Outcomes of a Multidisciplinary Clinic in Evaluating Recurrent Clostridioides difficile Infection Patients for Fecal Microbiota Transplant: A Retrospective Cohort Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Setting
2.2. Study Design
2.3. Study Population
2.4. FMT Procedure
2.5. Patient Follow-Up
2.6. Study Outcomes
2.7. Statistical Analysis
3. Results
3.1. Cohort Demographics
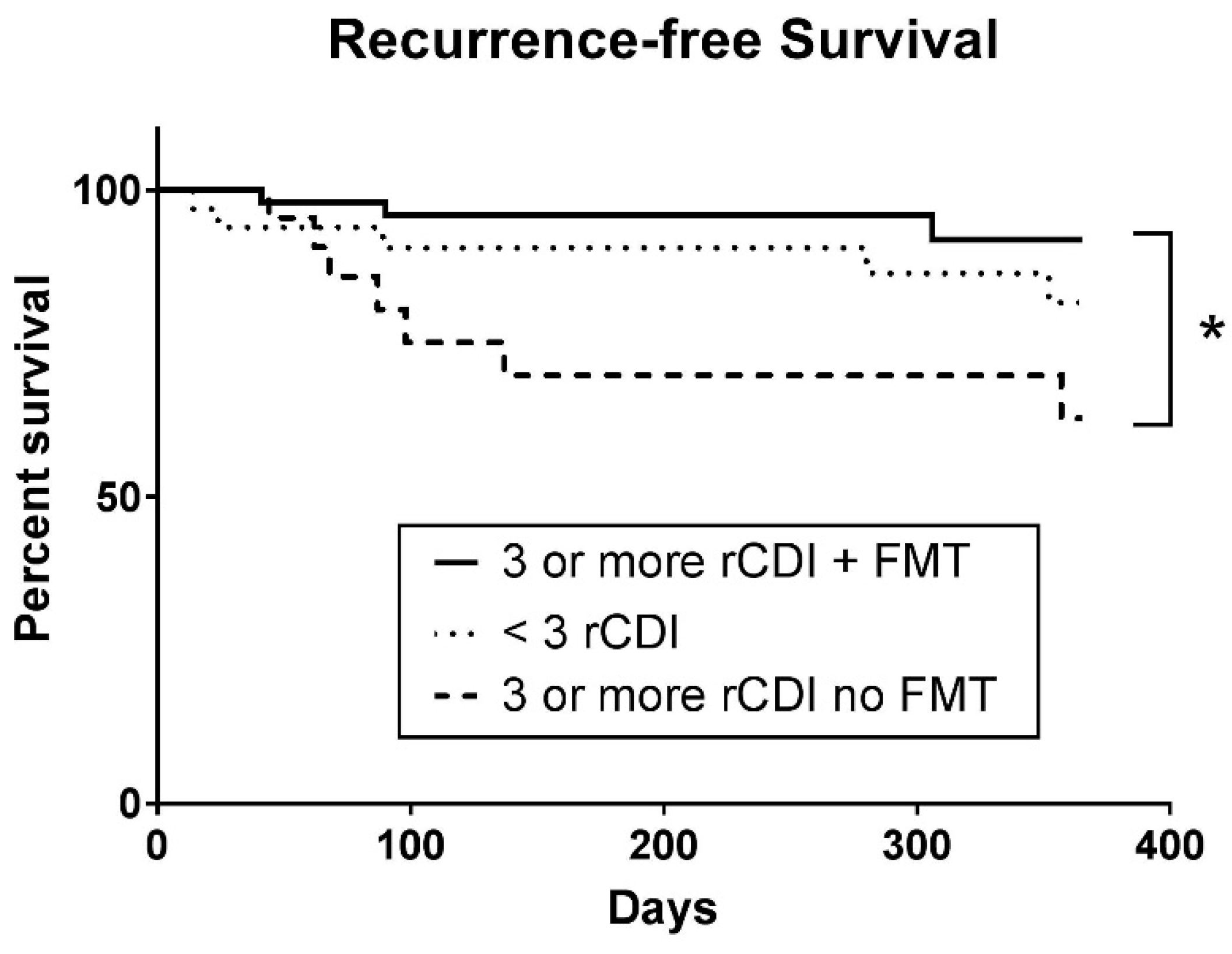
3.2. Primary Outcomes
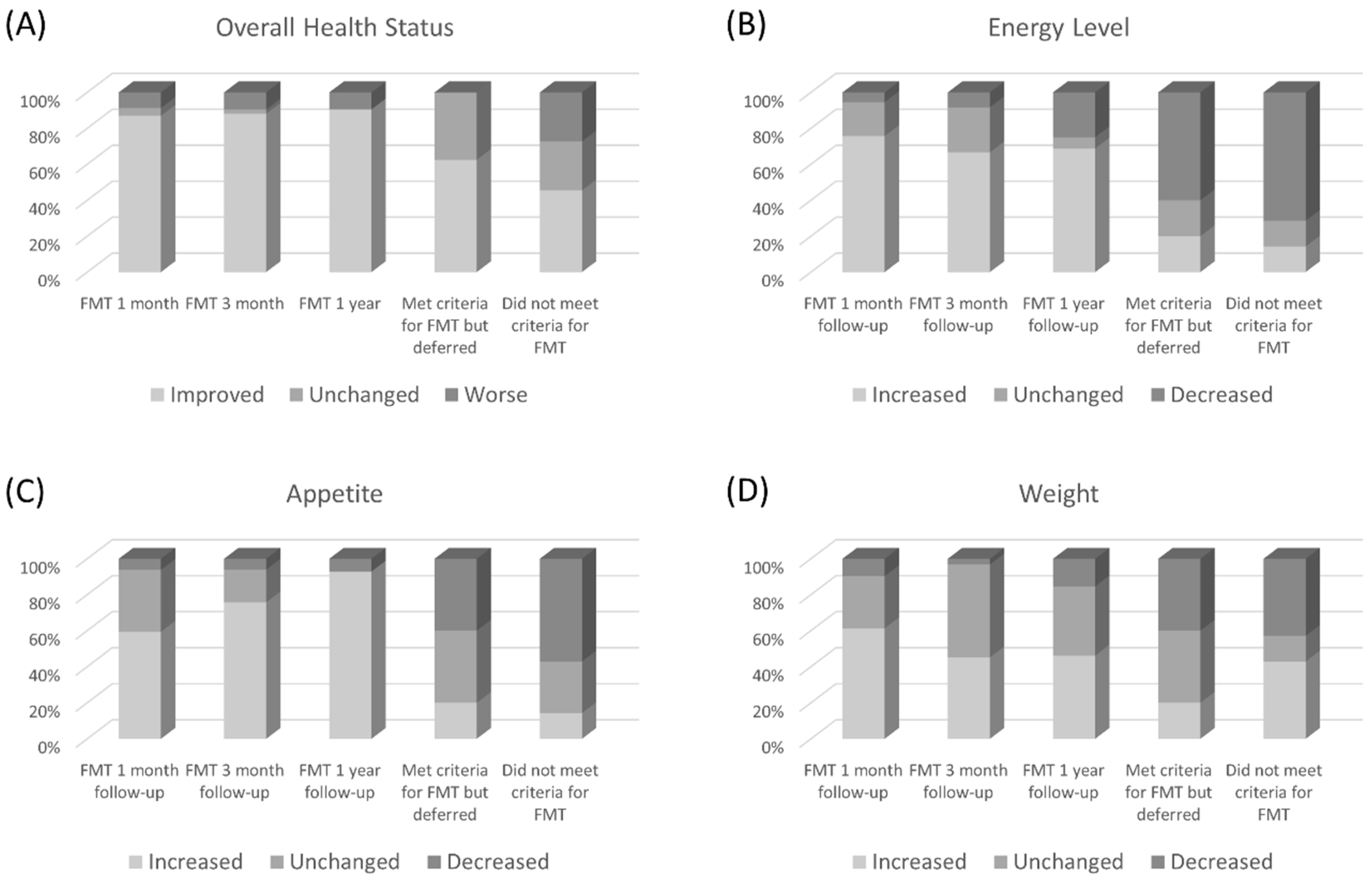
3.3. Secondary Outcomes
3.4. New Diagnoses Discovered from FMT Work-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Antibiotic Resistance Threats in the United States, 2013; CDC: Atlanta, GA, USA, 2013.
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care—Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Dubberke, E.R.; Olsen, M.A. Burden of Clostridium difficile on the Healthcare System. Clin. Infect. Dis. 2012, 55, S88–S92. [Google Scholar] [CrossRef]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar]
- Schwan, A.; Sjölin, S.; Trottestam, U.; Aronsson, B. Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet 1983, 2, 845. [Google Scholar] [CrossRef]
- Persky, S.E.; Brandt, L.J. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am. J. Gastroenterol. 2000, 95, 3283–3285. [Google Scholar]
- Aas, J.; Gessert, C.E.; Bakken, J.S. Recurrent Clostridium difficile colitis: Case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin. Infect. Dis. 2003, 36, 580–585. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. Available online: https://www.fda.gov/media/96562/download (accessed on 14 July 2019).
- Cammarota, G.; Masucci, L.; Ianiro, G.; Bibbò, S.; Dinoi, G.; Costamagna, G.; Sanguinetti, M.; Gasbarrini, A. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2015, 41, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Hocquart, M.; Lagier, J.-C.; Cassir, N.; Saidani, N.; Eldin, C.; Kerbaj, J.; Delord, M.; Valles, C.; Brouqui, P.; Raoult, D.; et al. Early Fecal Microbiota Transplantation Improves Survival in Severe Clostridium difficile Infections. Clin. Infect. Dis. 2018, 66, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Kassam, Z.; Bercik, P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: Experimental evidence and clinical implications. Curr. Opin. Microbiol. 2013, 16, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.-J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs. Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Moore, J.L.; Jordan, A.T.; Juttukonda, L.J.; Noto, M.J.; Nicholson, M.R.; Crews, J.D.; Semler, M.W.; Zhang, Y.; Ware, L.B.; et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat. Med. 2016, 22, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Niccum, B.A.; Stein, D.J.; Behm, B.W.; Hays, R.A. Zinc Deficiency and the Recurrence of Clostridium difficile Infection after Fecal Microbiota Transplant: A Retrospective Cohort Study. J. Nutr. Metab. 2018, 2018, 9682975. [Google Scholar] [CrossRef] [PubMed]
| Serum |
|---|
| Complete blood count |
| Comprehensive metabolic panel |
| Thyroid stimulating hormone |
| Vitamin A, B12, D and zinc |
| Immunoglobulin levels |
| Erythrocyte sedimentation rate and c-reactive protein |
| Serologies for human immunodeficiency virus, cytomegalovirus, Epstein-Barr virus, human T-cell lymphotropic virus, syphilis, and Strongyloides |
| Tissue transglutaminase IgA |
| Stool |
| Campylobacter |
| Cryptosporidium |
| Giardia |
| Norovirus |
| Shiga toxin |
| Clostridioides difficile toxin |
| Culture |
| Ova and parasite |
| Lactoferrin |
| Objectives |
|---|
| Clean the home with 10% bleach solution. |
| Exchange toothbrush if kept within six feet of the toilet. |
| Stop or decrease proton pump inhibitor if possible. |
| Wash hands with soap and water. |
| Use separate bathroom if there is more than one bathroom in the house. |
| Avoid any unnecessary antibiotics. |
| Variable | All Patients n = 113 | FMT n = 52 | Met Criteria but Not Treated with FMT n = 25 | Did not meet Criteria for FMT n = 36 | p Value |
|---|---|---|---|---|---|
| Age, mean (SD), y | 64 (18.5) | 67 (16.8) | 60 (21.1) | 62 (18.8) | 0.23 |
| Female sex, N (%) | 80 (71) | 41 (79) | 16 (64) | 23 (64) | 0.22 |
| Body mass index, mean | 26.12 | 27.04 | 25.79 | 25.02 | 0.29 |
| Body mass index, SD | 5.99 | 6.42 | 5.82 | 5.41 | |
| Mean number of C. difficile positive stools (SD) | 3 (1.50) | 3 (1.51) | 3 (1.33) | 2 (1.80) | 0.01 |
| Hospitalized in past year, N (%) | 74 (66) | 35 (67) | 17 (68) | 22 (61) | 0.80 |
| CDI related hospitalization, N (%) | 58 (51) | 27 (52) | 14 (56) | 17 (47) | 0.80 |
| Diabetes mellitus, N (%) | 23 (20) | 10 (19) | 6 (24) | 7 (19) | 0.88 |
| Hypertension, N (%) | 65 (58) | 30 (58) | 13 (52) | 22 (61) | 0.78 |
| Hyperlipidemia, N (%) | 49 (43) | 26 (50) | 6 (24) | 17 (47) | 0.08 |
| Gastrointestinal disorders, N (%) | 75 (66) | 33 (64) | 18 (72) | 24 (67) | 0.76 |
| IBD, N (%) | 14 (12) | 6 (12) | 5 (20) | 3 (8) | 0.38 |
| Ulcerative colitis, N | 6 | 2 | 2 | 2 (6) | |
| Indeterminate colitis, N | 3 | 2 | 1 | 0 | |
| Crohn’s disease, N | 4 | 2 | 2 | 0 | |
| Possible IBD, N | 1 | 0 | 0 | 1 | |
| Cardiac disorders, N (%) | 37 (33) | 14 (27) | 6 (24) | 17 (47) | 0.08 |
| Malignancy, N (%) | 29 (26) | 15 (29) | 7 (28) | 7 (19) | 0.58 |
| Pulmonary disorders, N (%) | 28 (25) | 7 (14) | 7 (28) | 14 (39) | 0.02 |
| Thyroid disorders, N (%) | 25 (22) | 12 (23) | 3 (52) | 10 (28) | 0.34 |
| Neurological disorders, N (%) | 25 (22) | 12 (23) | 7 (28) | 6 (17) | 0.56 |
| Renal disorders, N (%) | 21 (19) | 11 (21) | 4 (16) | 6 (17) | 0.81 |
| Immunosuppressive medications, N (%) | 27 (24) | 10 (19) | 9 (36) | 8 (22) | 0.26 |
| Antibiotics (non-CDI), N (%) | 79 (70) | 38 (73) | 17 (68) | 24 (67) | 0.79 |
| Acid suppressing agents, N (%) | 44 (39) | 20 (40) | 11 (44) | 13 (36) | 0.82 |
| At skilled nursing facility, N (%) | 16 (14) | 9 (17) | 2 (8) | 5 (14) | 0.55 |
| Reason for not doing FMT | Numbers |
|---|---|
| Resolved without FMT | 9 |
| Did not follow up in clinic | 6 |
| Patient preference | 5 |
| Deferred due to risk from procedure due to leukopenia | 1 |
| Deferred due to risk from procedure due to congestive heart failure | 1 |
| Unable to complete colon prep | 1 |
| Moved away | 1 |
| Diarrhea attributed to other cause | 1 |
| Gross Pathology (n =50) | n (%) |
|---|---|
| Normal | 11 (22) |
| Abnormal | 39 (78) |
| Diverticulosis | 22 (44) |
| Gross Inflammation | 19 (38) |
| Erythematous mucosa | 10 (20) |
| Colitis | 5 (10) |
| Ileal ulceration | 1 (2) |
| Pseudomembranes | 1 (2) |
| Mucosal congestion | 2 (4) |
| Angiodysplasia | 2 (4) |
| Neoplasm (new) | 5 (10) |
| Polyp(s) | 4 (8) |
| Mucosal edema | 1 (2) |
| Microscopic Pathology (n = 35) | n (%) |
| Normal | 19 (54) |
| Abnormal | 16 (46) |
| IBD | |
| Colon: IBD | 7 |
| Newly diagnosed IBD | 5 |
| Ileum: Ulcers, IBD | 1 |
| Microscopic colitis | |
| Collagenous colitis | 2 |
| Lymphocytic colitis | 2 |
| Focal increase in intraepithelial lymphocytes | 1 |
| Acute Inflammatory Process | |
| Mildly active acute colitis | 1 |
| Surface mucosal ulceration and epithelial regeneration | 1 |
| Mild nonspecific reactive epithelial changes | 1 |
| Neoplastic Process | |
| Tubular or tubulovillous adenoma | 4 |
| Moderately differentiated adenocarcinoma | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.H.; Chaplin, A.S.; Hays, R.A.; Kolling, G.L.; Vance, S.; Guerrant, R.L.; Archbald-Pannone, L.; Warren, C.A. Outcomes of a Multidisciplinary Clinic in Evaluating Recurrent Clostridioides difficile Infection Patients for Fecal Microbiota Transplant: A Retrospective Cohort Analysis. J. Clin. Med. 2019, 8, 1036. https://doi.org/10.3390/jcm8071036
Shin JH, Chaplin AS, Hays RA, Kolling GL, Vance S, Guerrant RL, Archbald-Pannone L, Warren CA. Outcomes of a Multidisciplinary Clinic in Evaluating Recurrent Clostridioides difficile Infection Patients for Fecal Microbiota Transplant: A Retrospective Cohort Analysis. Journal of Clinical Medicine. 2019; 8(7):1036. https://doi.org/10.3390/jcm8071036
Chicago/Turabian StyleShin, Jae Hyun, Ashley S. Chaplin, R. Ann Hays, Glynis L. Kolling, Sheila Vance, Richard L. Guerrant, Laurie Archbald-Pannone, and Cirle A. Warren. 2019. "Outcomes of a Multidisciplinary Clinic in Evaluating Recurrent Clostridioides difficile Infection Patients for Fecal Microbiota Transplant: A Retrospective Cohort Analysis" Journal of Clinical Medicine 8, no. 7: 1036. https://doi.org/10.3390/jcm8071036
APA StyleShin, J. H., Chaplin, A. S., Hays, R. A., Kolling, G. L., Vance, S., Guerrant, R. L., Archbald-Pannone, L., & Warren, C. A. (2019). Outcomes of a Multidisciplinary Clinic in Evaluating Recurrent Clostridioides difficile Infection Patients for Fecal Microbiota Transplant: A Retrospective Cohort Analysis. Journal of Clinical Medicine, 8(7), 1036. https://doi.org/10.3390/jcm8071036




