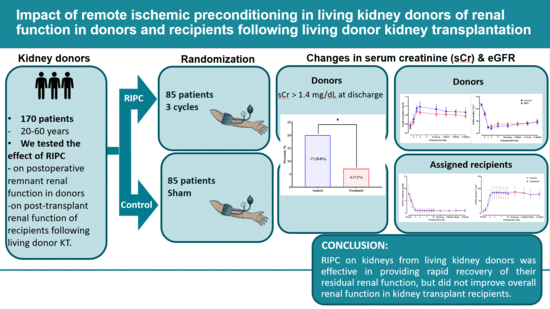
Impact of Remote Ischemic Preconditioning Conducted in Living Kidney Donors on Renal Function in Donors and Recipients Following Living Donor Kidney Transplantation: A Randomized Clinical Trial
Abstract
1. Introduction
2. Methods
2.1. Donors
2.2. Recipients
2.3. Intraoperative Fluid and Hemodynamic Management
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Suthanthiran, M.; Strom, T.B. Renal transplantation. N. Engl. J. Med. 1994, 331, 365–376. [Google Scholar] [CrossRef]
- Cohen, D.J.; St Martin, L.; Christensen, L.L.; Bloom, R.D.; Sung, R.S. Kidney and pancreas transplantation in the United States, 1995–2004. Am. J. Transplant. 2006, 6, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.A.; Roys, E.C.; Merion, R.M. Trends in organ donation and transplantation in the United States, 1999–2008. Am. J. Transplant. 2010, 10, 961–972. [Google Scholar] [CrossRef]
- Mjoen, G.; Hallan, S.; Hartmann, A.; Foss, A.; Midtvedt, K.; Oyen, O.; Reisaeter, A.; Pfeffer, P.; Jenssen, T.; Leivestad, T.; et al. Long-term risks for kidney donors. Kidney Int. 2014, 86, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Muzaale, A.D.; Massie, A.B.; Wang, M.C.; Montgomery, R.A.; McBride, M.A.; Wainright, J.L.; Segev, D.L. Risk of end-stage renal disease following live kidney donation. JAMA 2014, 311, 579–586. [Google Scholar] [CrossRef]
- Hostetter, T.H.; Olson, J.L.; Rennke, H.G.; Venkatachalam, M.A.; Brenner, B.M. Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. J. Am. Soc. Nephrol. 2001, 12, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.B.; Myers, B.D.; Derby, G.; Blouch, K.L.; Yan, J.; Ho, B.; Tan, J.C. Adaptive hyperfiltration in the aging kidney after contralateral nephrectomy. Am. J. Physiol. Renal Physiol. 2006, 291, F629–F634. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.W.; Hsu, K.H.; Lee, C.C.; Sun, C.Y.; Hsu, H.J.; Tsai, C.J.; Tzen, C.Y.; Wang, Y.C.; Lin, C.Y.; Wu, M.S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Miyamoto, Y.; Otagiri, M.; Maruyama, T. Update on the pharmacokinetics and redox properties of protein-bound uremic toxins. J. Pharm. Sci. 2011, 100, 3682–3695. [Google Scholar] [CrossRef]
- Lin, C.J.; Liu, H.L.; Pan, C.F.; Chuang, C.K.; Jayakumar, T.; Wang, T.J.; Chen, H.H.; Wu, C.J. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch. Med. Res. 2012, 43, 451–456. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Meijers, B.K.; Bammens, B.; De Moor, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008, 73, 1174–1180. [Google Scholar] [CrossRef]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef]
- Rossi, M.; Campbell, K.L.; Johnson, D.W.; Stanton, T.; Haluska, B.A.; Hawley, C.M.; Dimeski, G.; McWhinney, B.C.; Ungerer, J.P.; Kaisar, O.M.; et al. Uremic toxin development in living kidney donors: A longitudinal study. Transplantation 2014, 97, 548–554. [Google Scholar] [CrossRef]
- Lazaris, A.M.; Maheras, A.N.; Vasdekis, S.N.; Karkaletsis, K.G.; Charalambopoulos, A.; Kakisis, J.D.; Martikos, G.; Patapis, P.; Giamarellos-Bourboulis, E.J.; Karatzas, G.M.; et al. Protective effect of remote ischemic preconditioning in renal ischemia/reperfusion injury, in a model of thoracoabdominal aorta approach. J. Surg. Res. 2009, 154, 267–273. [Google Scholar] [CrossRef]
- Ali, Z.A.; Callaghan, C.J.; Lim, E.; Ali, A.A.; Nouraei, S.A.; Akthar, A.M.; Boyle, J.R.; Varty, K.; Kharbanda, R.K.; Dutka, D.P.; et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: A randomized controlled trial. Circulation 2007, 116, I98–I105. [Google Scholar] [CrossRef]
- Kierulf-Lassen, C.; Nieuwenhuijs-Moeke, G.J.; Krogstrup, N.V.; Oltean, M.; Jespersen, B.; Dor, F.J. Molecular Mechanisms of Renal Ischemic Conditioning Strategies. Eur. Surg. Res. 2015, 55, 151–183. [Google Scholar] [CrossRef]
- Rassaf, T.; Totzeck, M.; Hendgen-Cotta, U.B.; Shiva, S.; Heusch, G.; Kelm, M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ. Res. 2014, 114, 1601–1610. [Google Scholar] [CrossRef]
- Pickard, J.M.; Botker, H.E.; Crimi, G.; Davidson, B.; Davidson, S.M.; Dutka, D.; Ferdinandy, P.; Ganske, R.; Garcia-Dorado, D.; Giricz, Z.; et al. Remote ischemic conditioning: From experimental observation to clinical application: Report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res. Cardiol. 2015, 110, 453. [Google Scholar] [CrossRef]
- Carini, R.; Albano, E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology 2003, 125, 1480–1491. [Google Scholar] [CrossRef]
- Jaeschke, H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G15–G26. [Google Scholar] [CrossRef]
- Wu, J.; Feng, X.; Huang, H.; Shou, Z.; Zhang, X.; Wang, R.; Chen, Y.; Chen, J. Remote ischemic conditioning enhanced the early recovery of renal function in recipients after kidney transplantation: A randomized controlled trial. J. Surg. Res. 2014, 188, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, M.L.; Pattenden, C.J.; Barlow, A.D.; Hunter, J.P.; Lee, G.; Hosgood, S.A. A double blind randomized clinical trial of remote ischemic conditioning in live donor renal transplantation. Medicine (Baltimore) 2015, 94, e1316. [Google Scholar] [CrossRef]
- Souza Filho, M.V.; Loiola, R.T.; Rocha, E.L.; Simao, A.F.; Gomes, A.S.; Souza, M.H.; Ribeiro, R.A. Hind limb ischemic preconditioning induces an anti-inflammatory response by remote organs in rats. Braz. J. Med. Biol. Res. 2009, 42, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Franchello, A.; Gilbo, N.; David, E.; Ricchiuti, A.; Romagnoli, R.; Cerutti, E.; Salizzoni, M. Ischemic preconditioning (IP) of the liver as a safe and protective technique against ischemia/reperfusion injury (IRI). Am. J. Transplant. 2009, 9, 1629–1639. [Google Scholar] [CrossRef]
- Jassem, W.; Fuggle, S.V.; Cerundolo, L.; Heaton, N.D.; Rela, M. Ischemic preconditioning of cadaver donor livers protects allografts following transplantation. Transplantation 2006, 81, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. (2011) 2013, 3, 1–150. [Google Scholar]
- Solez, K.; Axelsen, R.A.; Benediktsson, H.; Burdick, J.F.; Cohen, A.H.; Colvin, R.B.; Croker, B.P.; Droz, D.; Dunnill, M.S.; Halloran, P.F.; et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int. 1993, 44, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Kim, S.O.; Kim, S.G.; Song, J.G.; Hwang, G.S. Cystatin-C is associated with partial recovery of kidney function and progression to chronic kidney disease in living kidney donors: Observational study. Medicine (Baltimore) 2017, 96, e6037. [Google Scholar] [CrossRef]
- Sakpal, T.V. Sample size estimation in clinical trial. Perspect. Clin. Res. 2010, 1, 67–69. [Google Scholar] [PubMed]
- Heusch, G.; Botker, H.E.; Przyklenk, K.; Redington, A.; Yellon, D. Remote ischemic conditioning. J. Am. Coll. Cardiol. 2015, 65, 177–195. [Google Scholar] [CrossRef]
- Gassanov, N.; Nia, A.M.; Caglayan, E.; Er, F. Remote ischemic preconditioning and renoprotection: From myth to a novel therapeutic option? J. Am. Soc. Nephrol. 2014, 25, 216–224. [Google Scholar] [CrossRef]
- Er, F.; Nia, A.M.; Dopp, H.; Hellmich, M.; Dahlem, K.M.; Caglayan, E.; Kubacki, T.; Benzing, T.; Erdmann, E.; Burst, V.; et al. Ischemic preconditioning for prevention of contrast medium-induced nephropathy: Randomized pilot RenPro Trial (Renal Protection Trial). Circulation 2012, 126, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk-van den Akker, J.M.; Warle, M.C.; van Zuilen, A.D.; Kloke, H.J.; Wever, K.E.; d’Ancona, F.C.; zdemir, D.M.; Wetzels, J.F.; Hoitsma, A.J. Urinary biomarkers after donor nephrectomy. Transpl. Int. 2015, 28, 544–552. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Anderson-Haag, T.; Israni, A.K.; Kalil, R.S.; Kimmel, P.L.; Kraus, E.S.; Kumar, R.; Posselt, A.A.; Pesavento, T.E.; Rabb, H.; et al. A prospective controlled study of living kidney donors: Three-year follow-up. Am. J. Kidney Dis. 2015, 66, 114–124. [Google Scholar] [CrossRef]
- Filler, G.; Bokenkamp, A.; Hofmann, W.; Le Bricon, T.; Martinez-Bru, C.; Grubb, A. Cystatin C as a marker of GFR—History, indications, and future research. Clin. Biochem. 2005, 38, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, H.; Wang, X.; Zhou, Z.; Luo, A.; Tian, Y. Remote ischemic preconditioning fails to improve early renal function of patients undergoing living-donor renal transplantation: A randomized controlled trial. Transplantation 2013, 95, e4–e6. [Google Scholar] [CrossRef]
- Botker, H.E.; Kharbanda, R.; Schmidt, M.R.; Bottcher, M.; Kaltoft, A.K.; Terkelsen, C.J.; Munk, K.; Andersen, N.H.; Hansen, T.M.; Trautner, S.; et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet 2010, 375, 727–734. [Google Scholar] [CrossRef]

| Control Group (n = 85) | RIPC Group (n = 85) | p-Value | |
|---|---|---|---|
| Preoperative characteristics | |||
| Age, year | 42.0 (33.0–49.0) | 44.0 (37.0–51.0) | 0.33 |
| Males, n (%) | 46 (54.1) | 26 (30.5) | <0.01 |
| Body mass index, kg/m2 | 24.0 ± 2.8 | 23.7 ± 2.9 | 0.45 |
| sCr, mg/dL | 0.8 (0.7–0.9) | 0.7 (0.6–0.8) | <0.01 |
| eGFR, mL/min/1.73 m2 | 106.6 ± 11.1 | 106.8 ± 10.6 | 0.90 |
| ASA PS 1/2 | 60/25 | 59/26 | 1.00 |
| Intraoperative characteristics | |||
| Operation time | 212.0 (190.0–235.0) | 205.0 (183.0–225.0) | 0.09 |
| HALS, n (%) | 66 (77.7) | 75 (88.2) | 0.10 |
| Kidney (right), n (%) | 34 (40.0) | 36 (42.4) | 0.88 |
| Plasmalyte, mL | 1302.1 ± 547.3 | 1205.9 ± 534.1 | 0.25 |
| Transfusion of RBC, n (%) | 0 (0) | 1 (1.2) | 1.00 |
| 20% Mannitol, mL | 160.0 (140.0–190.0) | 150.0 (14.0.0–175.0) | 0.33 |
| Urine output, mL | 270.0 (220.0–420.0) | 290.0 (190.0–400.0) | 0.37 |
| Mean blood pressure, mmHg | |||
| Before RIPC | 76.0 (71.0–89.0) | 78.0 (71.0–89.0) | 0.63 |
| After RIPC | 84.2 ± 9.6 | 85.4 ± 12.8 | 0.51 |
| Cystatin C, mg/L | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.39 |
| Control Group (n = 83) | RIPC Group (n = 85) | p-Value | |
|---|---|---|---|
| Preoperative characteristics | |||
| Age | 45.9 ± 13.4 | 47.6 ± 10.0 | 0.34 |
| Males, n (%) | 43 (50.6) | 46 (54.1) | 0.65 |
| Body mass index, kg/m2 | 22.6 ± 3.6 | 22.8 ± 3.3 | 0.68 |
| sCr, mg/dL | 7.9 (5.7–9.2) | 7.5 (6.0–9.1) | 0.53 |
| eGFR, mL/min/1.73 m2 | 7.0 (5.0–9.0) | 7.0 (5.0–9.0) | 0.41 |
| Duration of chronic renal failure, months | 72.9 ± 84.1 | 70.8 ± 58.4 | 0.22 |
| Dialysis, n (%) | 67 (78.8) | 64 (75.30) | 0.58 |
| Second transplantation, n (%) | 9 (10.8) | 6 (7.1) | 0.39 |
| ABO incompatibility, n (%) | 28 (33.7) | 25 (29.4) | 0.55 |
| Hypertension, n (%) | 76 (89.4) | 70 (82.4) | 0.19 |
| Cerebrovascular accident, n (%) | 2 (2.4) | 0 (0) | 0.50 |
| Diabetes mellitus, n (%) | 18 (21.2) | 22 (25.9) | 0.47 |
| Ischemic heart disease, n (%) | 4 (4.7) | 2 (2.4) | 0.68 |
| Intraoperative characteristics | |||
| Duration of operation, min | 267.0 (252.0–302.5) | 267.0 (230.0–310.0) | 0.51 |
| Plasmalyte, mL | 950 (700–1300) | 1000 (700–1260) | 0.83 |
| Transfusion of RBC, n (%) | 5 (6.0) | 3 (3.5) | 0.69 |
| 20% Mannitol, mL | 150 (125–170) | 150 (125–175) | 0.85 |
| Cold ischemic time, min | 41.0 (26.5–56.0) | 37.0 (22.0–55.0) | 0.23 |
| Warm ischemic time, min | 28.0 (24.0–31.0) | 25.0 (23.0–29.0) | <0.01 |
| Lowest mean blood pressure, mmHg | |||
| Before reperfusion | 78.0 (70.0–83.0) | 79.3 (71.0–87.3) | 0.30 |
| After reperfusion | 84.8 ± 11.4 | 87.0 ± 11.1 | 0.20 |
| Intraoperative inotropic use, n (%) | 12 | 10 | 0.77 |
| Dobutamine | 6 | 9 | - |
| Norepinephrine | 6 | 1 | - |
| Urine output, mL | 430 (260–745) | 440 (300–680) | 0.80 |
| Donors | Control Group (n = 85) | RIPC Group (n = 85) | p-Value |
| Progression to chronic kidney disease, n (%) | 6 (7.1) | 8 (9.4) | 0.78 |
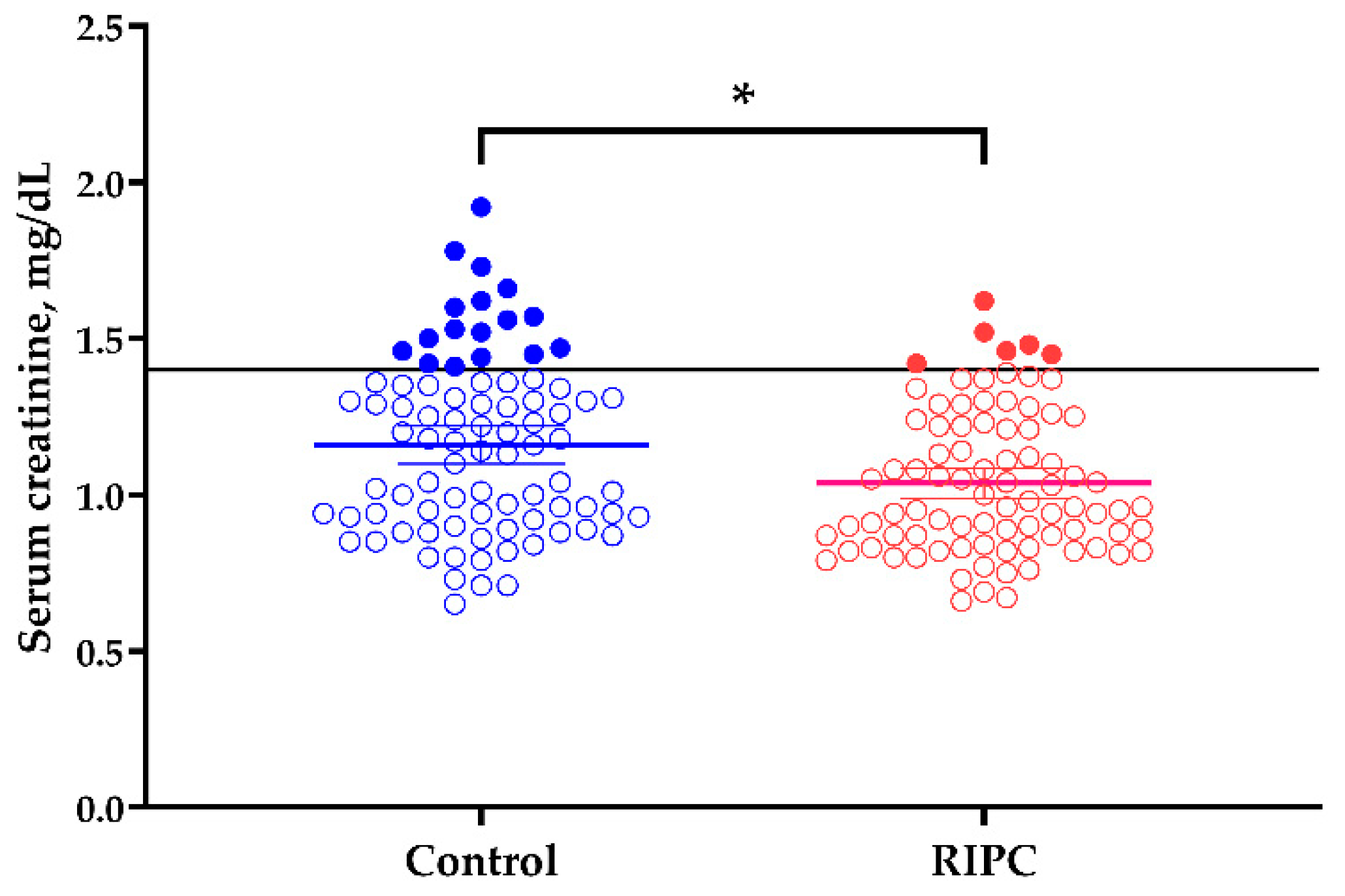
| Proportion with sCr ≥ 1.4 mg/dL at discharge, n (%) | 17 (20.0) | 6 (7.1) | <0.05 |
| Recipients | Control Group (n = 83) | Treated Group (n = 85) | p-Value |
| Time to 50% decrease in sCr, days | 1.1 ± 0.6 | 1.4 ± 3.2 | 0.58 |
| Delayed graft function, n (%) | 0 (0.0) | 2 (2.4) | 0.49 |
| Acute rejection, n (%) | 5 (6.0) | 5 (5.9) | 1.00 |
| Graft failure, n (%) | 1 (1.2) | 0 (0.0) | 0.99 |
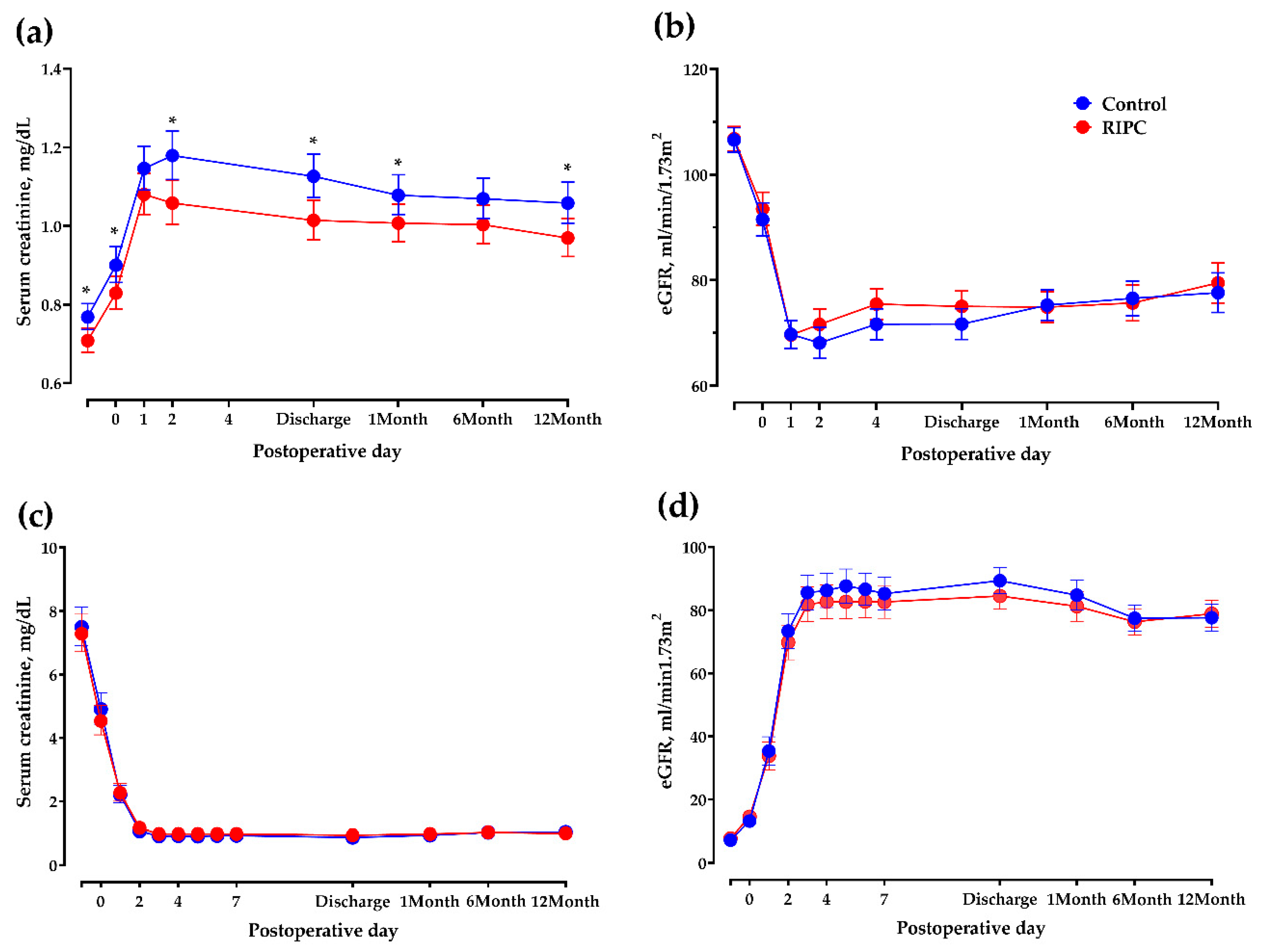
| eGFR at discharge, mL/min/1.73 m2 | 87.9 ± 17.7 | 83.9 ± 19.4 | 0.18 |
| Mean difference 1 | −3.23 (−8.62–2.16) | 0.14 | |
| eGFR at postoperative 1 year, mL/min/1.73 m2 | 77.0 ± 24.5 | 79.5 ± 18.2 | 0.49 |
| Mean difference 1 | 3.45 (−3.11–10.01) | 0.29 | |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| RIPC | 0.30 | 0.1–0.78 | 0.02 | 0.31 | 0.1–0.88 | 0.04 |
| Age | 0.95 | 0.91–0.99 | 0.02 | |||
| ASA PS | 0.80 | 0.27–2.07 | 0.66 | |||
| Body mass index | 1.17 | 1.00–1.37 | 0.05 | |||
| Operation time | 1.01 | 1.00–1.03 | 0.02 | |||
| Operation site | 1.10 | 0.45–2.80 | 0.83 | |||
| Systolic blood pressure | 1.03 | 1.00–1.07 | 0.07 | |||
| Albumin | 2.56 | 0.52–13.04 | 0.25 | |||
| eGFR | 0.95 | 0.91–0.99 | 0.01 | 0.94 | 0.90–0.99 | <0.01 |
| Cys C, mg/L | 1.91 | 1.39–2.75 | <0.01 | 2.17 | 1.44–3.44 | <0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, J.-Y.; Kim, S.-G.; Oh, J.; Kim, S.-O.; Go, Y.-J.; Hwang, G.-S.; Song, J.-G. Impact of Remote Ischemic Preconditioning Conducted in Living Kidney Donors on Renal Function in Donors and Recipients Following Living Donor Kidney Transplantation: A Randomized Clinical Trial. J. Clin. Med. 2019, 8, 713. https://doi.org/10.3390/jcm8050713
Bang J-Y, Kim S-G, Oh J, Kim S-O, Go Y-J, Hwang G-S, Song J-G. Impact of Remote Ischemic Preconditioning Conducted in Living Kidney Donors on Renal Function in Donors and Recipients Following Living Donor Kidney Transplantation: A Randomized Clinical Trial. Journal of Clinical Medicine. 2019; 8(5):713. https://doi.org/10.3390/jcm8050713
Chicago/Turabian StyleBang, Ji-Yeon, Sae-Gyeol Kim, Jimi Oh, Seon-Ok Kim, Yon-Ji Go, Gyu-Sam Hwang, and Jun-Gol Song. 2019. "Impact of Remote Ischemic Preconditioning Conducted in Living Kidney Donors on Renal Function in Donors and Recipients Following Living Donor Kidney Transplantation: A Randomized Clinical Trial" Journal of Clinical Medicine 8, no. 5: 713. https://doi.org/10.3390/jcm8050713
APA StyleBang, J.-Y., Kim, S.-G., Oh, J., Kim, S.-O., Go, Y.-J., Hwang, G.-S., & Song, J.-G. (2019). Impact of Remote Ischemic Preconditioning Conducted in Living Kidney Donors on Renal Function in Donors and Recipients Following Living Donor Kidney Transplantation: A Randomized Clinical Trial. Journal of Clinical Medicine, 8(5), 713. https://doi.org/10.3390/jcm8050713