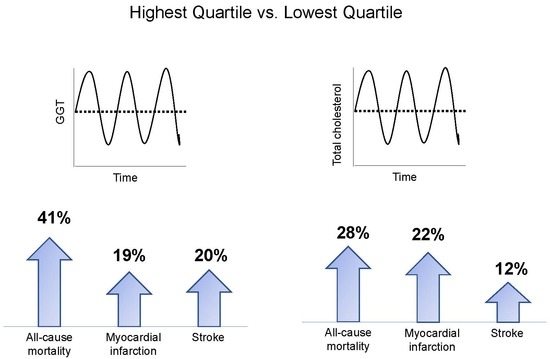
γ-Glutamyltransferase Variability and the Risk of Mortality, Myocardial Infarction, and Stroke: A Nationwide Population-Based Cohort Study
Abstract
:1. Introduction
2. Experimental Section
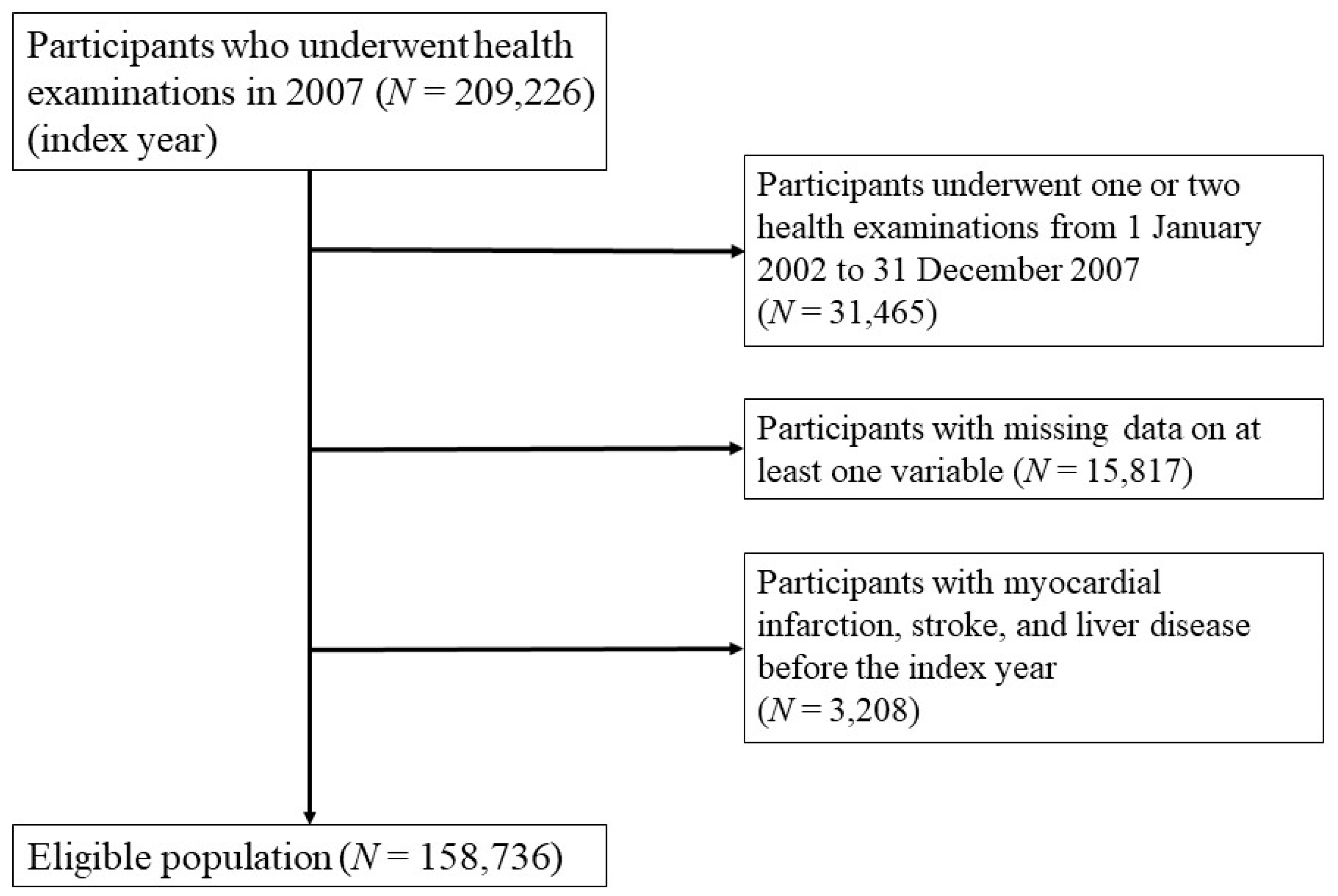
2.1. Study Participants
2.2. Measurements and Definitions
2.3. Definition of GGT Variability
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
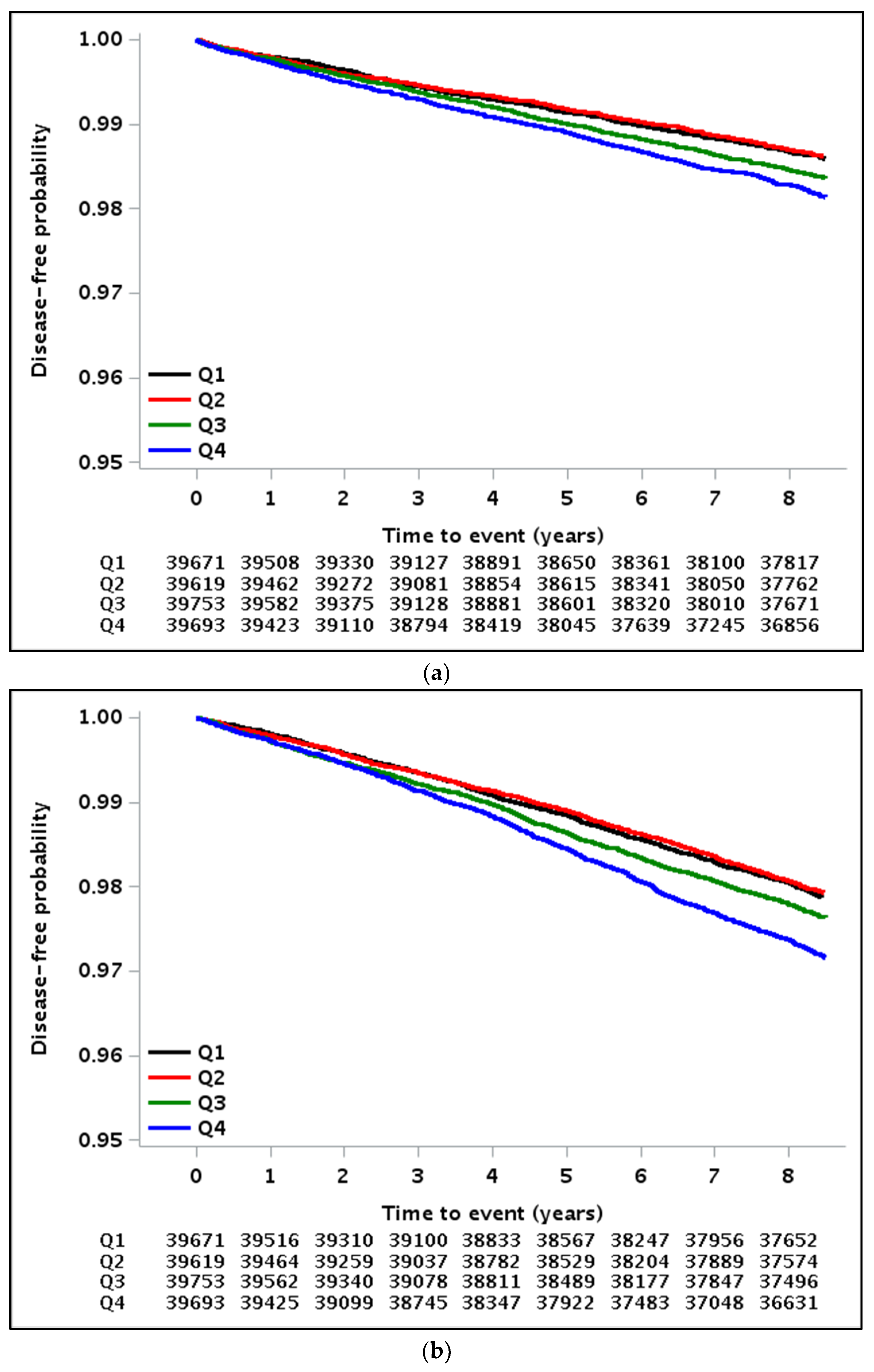
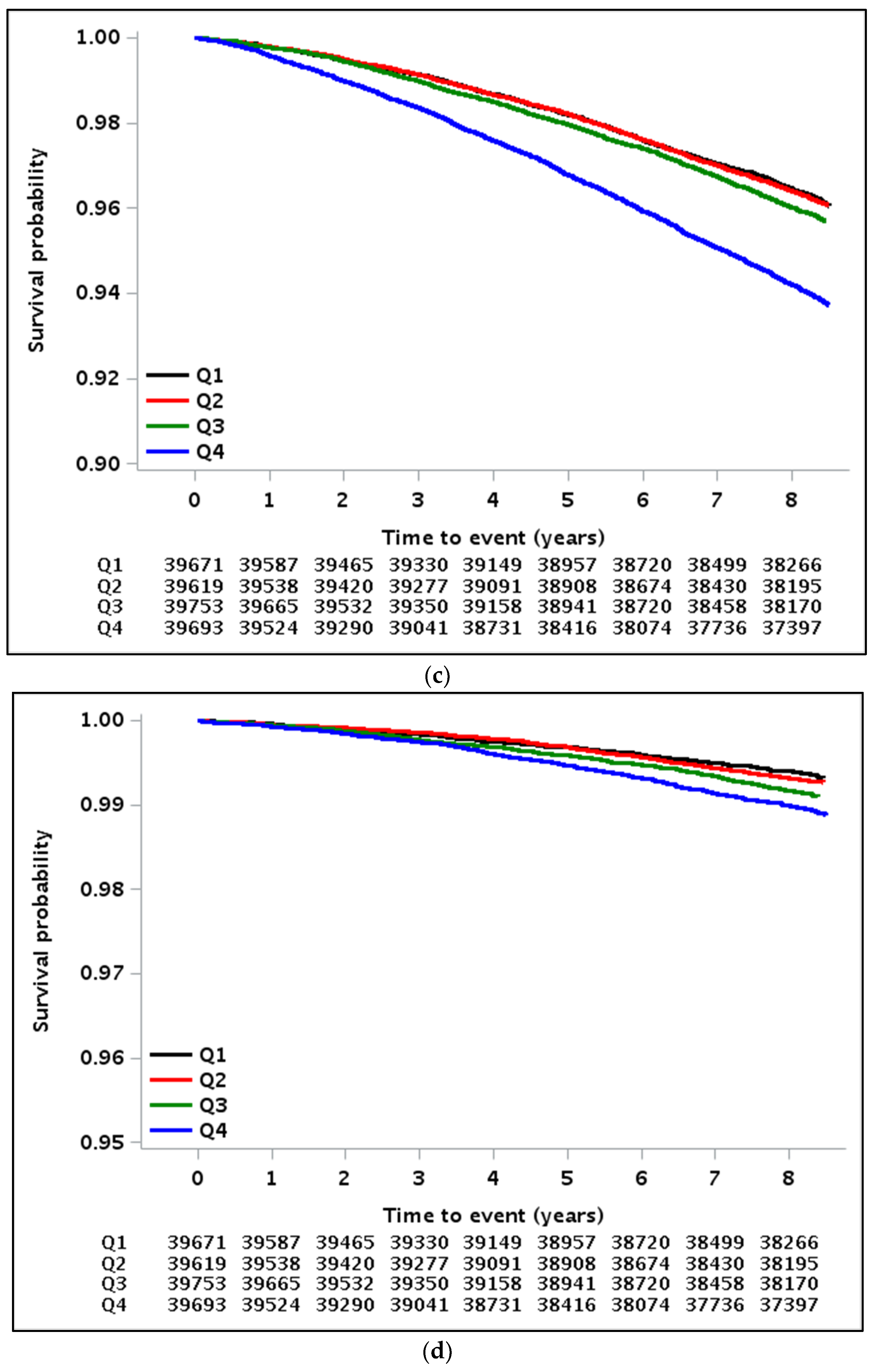
3.2. Implications of GGT Variability with All-Cause Mortality, MI, and Stroke
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hsieh, Y.T.; Tu, S.T.; Cho, T.J.; Chang, S.J.; Chen, J.F.; Hsieh, M.C. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: A 5.5-year prospective analysis. Eur. J. Clin. Investig. 2012, 42, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Hata, J.; Arima, H.; Rothwell, P.M.; Woodward, M.; Zoungas, S.; Anderson, C.; Patel, A.; Neal, B.; Glasziou, P.; Hamet, P.; et al. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: The ADVANCE trial. Circulation 2013, 128, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.Y.; Li, H.Y.; Pei, D.; Hsia, T.L.; Lu, K.C.; Tsai, L.Y.; Wei, J.N.; Su, C.C. Variability in hemoglobin A1c predicts all-cause mortality in patients with type 2 diabetes. J. Diabetes Its Complicat. 2012, 26, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Bouchi, R.; Babazono, T.; Mugishima, M.; Yoshida, N.; Nyumura, I.; Toya, K.; Hayashi, T.; Hanai, K.; Tanaka, N.; Ishii, A.; et al. Fluctuations in HbA1c are associated with a higher incidence of cardiovascular disease in Japanese patients with type 2 diabetes. J. Diabetes Investig. 2012, 3, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Takao, T.; Matsuyama, Y.; Yanagisawa, H.; Kikuchi, M.; Kawazu, S. Association between HbA1c variability and mortality in patients with type 2 diabetes. J. Diabetes Its Complicat. 2014, 28, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Arima, H.; Zoungas, S.; Ninomiya, T.; Cooper, M.; Hamet, P.; Mancia, G.; Poulter, N.; Harrap, S.; Woodward, M.; et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: The ADVANCE trial. Diabetes Care 2014, 37, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Han, K.; Kim, H.S.; Park, Y.M.; Kwon, H.S.; Yoon, K.H.; Lee, S.H. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: A nationwide population-based study. Eur. Heart J. 2017, 38, 3560–3566. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Breazna, A.; DeMicco, D.A.; Wun, C.C.; Messerli, F.H. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: Insights from the TNT trial. J. Am. Coll. Cardiol. 2015, 65, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Bakker, S.J.; Kootstra-Ros, J.E.; Gansevoort, R.T.; Dullaart, R.P. Circulating gamma glutamyltransferase and prediction of cardiovascular disease. Atherosclerosis 2015, 238, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, C.E.; Everhart, J.E. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009, 136, 477–485. [Google Scholar] [CrossRef]
- Targher, G. Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer—A narrative review. Clin. Chem. Lab. Med. 2010, 48, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Abbasi, A.; Adler, A.I. Gamma-glutamyl transferase and risk of type II diabetes: An updated systematic review and dose-response meta-analysis. Ann. Epidemiol. 2014, 24, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, G.; Ebrahim, S.; Shaper, A.G. Gamma-glutamyltransferase: Determinants and association with mortality from ischemic heart disease and all causes. Am. J. Epidemiol. 1995, 142, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ruttmann, E.; Brant, L.J.; Concin, H.; Diem, G.; Rapp, K.; Ulmer, H.; Vorarlberg Health Monitoring and Promotion Program Study Group. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: An epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 2005, 112, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Han, K.; Park, S.; Chung, H.S.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Implication of liver enzymes on incident cardiovascular diseases and mortality: A nationwide population-based cohort study. Sci. Rep. 2018, 8, 3764. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S. Thirty years of national health insurance in South Korea: Lessons for achieving universal health care coverage. Health Policy Plan 2009, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Y.; Wei, L.J.; Ying, Z. Checking the Cox Model with Cumulative Sums of Martingale-Based Residuals. Biometrika 1993, 80, 557–572. [Google Scholar] [CrossRef]
- Lee, D.S.; Evans, J.C.; Robins, S.J.; Wilson, P.W.; Albano, I.; Fox, C.S.; Wang, T.J.; Benjamin, E.J.; D’Agostino, R.B.; Vasan, R.S. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 127–133. [Google Scholar] [CrossRef]
- Fraser, A.; Harris, R.; Sattar, N.; Ebrahim, S.; Smith, G.D.; Lawlor, D.A. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: Analysis of the British Women’s Heart and Health Study and Meta-Analysis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2729–2735. [Google Scholar] [CrossRef]
- Andre, P.; Balkau, B.; Born, C.; Charles, M.A.; Eschwege, E.; DESIR Study Group. Three-year increase of gamma-glutamyltransferase level and development of type 2 diabetes in middle-aged men and women: The DESIR Cohort. Diabetologia 2006, 49, 2599–2603. [Google Scholar] [CrossRef]
- Yadav, D.; Lee, M.Y.; Kim, J.Y.; Ryu, H.; Huh, J.H.; Bae, K.S.; Ahn, S.V.; Chung, C.H.; Park, J.T.; Koh, S.B. Combined Effect of Initial and Longitudinal Increases in gamma-Glutamyltransferase on Incident Metabolic Syndrome: ARIRANG Study. Yonsei Med. J. 2017, 58, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Strasak, A.M.; Kelleher, C.C.; Klenk, J.; Brant, L.J.; Ruttmann, E.; Rapp, K.; Concin, H.; Diem, G.; Pfeiffer, K.P.; Ulmer, H.; et al. Longitudinal change in serum gamma-glutamyltransferase and cardiovascular disease mortality: A prospective population-based study in 76,113 Austrian adults. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1857–1865. [Google Scholar] [CrossRef]
- De Oliveira e Silva, E.R.; Foster, D.; McGee Harper, M.; Seidman, C.E.; Smith, J.D.; Breslow, J.L.; Brinton, E.A. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation 2000, 102, 2347–2352. [Google Scholar] [CrossRef]
- Langer, R.D.; Criqui, M.H.; Reed, D.M. Lipoproteins and blood pressure as biological pathways for effect of moderate alcohol consumption on coronary heart disease. Circulation 1992, 85, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A. In vino veritas: Alcohol and heart disease. Am. J. Med. Sci. 2005, 329, 124–135. [Google Scholar] [PubMed]
- Gronbaek, M.; Johansen, D.; Becker, U.; Hein, H.O.; Schnohr, P.; Jensen, G.; Vestbo, J.; Sørensen, T.I. Changes in alcohol intake and mortality: A longitudinal population-based study. Epidemiology 2004, 15, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Leppala, J.M.; Paunio, M.; Virtamo, J.; Fogelholm, R.; Albanes, D.; Taylor, P.R.; Heinonen, O.P. Alcohol consumption and stroke incidence in male smokers. Circulation 1999, 100, 1209–1214. [Google Scholar] [CrossRef]
- Barbaresko, J.; Rienks, J.; Nothlings, U. Lifestyle Indices and Cardiovascular Disease Risk: A Meta-analysis. Am. J. Prev. Med. 2018, 55, 555–564. [Google Scholar] [CrossRef]
- Mason, J.E.; Starke, R.D.; Van Kirk, J.E. Gamma-glutamyl transferase: A novel cardiovascular risk biomarker. Prev. Cardiol. 2010, 13, 36–41. [Google Scholar] [CrossRef]
- Emdin, M.; Pompella, A.; Paolicchi, A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: Triggering oxidative stress within the plaque. Circulation 2005, 112, 2078–2080. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
| Q1 | Q2 | Q3 | Q4 | p Value | |
|---|---|---|---|---|---|
| N | 39,671 | 39,619 | 39,753 | 39,693 | |
| Age (years) | 56.3 ± 8.9 | 55.3 ± 8.5 | 55.5 ± 8.6 | 56.3 ± 8.8 | <0.001 |
| Sex (male) (n, %) | 21,593 (54.4) | 24,094 (60.8) | 24,006 (60.4) | 23,543 (59.3) | <0.001 |
| Body mass index (kg/m2) | 23.8 ± 2.8 | 24.0 ± 2.8 | 24.1 ± 2.9 | 24.0 ± 2.9 | <0.001 |
| Systolic BP (mmHg) | 124.8 ± 15.6 | 125.4 ± 15.5 | 126.0 ± 15.7 | 126.7 ± 16.1 | <0.001 |
| Diastolic BP (mmHg) | 77.7 ± 10.1 | 78.3 ± 10.1 | 78.6 ± 10.2 | 78.8 ± 10.3 | <0.001 |
| Total cholesterol (mg/dL) | 198.0 ± 35.3 | 198.8 ± 35.7 | 199.4 ± 36.4 | 198.7 ± 38.4 | <0.001 |
| Fasting plasma glucose (mmol/L) | 5.40 ± 1.31 | 5.45 ± 1.38 | 5.51 ± 1.43 | 5.59 ± 1.57 | <0.001 |
| Mean GGT (IU/L) | 27.0 ± 25.6 | 32.0 ± 31.1 | 37.0 ± 38.6 | 52.9 ± 59.3 | <0.001 |
| GGT-CV (%) | 10.67 ± 3.62 | 19.87 ± 2.36 | 29.09 ± 3.19 | 51.94 ± 18.71 | <0.001 |
| Current smoker (n, %) | 6938 (17.5) | 8269 (20.9) | 8353 (21.0) | 8657 (21.8) | <0.001 |
| Alcohol consumption (n, %) | 15,255 (38.5) | 17,500 (44.2) | 17,812 (44.8) | 17,854 (45.0) | <0.001 |
| Regular exercise (n, %) | 4176 (10.5) | 3940 (9.9) | 3946 (9.9) | 4175 (10.5) | 0.002 |
| Income (lower 10%) (n, %) | 2932 (7.4) | 3099 (7.8) | 3248 (8.2) | 3412 (8.6) | <0.001 |
| Hypertension (n, %) | 20,347 (51.3) | 21,981 (55.5) | 22,733 (57.2) | 23,630 (59.5) | <0.001 |
| Diabetes mellitus (n, %) | 5164 (13.0) | 6001 (15.1) | 6743 (17.0) | 7720 (19.4) | <0.001 |
| Dyslipidemia (n, %) | 10,347 (26.1) | 11,153 (28.2) | 11,878 (29.9) | 12,607 (31.8) | <0.001 |
| GGT Variability (Coefficient of Variation) | Total Cholesterol Variability (Coefficient of Variation) | |||||||
|---|---|---|---|---|---|---|---|---|
| Events (n) | Follow-Up Duration (Person Years) | Incidence Rate (per 1000 Person-Years) | Adjusted HR a (95% CI) | Events (n) | Follow-Up Duration (Person Years) | Incidence Rate (per 1000 Person-Years) | Adjusted HR b (95% CI) | |
| Myocardial infarction | ||||||||
| Q1 | 550 | 326,982 | 1.68 | 1 (ref) | 541 | 326,588 | 1.66 | 1 (ref) |
| Q2 | 538 | 326,090 | 1.65 | 1.00 (0.89–1.12) | 564 | 326,328 | 1.73 | 1.09 (0.97–1.23) |
| Q3 | 638 | 326,175 | 1.96 | 1.14 (1.02–1.28) | 563 | 326,019 | 1.73 | 1.04 (0.92–1.17) |
| Q4 | 710 | 322,430 | 2.20 | 1.19 (1.06–1.34) | 768 | 322,743 | 2.38 | 1.22 (1.08–1.37) |
| p for trend | <0.001 | 0.003 | ||||||
| Stroke | ||||||||
| Q1 | 825 | 326,351 | 2.53 | 1 (ref) | 855 | 325,624 | 2.63 | 1 (ref) |
| Q2 | 803 | 325,372 | 2.47 | 1.04 (0.94–1.14) | 780 | 325,806 | 2.39 | 1.00 (0.91–1.10) |
| Q3 | 923 | 325,408 | 2.84 | 1.14 (1.04–1.25) | 847 | 325,337 | 2.60 | 1.01 (0.92–1.11) |
| Q4 | 1091 | 321,593 | 3.39 | 1.20 (1.10–1.32) | 1160 | 321,957 | 3.60 | 1.12 (1.02–1.23) |
| p for trend | <0.001 | 0.020 | ||||||
| All-cause mortality | ||||||||
| Q1 | 1550 | 329,166 | 4.71 | 1 (ref) | 1595 | 328,660 | 4.85 | 1 (ref) |
| Q2 | 1544 | 328,170 | 4.70 | 1.08 (1.01–1.16) | 1533 | 328,516 | 4.67 | 1.07 (1.00–1.15) |
| Q3 | 1713 | 328,611 | 5.21 | 1.14 (1.06–1.22) | 1683 | 328,233 | 5.13 | 1.08 (1.01–1.16) |
| Q4 | 2500 | 325,122 | 7.69 | 1.41 (1.33–1.51) | 2496 | 325,660 | 7.66 | 1.28 (1.20–1.37) |
| p for trend | <0.001 | <0.001 | ||||||
| Cardiovascular disease-related mortality | ||||||||
| Q1 | 261 | 329,166 | 0.79 | 1 (ref) | 303 | 328,660 | 0.92 | 1 (ref) |
| Q2 | 287 | 28,170 | 0.87 | 1.22 (1.03–1.44) | 280 | 328,516 | 0.85 | 1.05 (0.89–1.24) |
| Q3 | 356 | 328,611 | 1.08 | 1.43 (1.22–1.68) | 285 | 328,233 | 0.87 | 0.98 (0.83–1.15) |
| Q4 | 434 | 325,122 | 1.33 | 1.52 (1.30–1.78) | 470 | 325,660 | 1.44 | 1.22 (1.05–1.42) |
| p for trend | <0.001 | 0.022 | ||||||
| GGT Variability (Standard Deviation) | GGT Variability (Variability Independent of the Mean) | |||||||
|---|---|---|---|---|---|---|---|---|
| Events (n) | Follow-Up Duration (Person Years) | Incidence Rate (per 1000 Person-Years) | Adjusted HR a (95% CI) | Events (n) | Follow-Up Duration (Person Years) | Incidence Rate (per 1000 Person-Years) | Adjusted HR a (95% CI) | |
| Myocardial infarction | ||||||||
| Q1 | 446 | 328,049 | 1.36 | 1 (ref) | 532 | 326,982 | 1.63 | 1 (ref) |
| Q2 | 559 | 327,378 | 1.71 | 1.13 (1.00–1.28) | 539 | 327,193 | 1.65 | 1.01 (0.90–1.14) |
| Q3 | 732 | 324,612 | 2.26 | 1.38 (1.22–1.56) | 633 | 325,704 | 1.94 | 1.14 (1.02-1.28) |
| Q4 | 699 | 321,638 | 2.17 | 1.23 (1.07–1.41) | 732 | 321,799 | 2.27 | 1.22 (1.08–1.37) |
| p for trend | <0.001 | <0.001 | ||||||
| Stroke | ||||||||
| Q1 | 707 | 327,414 | 2.16 | 1 (ref) | 790 | 326,411 | 2.42 | 1 (ref) |
| Q2 | 852 | 326,600 | 2.61 | 1.16 (1.05–1.28) | 811 | 326,513 | 2.48 | 1.07 (0.97–1.18) |
| Q3 | 950 | 324,170 | 2.93 | 1.25 (1.13–1.39) | 923 | 324,827 | 2.84 | 1.18 (1.07–1.30) |
| Q4 | 1133 | 320,540 | 3.53 | 1.44 (1.29–1.61) | 1118 | 320,973 | 3.48 | 1.26 (1.15–1.39) |
| p for trend | <0.001 | <0.001 | ||||||
| All-cause mortality | ||||||||
| Q1 | 1388 | 329,799 | 4.21 | 1 (ref) | 1524 | 329,081 | 4.63 | 1 (ref) |
| Q2 | 1582 | 329,472 | 4.80 | 1.11 (1.03-1.19) | 1529 | 329,279 | 4.64 | 1.07 (0.99–1.15) |
| Q3 | 1839 | 327,528 | 5.61 | 1.27 (1.18–1.37) | 1688 | 328,129 | 5.14 | 1.15 (1.07–1.23) |
| Q4 | 2498 | 324,270 | 7.70 | 1.53 (1.42–1.65) | 2566 | 324,580 | 7.91 | 1.44 (1.34–1.53) |
| p for trend | <0.001 | <0.001 | ||||||
| Cardiovascular disease-related mortality | ||||||||
| Q1 | 245 | 329,799 | 0.74 | 1 (ref) | 255 | 329,081 | 0.77 | 1 (ref) |
| Q2 | 305 | 329,472 | 0.93 | 1.24 (1.04–1.46) | 292 | 329,279 | 0.89 | 1.24 (1.05–1.46) |
| Q3 | 373 | 327,528 | 1.14 | 1.54 (1.30–1.81) | 349 | 328,129 | 1.06 | 1.44 (1.23–1.70) |
| Q4 | 415 | 324,270 | 1.28 | 1.74 (1.45–2.08) | 442 | 324,580 | 1.36 | 1.60 (1.37–1.88) |
| p for trend | <0.0001 | <0.0001 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.S.; Lee, J.S.; Kim, J.A.; Roh, E.; Lee, Y.B.; Hong, S.H.; Yoo, H.J.; Baik, S.H.; Kim, N.H.; Seo, J.A.; et al. γ-Glutamyltransferase Variability and the Risk of Mortality, Myocardial Infarction, and Stroke: A Nationwide Population-Based Cohort Study. J. Clin. Med. 2019, 8, 832. https://doi.org/10.3390/jcm8060832
Chung HS, Lee JS, Kim JA, Roh E, Lee YB, Hong SH, Yoo HJ, Baik SH, Kim NH, Seo JA, et al. γ-Glutamyltransferase Variability and the Risk of Mortality, Myocardial Infarction, and Stroke: A Nationwide Population-Based Cohort Study. Journal of Clinical Medicine. 2019; 8(6):832. https://doi.org/10.3390/jcm8060832
Chicago/Turabian StyleChung, Hye Soo, Ji Sung Lee, Jung A. Kim, Eun Roh, You Bin Lee, So Hyeon Hong, Hye Jin Yoo, Sei Hyun Baik, Nan Hee Kim, Ji A Seo, and et al. 2019. "γ-Glutamyltransferase Variability and the Risk of Mortality, Myocardial Infarction, and Stroke: A Nationwide Population-Based Cohort Study" Journal of Clinical Medicine 8, no. 6: 832. https://doi.org/10.3390/jcm8060832