Investigation of the Proportion of Diagnosed People Living with HIV/AIDS among Foreign Residents in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Descriptions
2.2. Balance Equation Model
2.3. Ethical Considerations
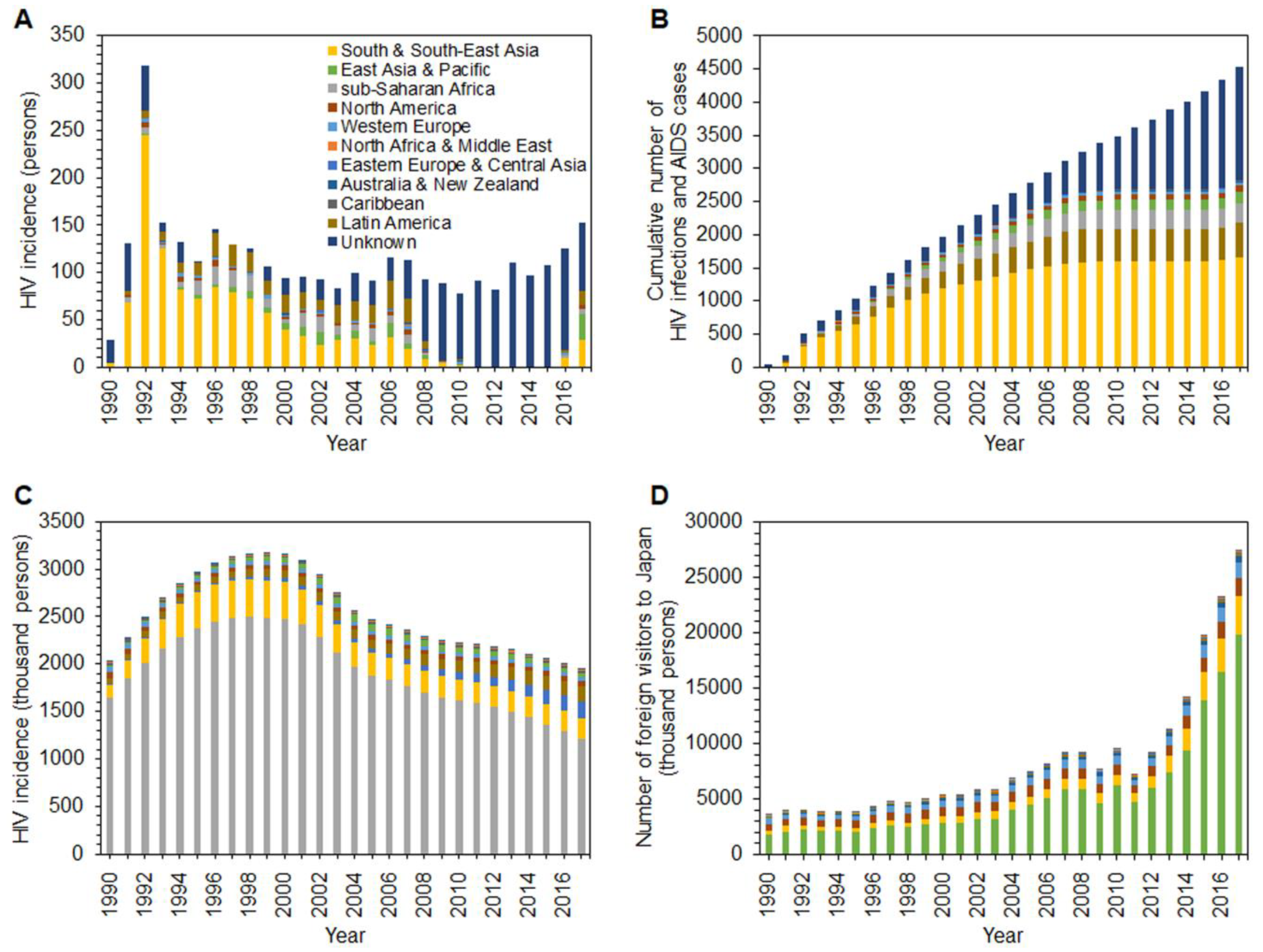
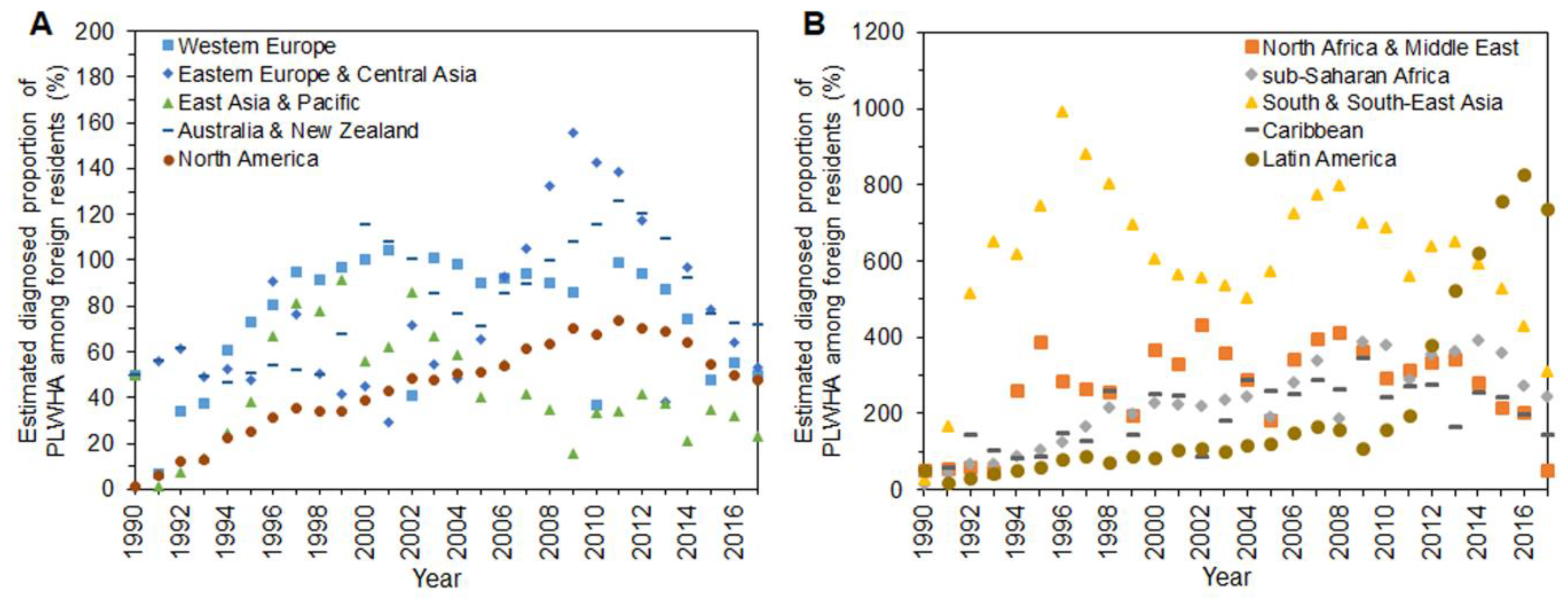
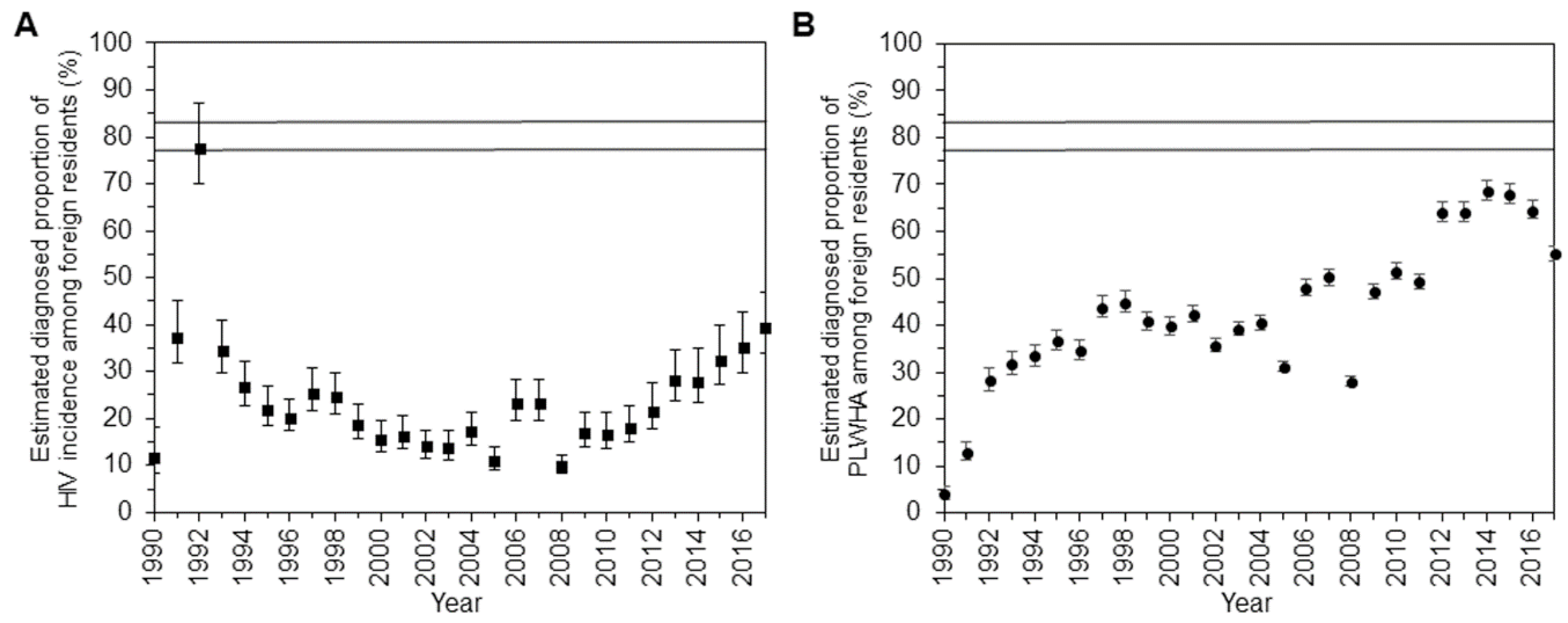
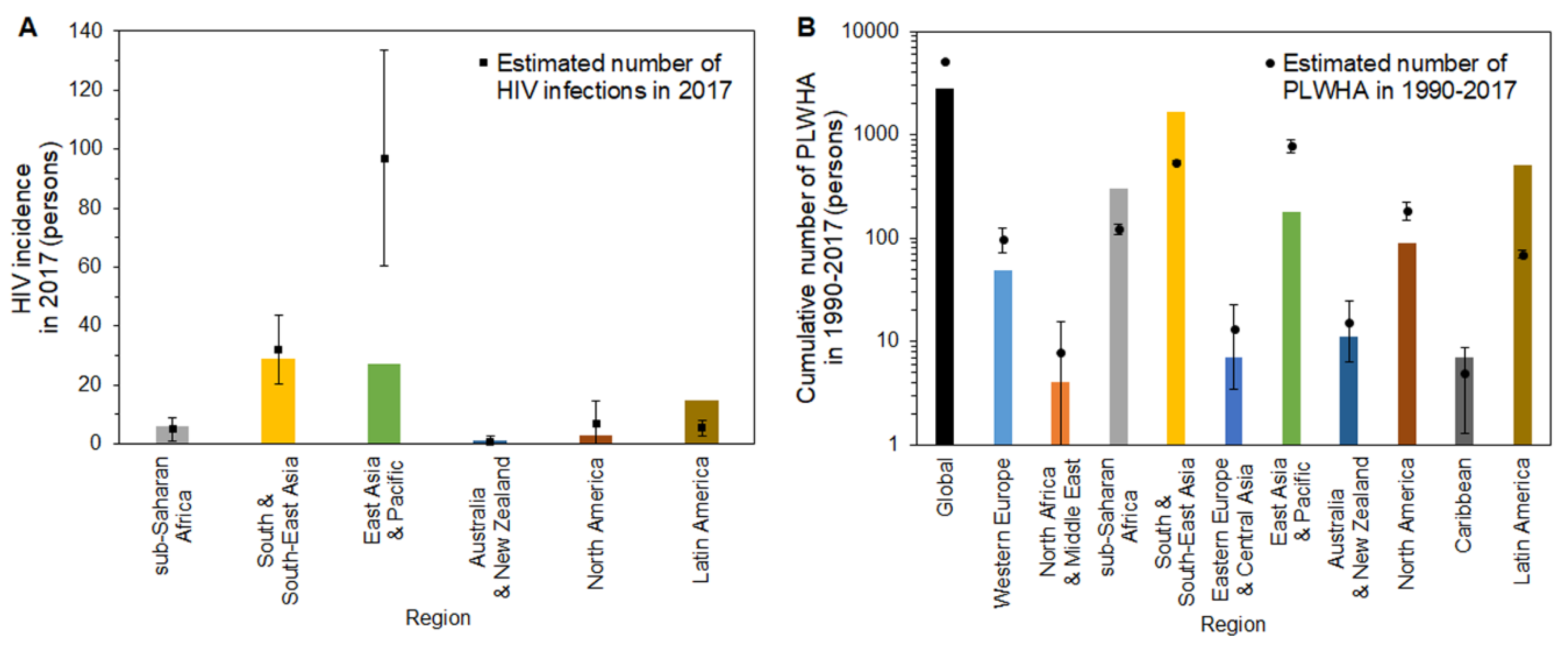
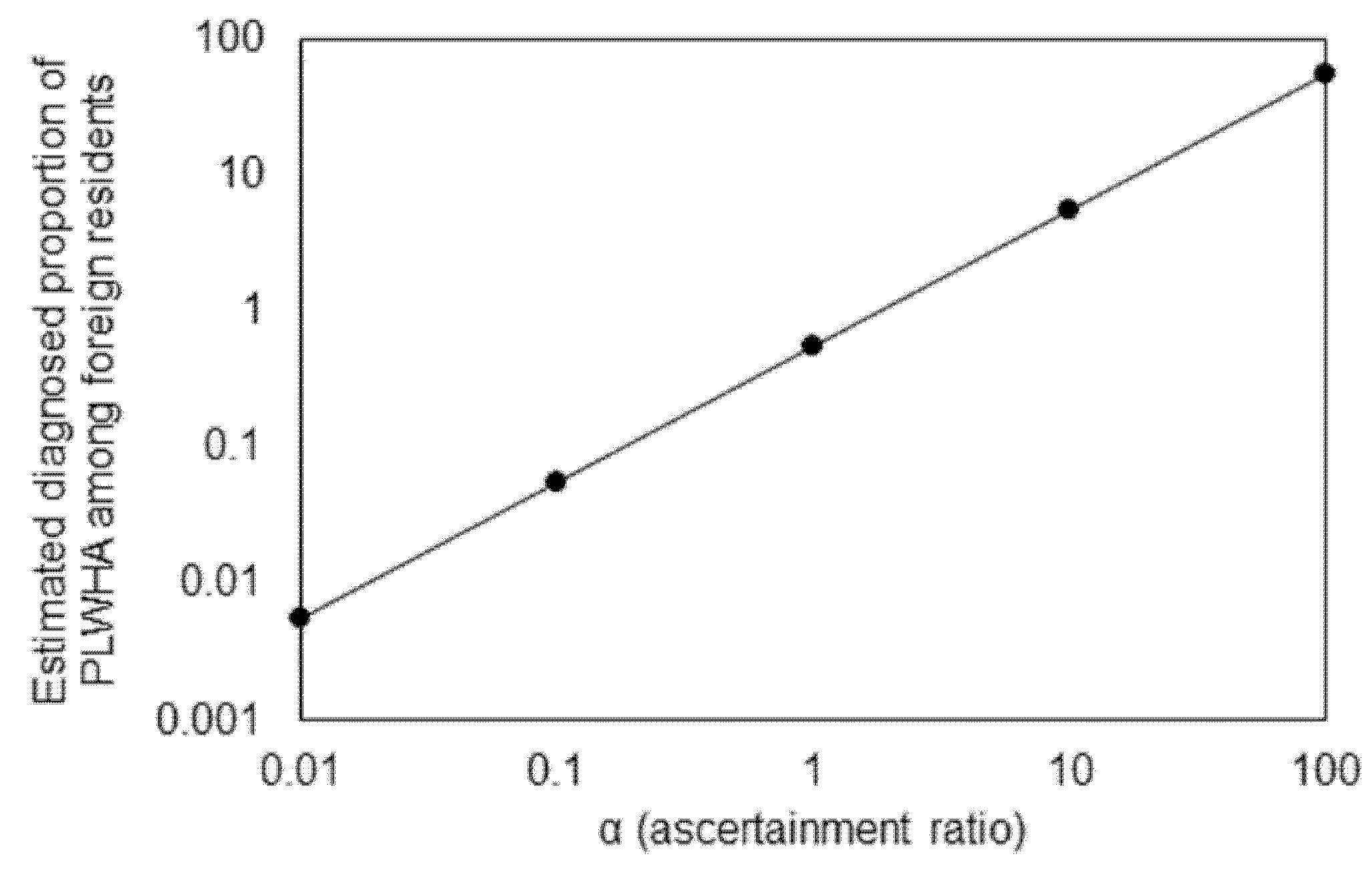
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Average Length of Stay in Japan by Those from Each Country of Origin
Appendix A.2. Number of Foreign Nationals Entering Japan, by Country
Appendix A.3. Classification of Regions
References
- Jones, J.; Sullivan, P.S.; Curran, J.W. Progress in the HIV epidemic: Identifying goals and measuring success. PLoS Med. 2019, 16, e1002729. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011, 365, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N. Engl. J. Med. 2016, 375, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Bavinton, B.R.; Pinto, A.N.; Phanuphak, N.; Grinsztejn, B.; Prestage, G.P.; Zablotska-Manos, I.B.; Jin, F.; Fairley, C.K.; Moore, R.; Roth, N.; et al. Viral suppression and HIV transmission in serodiscordant male couples: An international, prospective, observational, cohort study. Lancet HIV 2018, 5, e438–e447. [Google Scholar] [CrossRef]
- UNAIDS. Global HIV & AIDS Statistics—2018 Fact Sheet; UNAIDS: Geneva, Switzerland, 2018; Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 25 April 2019).
- UNAIDS. UNAIDS Data 2018; UNAIDS: Geneva, Switzerland, 2018; Available online: https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf (accessed on 25 April 2019).
- UNAIDS. 90-90-90—An Ambitious Treatment Target to Help end the AIDS Epidemic; UNAIDS: Geneva, Switzerland, 2017; Available online: https://www.unaids.org/en/resources/documents/2017/90-90-90 (accessed on 25 April 2019).
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; van Lunzen, J.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016, 316, 171–181. [Google Scholar] [CrossRef]
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; Degen, O.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicenter, prospective, observational study. Lancet 2019, 393, 2428–2438. [Google Scholar] [CrossRef]
- Cohen, M.S. Successful treatment of HIV eliminates sexual transmission. Lancet 2019. [Google Scholar] [CrossRef]
- Granich, R.; Williams, B.; Montaner, J.; Zuniga, J.M. 90-90-90 and ending AIDS: Necessary and feasible. Lancet 2017, 390, 341–343. [Google Scholar] [CrossRef]
- UNAIDS. Fast-Track—Ending the AIDS Epidemic by 2030; UNAIDS: Geneva, Switzerland, 2014; Available online: https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf (accessed on 25 April 2019).
- Piot, P.; Abdool Karim, S.S.; Hecht, R.; Legido-Quigley, H.; Buse, K.; Stover, J.; Resch, S.; Ryckman, T.; Møgedal, S.; Dybul, M.; et al. Defeating AIDS—Advancing global health. Lancet 2015, 386, 171–218. [Google Scholar] [CrossRef]
- UNAIDS. UNAIDS 2016-2021 Strategy: On the Fast-Track to End AIDS; UNAIDS: Geneva, Switzerland, 2015; Available online: https://www.unaids.org/sites/default/files/media_asset/20151027_UNAIDS_PCB37_15_18_EN_rev1.pdf (accessed on 25 April 2019).
- Piot, P.; Bartos, M.; Larson, H.; Zewdie, D.; Mane, P. Coming to terms with complexity: A call to action for HIV prevention. Lancet 2008, 372, 845–859. [Google Scholar] [CrossRef]
- Nemoto, T. HIV/AIDS surveillance and prevention studies in Japan: Summary and recommendations. AIDS Educ. Prev. 2004, 16, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Report to UNAIDS—HIV/AIDS Trends in Japan, April 2016. Available online: http://www.unaids.org/en/file/110930/download?token=VdC4yp-g (accessed on 25 April 2019).
- Iwamoto, A.; Taira, R.; Yokomaku, Y.; Koibuchi, T.; Rahman, M.; Izumi, Y.; Tadokoro, K. The HIV care cascade: Japanese perspectives. PLoS ONE 2017, 12, e0174360. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H. Estimating the incidence and diagnosed proportion of HIV infections in Japan: A statistical modeling study. PeerJ 2019, 7, e6275. [Google Scholar] [CrossRef] [PubMed]
- Suguimoto, S.P.; Techasrivichien, T.; Musumari, P.M.; El-saaidi, C.; Lukhele, B.W.; Ono-Kihara, M.; Kihara, M. Changing patterns of HIV epidemic in 30 years in East Asia. Curr. HIV/AIDS Rep. 2014, 11, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.; Li, J.; Shibuya, K. Projecting HIV transmission in Japan. PLoS ONE 2012, 7, e43473. [Google Scholar] [CrossRef] [PubMed]
- Sherriff, N.; Koerner, J.; Kaneko, N.; Shiono, S.; Takaku, M.; Boseley, R.; Ichikawa, S. Everywhere in Japan: An international approach to working with commercial gay businesses in HIV prevention. Health Promot. Int. 2017, 32, 522–534. [Google Scholar] [CrossRef]
- Ezoe, S.; Morooka, T.; Noda, T.; Sabin, M.L.; Koike, S. Population size estimation of men who have sex with men through the network scale-up method in Japan. PLoS ONE 2012, 7, e31184. [Google Scholar] [CrossRef]
- The Tuberculosis and Infectious Diseases Control Division, Ministry of Health, Labour and Welfare, Japan. Annual Report of the National AIDS Surveillance Committee. Available online: http://api-net.jfap.or.jp/status/2017/17nenpo/17nenpo_menu.html (accessed on 21 December 2018). (In Japanese).
- Kinoshita, M.; Oka, S. Migrant patients living with HIV/AIDS in Japan: Review of factors associated with high dropout rate in a leading medical institution in Japan. PLoS ONE 2018, 13, e0205184. [Google Scholar] [CrossRef]
- Ministry of Justice, Japan. The Annual Report of Statistics on Legal Migrants. Available online: http://www.moj.go.jp/housei/toukei/toukei_ichiran_nyukan.html (accessed on 21 December 2018). (In Japanese).
- Ministry of Justice, Japan. The Transition of Foreign Residents by Nationality and Region. Available online: http://www.moj.go.jp/content/001269620.pdf (accessed on 25 April 2019). (In Japanese).
- Ministry of Justice, Japan. Law for Partial Amendment to Immigration Control and Refugee Recognition Act and Act for Establishment of the Ministry of Justice. Available online: http://www.moj.go.jp/nyuukokukanri/kouhou/nyuukokukanri05_00017.html (accessed on 25 April 2019). (In Japanese).
- UNAIDS. AIDSinfo. Available online: http://aidsinfo.unaids.org/ (accessed on 31 October 2018).
- Global Health Data Exchange. GBD Results Tool. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 31 October 2018).
- GBD 2015 HIV Collaborators. Estimates of Global, Regional, and National Incidence, Prevalence, and Mortality of HIV, 1980–2015: The Global Burden of Disease Study 2015. Lancet HIV 2016, 3, e361–e387. [Google Scholar] [CrossRef]
- Kelly, S.L.; Wilson, D.P. GBD 2015 and HIV estimates from the Optima model. Lancet HIV 2016, 3, e558. [Google Scholar] [CrossRef]
- Population Division, Department of Economic and Social Affairs, United Nations Secretariat. The 2017 Revision of World Population Prospects. Available online: https://population.un.org/wpp/ (accessed on 12 November 2018).
- UNAIDS and WHO. Report on the Global HIV/AIDS Epidemic—June 1998; UNAIDS and WHO: Geneva, Switzerland, 1998; Available online: http://data.unaids.org/pub/report/1998/19981125_global_epidemic_report_en.pdf (accessed on 31 October 2018).
- Uretsky, E. ‘Mobile men with money’: The socio-cultural and politico-economic context of ‘high-risk’ behaviour among wealthy businessmen and government officials in urban China. Cult. Health. Sex. 2008, 10, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Aggleton, P.; Bell, S.A.; Kelly-Hanku, A. ‘Mobile men with money’: HIV prevention and the erasure of difference. Glob. Public Health 2014, 9, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.T.; Ng, K.T.; Ong, L.Y.; Chook, J.B.; Chan, K.G.; Takebe, Y.; Kamarulzaman, A.; Tee, K.K. Cross-border sexual transmission of the newly emerging HIV-1 clade CRF51_01B. PLoS ONE 2014, 9, e111236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peach, E.; Lemoh, C.; Stoove, M.; Agius, P.; El Hayek, C.; Higgins, N.; Hellard, M. Aiming for 90-90-90 – the importance of understanding the risk factors for HIV exposure and advanced HIV infection in migrant populations and other groups who do not report male-to-male sex. Sex Health 2018, 15, 441–450. [Google Scholar] [CrossRef] [PubMed]
- McMahon, T.; Ward, P.R. HIV among immigrants living in high-income countries: A realist review of evidence to guide targeted approaches to behavioural HIV prevention. Syst. Rev. 2012, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.D.; Nakamanya, S.; Kyomuhangi, R.; Amurwon, J.; Namara, G.; Amuron, B.; Nabiryo, C.; Birungi, J.; Wolff, B.; Jaffar, S.; et al. The experience of “medicine companions” to support adherence to antiretroviral therapy: Quantitative and qualitative data from a trial population in Uganda. AIDS Care 2010, 22, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Beyrer, C.; Baral, S.D.; Walker, D.; Wirtz, A.L.; Johns, B.; Sifakis, F. The expanding epidemics of HIV type 1 among men who have sex with men in low- and middle-income countries: Diversity and consistency. Epidemiol. Rev. 2010, 32, 137–151. [Google Scholar] [CrossRef]
- Norredam, M.; Nielsen, S.S.; Krasnik, A. Migrants’ utilization of somatic healthcare services in Europe—A systematic review. Eur. J. Public Health 2010, 20, 555–563. [Google Scholar] [CrossRef]
- Hernando, V.; Alvárez-del Arco, D.; Alejos, B.; Monge, S.; Amato-Gauci, A.J.; Noori, T.; Pharris, A.; del Amo, J. HIV infection in migrant populations in the European Union and European Economic Area in 2007–2012: An epidemic on the move. J. Acquir. Immune Defic. Syndr. 2015, 70, 204–211. [Google Scholar] [CrossRef]
- Deen, L.; Cowan, S.; Wejse, C.; Petersen, J.H.; Norredam, M. Refugees and family-reunified immigrants have a high incidence of HIV diagnosis and late presentation compared with Danish born: A nationwide register-based cohort study. Infection 2018, 46, 659–667. [Google Scholar] [CrossRef]
- Redditt, V.J.; Janakiram, P.; Graziano, D.; Rashid, M. Health status of newly arrived refugees in Toronto, Ont: Part 1: Infectious diseases. Can. Fam. Physician 2015, 61, e303–e309. [Google Scholar] [PubMed]
- Pottie, K.; Janakiram, P.; Topp, P.; McCarthy, A. Prevalence of selected preventable and treatable diseases among government-assisted refugees: Implications for primary care providers. Can. Fam. Physician 2007, 53, 1928–1934. [Google Scholar] [PubMed]
- The Lancet Global Health. Bridging the global health gap. Lancet Glob. Health 2016, 4, e579. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Special Report, HIV and Migrants. Monitoring Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2017 Progress Report; ECDC: Stockholm, Sweden, 2017; Available online: https://ecdc.europa.eu/sites/portal/files/documents/HIV%20and%20migrants.pdf (accessed on 25 April 2019).
- Gunaratnam, P.; Heywood, A.E.; McGregor, S.; Jamil, M.S.; McManus, H.; Mao, L.; Lobo, R.; Brown, G.; Hellard, M.; Marukutira, T.; et al. HIV diagnoses in migrant populations in Australia—A changing epidemiology. PLoS ONE 2019, 14, e0212268. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, W.; Nöstlinger, C.; Hospers, H.; Laga, M. International mobility, sexual behaviour and HIV-related characteristics of men who have sex with men residing in Belgium. BMC Public Health 2013, 13, 968. [Google Scholar] [CrossRef] [PubMed]
- Richens, J. Sexually transmitted infections and HIV among travellers: A review. Travel Med. Infect. Dis. 2006, 4, 184–195. [Google Scholar] [CrossRef]
- Lau, J.T.; Cai, W.; Tsui, H.Y.; Chen, L.; Cheng, J.; Lin, C.; Gu, J.; Hao, C. Unprotected anal intercourse behavior and intention among male sex workers in Shenzhen serving cross-boundary male clients coming from Hong Kong, China—Prevalence and associated factors. AIDS Care 2012, 24, 59–70. [Google Scholar] [CrossRef]
- Mao, J.; Tang, W.; Liu, C.; Wong, N.S.; Tang, S.; Wei, C.; Tucker, J.D. Sex tourism among Chinese men who have sex with men: A cross-sectional observational study. BMC Public Health 2018, 18, 306. [Google Scholar] [CrossRef]
- Zabin, L.S.; Emerson, M.R.; Nan, L.; Chaohua, L.; Ersheng, G.; Minh, N.H.; Chuang, Y.L.; Hurng, B.S.; Bishai, D.; Blum, R.W. Levels of change in adolescent sexual behavior in three Asian cities. Stud. Fam. Plan. 2009, 40, 1–12. [Google Scholar] [CrossRef]
- The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med. 2015, 373, 795–807. [Google Scholar] [CrossRef]
- Alvarez-del Arco, D.; Monge, S.; Azcoaga, A.; Rio, I.; Hernando, V.; Gonzalez, C.; Alejos, B.; Caro, A.M.; Perez-Cachafeiro, S.; Ramirez-Rubio, O.; et al. HIV testing and counselling for migrant populations living in high-income countries: A systematic review. Eur. J. Public Health 2013, 23, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Baggaley, R.F.; Irvine, M.A.; Leber, W.; Cambiano, V.; Figueroa, J.; McMullen, H.; Anderson, J.; Santos, A.C.; Terris-Prestholt, F.; Miners, A.; et al. Cost-effectiveness of screening for HIV in primary care: A health economics modelling analysis. Lancet HIV 2017, 4, e465–e474. [Google Scholar] [CrossRef]
- Esteban-Vasallo, M.D.; Morán-Arribas, M.; García-Riolobos, C.; Domínguez-Berjón, M.F.; Rico-Bermejo, J.; Collado-González, S.; Jiménez-García, R.; Guionnet, A.; de la Fuente, B.P.; El Kertat, R.; et al. Targeted rapid HIV testing in public primary care services in Madrid. Are we reaching the vulnerable populations? Int. J. Infect. Dis. 2014, 19, 39–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pottie, K.; Lotfi, T.; Kilzar, L.; Howeiss, P.; Rizk, N.; Akl, E.A.; Dias, S.; Biggs, B.A.; Christensen, R.; Rahman, P.; et al. The effectiveness and cost-effectiveness of screening for HIV in migrants in the EU/EEA: A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 1700. [Google Scholar] [CrossRef] [PubMed]
- Creek, T.L.; Ntumy, R.; Seipone, K.; Smith, M.; Mogodi, M.; Smit, M.; Legwaila, K.; Molokwane, I.; Tebele, G.; Mazhani, L.; et al. Successful introduction of routine opt-out HIV testing in antenatal care in Botswana. J. Acquir. Immune Defic. Syndr. 2007, 45, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Sanders, G.D.; Bayoumi, A.M.; Sundaram, V.; Bilir, S.P.; Neukermans, C.P.; Rydzak, C.E.; Douglass, L.R.; Lazzeroni, L.C.; Holodniy, M.; Owens, D.K. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N. Engl. J. Med. 2005, 352, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Branson, B.M.; Handsfield, H.H.; Lampe, M.A.; Janssen, R.S.; Taylor, A.W.; Lyss, S.B.; Clark, J.E. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm. Rep. 2006, 55, 1–17. [Google Scholar] [PubMed]
- Hamill, M.; Burgoine, K.; Farrell, F.; Hemelaar, J.; Patel, G.; Welchew, D.E.; Jaffe, H.W. Time to move towards opt-out testing for HIV in the UK. BMJ 2007, 334, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Beyrer, C.; Malinowska-Sempruch, K.; Kamarulzaman, A.; Kazatchkine, M.; Sidibe, M.; Strathdee, S.A. Time to act: A call for comprehensive responses to HIV in people who use drugs. Lancet 2010, 376, 551–563. [Google Scholar] [CrossRef]
- Beyrer, C.; Baral, S.D.; van Griensven, F.; Goodreau, S.M.; Chariyalertsak, S.; Wirtz, A.L.; Brookmeyer, R. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012, 380, 367–377. [Google Scholar] [CrossRef]
- Baral, S.; Beyrer, C.; Muessig, K.; Poteat, T.; Wirtz, A.L.; Decker, M.R.; Sherman, S.G.; Kerrigan, D. Burden of HIV among female sex workers in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 538–549. [Google Scholar] [CrossRef]
- Valverde, E.E.; Oster, A.M.; Xu, S.; Wertheim, J.O.; Hernandez, A.L. HIV Transmission Dynamics among Foreign-Born Persons in the United States. J. Acquir. Immune Defic. Syndr. 2017, 76, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Fakoya, I.; Álvarez-del Arco, D.; Woode-Owusu, M.; Monge, S.; Rivero-Montesdeoca, Y.; Delpech, V.; Rice, B.; Noori, T.; Pharris, A.; Amato-Gauci, A.J.; et al. A systematic review of post-migration acquisition of HIV among migrants from countries with generalised HIV epidemics living in Europe: Implications for effectively managing HIV prevention programmes and policy. BMC Public Health 2015, 15, 561. [Google Scholar] [CrossRef] [PubMed]
- Rice, B.D.; Elford, J.; Yin, Z.; Delpech, V.C. A new method to assign country of HIV infection among heterosexuals born abroad and diagnosed with HIV. AIDS 2012, 26, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Piot, P.; Russell, S.; Larson, H. Good politics, bad politics: The experience of AIDS. Am. J. Public Health 2007, 97, 1934–1936. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, K.; Nishiura, H.; Imamura, A. Investigation of the Proportion of Diagnosed People Living with HIV/AIDS among Foreign Residents in Japan. J. Clin. Med. 2019, 8, 804. https://doi.org/10.3390/jcm8060804
Shimizu K, Nishiura H, Imamura A. Investigation of the Proportion of Diagnosed People Living with HIV/AIDS among Foreign Residents in Japan. Journal of Clinical Medicine. 2019; 8(6):804. https://doi.org/10.3390/jcm8060804
Chicago/Turabian StyleShimizu, Kazuki, Hiroshi Nishiura, and Akifumi Imamura. 2019. "Investigation of the Proportion of Diagnosed People Living with HIV/AIDS among Foreign Residents in Japan" Journal of Clinical Medicine 8, no. 6: 804. https://doi.org/10.3390/jcm8060804
APA StyleShimizu, K., Nishiura, H., & Imamura, A. (2019). Investigation of the Proportion of Diagnosed People Living with HIV/AIDS among Foreign Residents in Japan. Journal of Clinical Medicine, 8(6), 804. https://doi.org/10.3390/jcm8060804





