, H.; Chan, D.C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 2013, 24, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Bioenergetic role of mitochondrial fusion and fission. Biochim. Biophys. Acta 2012, 1817, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd. Mitochondrial dynamism and heart disease: Changing shape and shaping change. EMBO Mol. Med. 2015, 7, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Radde, B.N.; Ivanova, M.M.; Mai, H.X.; Salabei, J.K.; Hill, B.G.; Klinge, C.M. Bioenergetic differences between MCF-7 and T47D breast cancer cells and their regulation by oestradiol and tamoxifen. Biochem. J. 2015, 465, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Shue, Y.T.; Lim, J.S.; Sage, J. Tumor heterogeneity in small cell lung cancer defined and investigated in pre-clinical mouse models. Transl. Lung Cancer Res. 2018, 7, 21–31. [Google Scholar] [CrossRef]
- Byers, L.A.; Rudin, C.M. Small cell lung cancer: Where do we go from here? Cancer 2015, 121, 664–672. [Google Scholar] [CrossRef]
- Ma, P.C.; Blaszkowsky, L.; Bharti, A.; Ladanyi, A.; Kraeft, S.K.; Bruno, A.; Skarin, A.T.; Chen, L.B.; Salgia, R. Circulating tumor cells and serum tumor biomarkers in small cell lung cancer. Anticancer Res. 2003, 23, 49–62. [Google Scholar] [PubMed]
- Bunn, P.A., Jr.; Minna, J.D.; Augustyn, A.; Gazdar, A.F.; Ouadah, Y.; Krasnow, M.A.; Berns, A.; Brambilla, E.; Rekhtman, N.; Massion, P.P.; et al. Small Cell Lung Cancer: Can Recent Advances in Biology and Molecular Biology Be Translated into Improved Outcomes? J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe Lo, R.; Macerelli, M.; Platania, M.; Zilembo, N.; Vitali, M.; Signorelli, D.; Proto, C.; Ganzinelli, M.; Gallucci, R.; Agustoni, F.; et al. Small-Cell Lung Cancer: Clinical Management and Unmet Needs New Perspectives for an Old Problem. Curr. Drug Targets 2017, 18, 341–362. [Google Scholar]
- Carney, D.N. The biology of lung cancer. Curr. Opin. Pulm. Med. 1995, 1, 271–277. [Google Scholar] [CrossRef]
- Sattler, M.; Salgia, R. Molecular and cellular biology of small cell lung cancer. Semin. Oncol. 2003, 30, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod. Pathol. 2012, 25, S18–S30. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretic, L.; Kong, G.; Leenders, F.; Lu, X.; Fernandez-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Salgia, R.; Mambetsariev, I.; Hewelt, B.; Achuthan, S.; Li, H.; Poroyko, V.; Wang, Y.; Sattler, M. Modeling small cell lung cancer (SCLC) biology through deterministic and stochastic mathematical models. Oncotarget 2018, 9, 26226–26242. [Google Scholar] [CrossRef]
- Weibel, E.R. Fractal geometry: A design principle for living organisms. Am. J. Physiol. 1991, 261, L361–L369. [Google Scholar] [CrossRef]
- Ahmed, E. Fractals and chaos in cancer models. Int. J. Theor. Phys. 1993, 32, 353–355. [Google Scholar] [CrossRef]
- Altemeier, W.A.; McKinney, S.; Glenny, R.W. Fractal nature of regional ventilation distribution. J. Appl. Physiol. (1985) 2000, 88, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Glenny, R.W.; Robertson, H.T. Fractal properties of pulmonary blood flow: Characterization of spatial heterogeneity. J. Appl. Physiol. (1985) 1990, 69, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Glenny, R.W.; Robertson, H.T. Fractal modeling of pulmonary blood flow heterogeneity. J. Appl. Physiol. (1985) 1991, 70, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Moledina, S.; de Bruyn, A.; Schievano, S.; Owens, C.M.; Young, C.; Haworth, S.G.; Taylor, A.M.; Schulze-Neick, I.; Muthurangu, V. Fractal branching quantifies vascular changes and predicts survival in pulmonary hypertension: A proof of principle study. Heart 2011, 97, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Mishima, M.; Hirai, T.; Itoh, H.; Nakano, Y.; Sakai, H.; Muro, S.; Nishimura, K.; Oku, Y.; Chin, K.; Ohi, M.; et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 1999, 96, 8829–8834. [Google Scholar] [CrossRef] [PubMed]
- Boser, S.R.; Park, H.; Perry, S.F.; Menache, M.G.; Green, F.H. Fractal geometry of airway remodeling in human asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Sauret, V.; Halson, P.M.; Brown, I.W.; Fleming, J.S.; Bailey, A.G. Study of the three-dimensional geometry of the central conducting airways in man using computed tomographic (CT) images. J. Anat. 2002, 200, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Horsfield, K. Diameters, generations, and orders of branches in the bronchial tree. J. Appl. Physiol. (1985) 1990, 68, 457–461. [Google Scholar] [CrossRef]
- Iber, D.; Menshykau, D. The control of branching morphogenesis. Open Biol. 2013, 3, 130088. [Google Scholar] [CrossRef]
- Kitaoka, H.; Takaki, R.; Suki, B. A three-dimensional model of the human airway tree. J. Appl. Physiol. (1985) 1999, 87, 2207–2217. [Google Scholar] [CrossRef]
- Weibel, E.R. What makes a good lung? Swiss Med. Wkly. 2009, 139, 375–386. [Google Scholar]
- Fleury, V. [A fundamental symmetry between morphogenesis and function of branched organs]. Compt. Rendus Acad. Sci. III 2001, 324, 405–412. [Google Scholar]
- Oxnard, G.R.; Zhao, B.; Sima, C.S.; Ginsberg, M.S.; James, L.P.; Lefkowitz, R.A.; Guo, P.; Kris, M.G.; Schwartz, L.H.; Riley, G.J. Variability of lung tumor measurements on repeat computed tomography scans taken within 15 min. J. Clin. Oncol. 2011, 29, 3114–3119. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Guo, M.; Jackman, D.M.; DiPiro, P.J.; Yap, J.T.; Ho, T.K.; Hatabu, H.; Janne, P.A.; Van den Abbeele, A.D.; Johnson, B.E. CT tumor volume measurement in advanced non-small-cell lung cancer: Performance characteristics of an emerging clinical tool. Acad. Radiol. 2011, 18, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.M.; van Klaveren, R.J.; de Bock, G.H.; Leusveld, A.L.; Dorrius, M.D.; Zhao, Y.; Wang, Y.; de Koning, H.J.; Scholten, E.T.; Verschakelen, J.; et al. Role of baseline nodule density and changes in density and nodule features in the discrimination between benign and malignant solid indeterminate pulmonary nodules. Eur. J. Radiol. 2009, 70, 492–498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gould, M.K.; Ananth, L.; Barnett, P.G. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007, 131, 383–388. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, A.; Tammemagi, M.C.; Mayo, J.R.; Roberts, H.; Liu, G.; Soghrati, K.; Yasufuku, K.; Martel, S.; Laberge, F.; Gingras, M.; et al. Probability of cancer in pulmonary nodules detected on first screening CT. N. Engl. J. Med. 2013, 369, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.M.; van Klaveren, R.J.; de Bock, G.H.; Leusveld, A.; Zhao, Y.; Wang, Y.; Vliegenthart, R.; de Koning, H.J.; Scholten, E.T.; Verschakelen, J.; et al. Limited value of shape, margin and CT density in the discrimination between benign and malignant screen detected solid pulmonary nodules of the NELSON trial. Eur. J. Radiol. 2008, 68, 347–352. [Google Scholar] [CrossRef]
- Vittitoe, N.F.; Baker, J.A.; Floyd, C.E., Jr. Fractal texture analysis in computer-aided diagnosis of solitary pulmonary nodules. Acad. Radiol. 1997, 4, 96–101. [Google Scholar] [CrossRef]
- Xue, X.; Yang, Y.; Huang, Q.; Cui, F.; Lian, Y.; Zhang, S.; Yao, L.; Peng, W.; Li, X.; Pang, P.; et al. Use of a Radiomics Model to Predict Tumor Invasiveness of Pulmonary Adenocarcinomas Appearing as Pulmonary Ground-Glass Nodules. BioMed Res. Int. 2018, 2018, 9. [Google Scholar] [CrossRef]
- Hung, S.H.; Lin, C.Y.; Lee, J.Y.; Tseng, H. Computed tomography image characteristics of metastatic lymph nodes in patients with squamous cell carcinoma of the head and neck. Auris Nasus Larynx 2012, 39, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Hayano, K.; Lee, S.H.; Yoshida, H.; Zhu, A.X.; Sahani, D.V. Fractal analysis of contrast-enhanced CT images to predict survival of patients with hepatocellular carcinoma treated with sunitinib. Dig. Dis. Sci. 2014, 59, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Kido, S.; Kuriyama, K.; Higashiyama, M.; Kasugai, T.; Kuroda, C. Fractal analysis of internal and peripheral textures of small peripheral bronchogenic carcinomas in thin-section computed tomography: Comparison of bronchioloalveolar cell carcinomas with nonbronchioloalveolar cell carcinomas. J. Comput. Assist. Tomogr. 2003, 27, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Michallek, F.; Dewey, M. Fractal analysis in radiological and nuclear medicine perfusion imaging: A systematic review. Eur. Radiol. 2014, 24, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Inubushi, M.; Wagatsuma, K.; Nagao, M.; Murata, T.; Koyama, M.; Koizumi, M.; Sasaki, M. FDG uptake heterogeneity evaluated by fractal analysis improves the differential diagnosis of pulmonary nodules. Eur. J. Radiol. 2014, 83, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Streba, C.T.; Pirici, D.; Vere, C.C.; Mogoanta, L.; Comanescu, V.; Rogoveanu, I. Fractal analysis differentiation of nuclear and vascular patterns in hepatocellular carcinomas and hepatic metastasis. Romanian J. Morphol. Embryol. 2011, 52, 845–854. [Google Scholar] [CrossRef]
- Bikou, O.; Delides, A.; Drougou, A.; Nonni, A.; Patsouris, E.; Pavlakis, K. Fractal Dimension as a Diagnostic Tool of Complex Endometrial Hyperplasia and Well-differentiated Endometrioid Carcinoma. In Vivo 2016, 30, 681–690. [Google Scholar] [PubMed]
- Bose, P.; Brockton, N.T.; Guggisberg, K.; Nakoneshny, S.C.; Kornaga, E.; Klimowicz, A.C.; Tambasco, M.; Dort, J.C. Fractal analysis of nuclear histology integrates tumor and stromal features into a single prognostic factor of the oral cancer microenvironment. BMC Cancer 2015, 15, 409. [Google Scholar] [CrossRef]
- Jitaree, S.; Phinyomark, A.; Boonyaphiphat, P.; Phukpattaranont, P. Cell type classifiers for breast cancer microscopic images based on fractal dimension texture analysis of image color layers. Scanning 2015, 37, 145–151. [Google Scholar] [CrossRef]
- Rajkovic, N.; Li, X.; Plataniotis, K.N.; Kanjer, K.; Radulovic, M.; Milosevic, N.T. The Pan-Cytokeratin Staining Intensity and Fractal Computational Analysis of Breast Tumor Malignant Growth Patterns Prognosticate the Occurrence of Distant Metastasis. Front. Oncol. 2018, 8, 348. [Google Scholar] [CrossRef]
- Ushenko, A.; Yermolenko, S.; Prydij, A.; Guminetsky, S.; Gruia, I.; Toma, O.; Vladychenko, K. Statistical and fractal approaches in laser polarimetry diagnostics of the cancer prostate tissues—art. no. 70082C. In Proceedings of the SPIE—The International Society for Optical Engineering (Proceedings of SPIE), Chernivtsi, Ukraine, 11 September 2007. [Google Scholar]
- Sabo, E.; Boltenko, A.; Sova, Y.; Stein, A.; Kleinhaus, S.; Resnick, M.B. Microscopic analysis and significance of vascular architectural complexity in renal cell carcinoma. Clin. Cancer Res. 2001, 7, 533–537. [Google Scholar] [PubMed]
- Stefanescu, D.; Streba, C.; Cartana, E.T.; Saftoiu, A.; Gruionu, G.; Gruionu, L.G. Computer Aided Diagnosis for Confocal Laser Endomicroscopy in Advanced Colorectal Adenocarcinoma. PLoS ONE 2016, 11, e0154863. [Google Scholar] [CrossRef] [PubMed]
- Franzen, L.E.; Hahn-Stromberg, V.; Edvardsson, H.; Bodin, L. Characterization of colon carcinoma growth pattern by computerized morphometry: Definition of a complexity index. Int. J. Mol. Med. 2008, 22, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Pribic, J.; Vasiljevic, J.; Kanjer, K.; Konstantinovic, Z.N.; Milosevic, N.; Vukosavljevic, D.N.; Radulovic, M.; Zivic, N. Fractal dimension and lacunarity of tumor microscopic images as prognostic indicators of clinical outcome in early breast cancer. Biomark. Med. 2015, 9, 1277–1279. [Google Scholar]
- Hernández Velázquez, J.d.D.; Mejia-Rosales, S.; Gama Goicochea, A. Fractal properties of biophysical models of pericellular brushes can be used to differentiate between cancerous and normal cervical epithelial cells. Colloids Surf. B Biointerfaces 2018, 170, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Delides, A.; Ioannis, P.; Alegakis, T.; Kyroudi, A.; Banis, C.; Pavlaki, A.; Helidonis, E.; Kittas, C. Fractal dimension as a prognostic factor for laryngeal carcinoma. Anticancer Res. 2005, 25, 2141–2144. [Google Scholar] [PubMed]
- Goutzanis, L.; Papadogeorgakis, N.; Pavlopoulos, P.M.; Katti, K.; Petsinis, V.; Plochoras, I.; Pantelidaki, C.; Kavantzas, N.; Patsouris, E.; Alexandridis, C. Nuclear fractal dimension as a prognostic factor in oral squamous cell carcinoma. Oral Oncol. 2008, 44, 345–353. [Google Scholar] [CrossRef]
- Dey, P. Basic principles and applications of fractal geometry in pathology: A review. Anal. Quant. Cytol. Histol. 2005, 27, 284–290. [Google Scholar]
- Gheonea, D.I.; Streba, C.T.; Vere, C.C.; Serbanescu, M.; Pirici, D.; Comanescu, M.; Streba, L.A.; Ciurea, M.E.; Mogoanta, S.; Rogoveanu, I. Diagnosis system for hepatocellular carcinoma based on fractal dimension of morphometric elements integrated in an artificial neural network. Biomed Res. Int. 2014, 2014, 239706. [Google Scholar] [CrossRef]
- Sturgis, C.D.; Nassar, D.L.; D’Antonio, J.A.; Raab, S.S. Cytologic features useful for distinguishing small cell from non-small cell carcinoma in bronchial brush and wash specimens. Am. J. Clin. Pathol. 2000, 114, 197–202. [Google Scholar] [CrossRef]
- Nicholson, S.A.; Beasley, M.B.; Brambilla, E.; Hasleton, P.S.; Colby, T.V. Small cell lung carcinoma (SCLC): A clinicopathologic study of 100 cases with surgical specimens. Am. J. Surg. Pathol. 2002, 26, 1184–1197. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Leporati, P.; Rotondi, M.; Chiovato, L. Expanding the therapeutic spectrum of metformin: From diabetes to cancer. J. Endocrinol. Investig. 2015, 38, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, B.; Newick, K.; Nelson, K.J.; Wozniak, A.N.; Beuschel, S.; Leavitt, B.; Bhave, A.; Butnor, K.; Koenig, A.; Chouchani, E.T.; et al. Disabling Mitochondrial Peroxide Metabolism via Combinatorial Targeting of Peroxiredoxin 3 as an Effective Therapeutic Approach for Malignant Mesothelioma. PLoS ONE 2015, 10, e0127310. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.S.; Baty, J.W.; Dong, L.F.; Bezawork-Geleta, A.; Endaya, B.; Goodwin, J.; Bajzikova, M.; Kovarova, J.; Peterka, M.; Yan, B.; et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015, 21, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Bryant, J.L.; Meredith, S.L.; Williams, K.J.; White, A. Targeting hypoxia in the treatment of small cell lung cancer. Lung Cancer 2014, 86, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Klameth, L.; Rath, B.; Hochmaier, M.; Moser, D.; Redl, M.; Mungenast, F.; Gelles, K.; Ulsperger, E.; Zeillinger, R.; Hamilton, G. Small cell lung cancer: Model of circulating tumor cell tumorospheres in chemoresistance. Sci. Rep. 2017, 7, 5337. [Google Scholar] [CrossRef]
- Khalbuss, W.E.; Yang, H.; Lian, Q.; Elhosseiny, A.; Pantanowitz, L.; Monaco, S.E. The cytomorphologic spectrum of small-cell carcinoma and large-cell neuroendocrine carcinoma in body cavity effusions: A study of 68 cases. Cytojournal 2011, 8, 18. [Google Scholar] [CrossRef]
- Jolly, M.K.; Boareto, M.; Debeb, B.G.; Aceto, N.; Farach-Carson, M.C.; Woodward, W.A.; Levine, H. Inflammatory breast cancer: A model for investigating cluster-based dissemination. NPJ Breast Cancer 2017, 3, 21. [Google Scholar] [CrossRef]

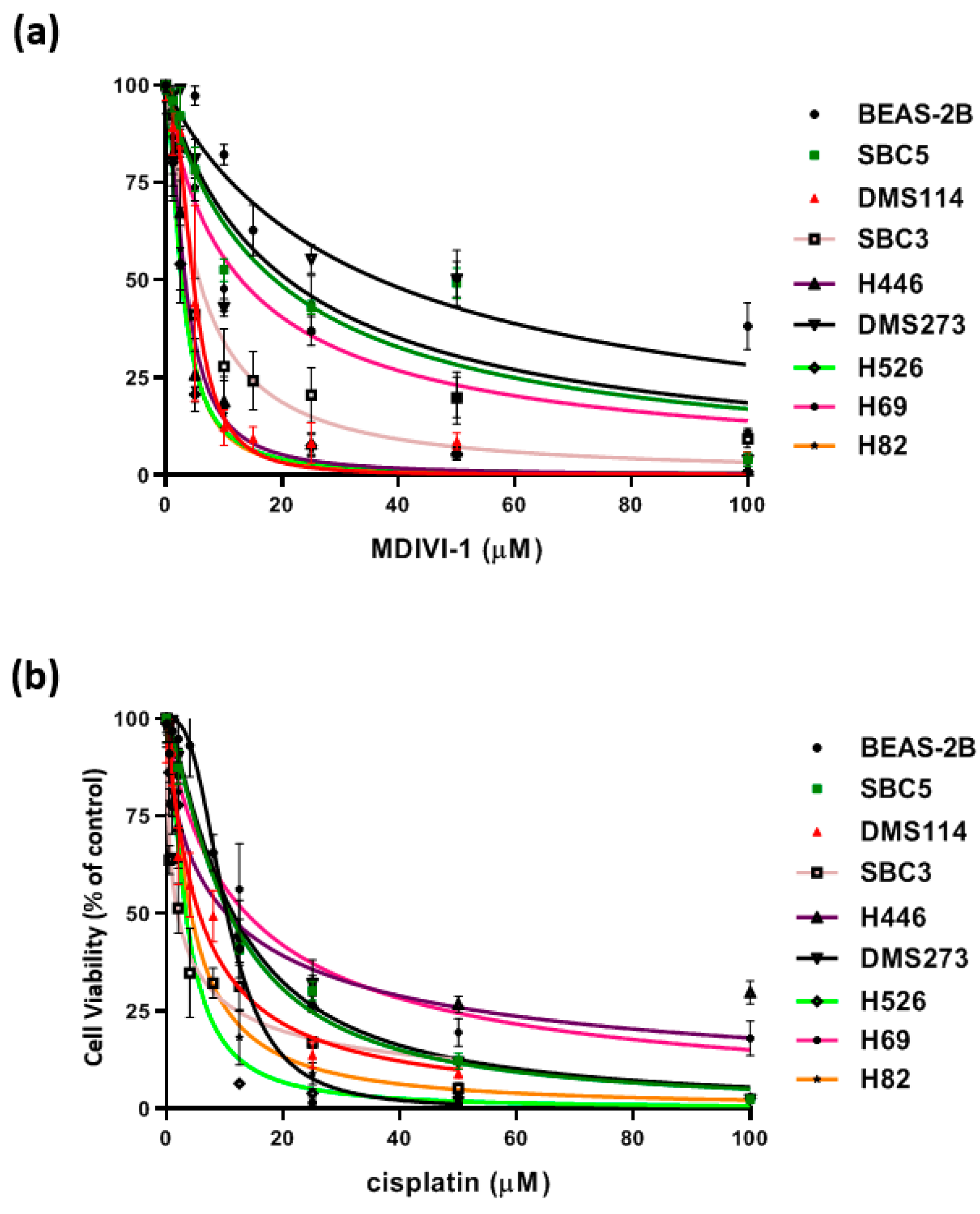
| Drug EC50 | Beas-2B (Control) | H69 | H82 | H446 | H526 | SBC3 | SBC5 | DMS114 | DMS273 |
|---|---|---|---|---|---|---|---|---|---|
| mdivi-1 μM | 36.9 | 13.01 | 2.889 | 3.356 | 2.738 | 5.763 | 18.83 | 4.735 | 21.07 |
| Cisplatin μM | 10.37 | 13.76 | 4.218 | 10.37 | 3.357 | 1.940 | 10.76 | 5.626 | 11.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mambetsariev, I.; Mirzapoiazova, T.; Lennon, F.; Jolly, M.K.; Li, H.; Nasser, M.W.; Vora, L.; Kulkarni, P.; Batra, S.K.; Salgia, R. Small Cell Lung Cancer Therapeutic Responses Through Fractal Measurements: From Radiology to Mitochondrial Biology. J. Clin. Med. 2019, 8, 1038. https://doi.org/10.3390/jcm8071038
Mambetsariev I, Mirzapoiazova T, Lennon F, Jolly MK, Li H, Nasser MW, Vora L, Kulkarni P, Batra SK, Salgia R. Small Cell Lung Cancer Therapeutic Responses Through Fractal Measurements: From Radiology to Mitochondrial Biology. Journal of Clinical Medicine. 2019; 8(7):1038. https://doi.org/10.3390/jcm8071038
Chicago/Turabian StyleMambetsariev, Isa, Tamara Mirzapoiazova, Frances Lennon, Mohit Kumar Jolly, Haiqing Li, Mohd W. Nasser, Lalit Vora, Prakash Kulkarni, Surinder K. Batra, and Ravi Salgia. 2019. "Small Cell Lung Cancer Therapeutic Responses Through Fractal Measurements: From Radiology to Mitochondrial Biology" Journal of Clinical Medicine 8, no. 7: 1038. https://doi.org/10.3390/jcm8071038
APA StyleMambetsariev, I., Mirzapoiazova, T., Lennon, F., Jolly, M. K., Li, H., Nasser, M. W., Vora, L., Kulkarni, P., Batra, S. K., & Salgia, R. (2019). Small Cell Lung Cancer Therapeutic Responses Through Fractal Measurements: From Radiology to Mitochondrial Biology. Journal of Clinical Medicine, 8(7), 1038. https://doi.org/10.3390/jcm8071038








