Does Herpes Zoster Increase the Risk of Stroke and Myocardial Infarction? A Comprehensive Review
Abstract
1. Introduction
2. Studies Investigating the Association Between HZ and CVD or Cerebrovascular Disease
3. Herpes Zoster and Risk of Stroke or Myocardial Infarction
4. Herpes Zoster Ophthalmicus and Risk of Stroke
5. Post-Herpes Zoster Length of Follow-Up and Age Stratification with Associated Risk of CVD
6. Varicella Zoster Virus Vasculopathy and Other Potential Mechanisms of Herpes Zoster, Such as CVD Risks
7. Limitations of Current Observational Studies
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vrcek, I.; Choudhury, E.; Durairaj, V. Herpes zoster ophthalmicus: A review for the internist. Am. J. Med. 2017, 130, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J.; Pacou, M.; Gautier, A.; Brown, M.M. Herpes zoster as a risk factor for stroke and tia: A retrospective cohort study in the uk. Neurology 2014, 83, e27–e33. [Google Scholar] [CrossRef]
- Kang, J.H.; Ho, J.D.; Chen, Y.H.; Lin, H.C. Increased risk of stroke after a herpes zoster attack: A population-based follow-up study. Stroke 2009, 40, 3443–3448. [Google Scholar] [CrossRef]
- Sundstrom, K.; Weibull, C.E.; Soderberg-Lofdal, K.; Bergstrom, T.; Sparen, P.; Arnheim-Dahlstrom, L. Incidence of herpes zoster and associated events including stroke--a population-based cohort study. BMC Infect. Dis. 2015, 15, 488. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Wollan, P.C.; Nagel, M.A.; Gilden, D. Risk of stroke and myocardial infarction after herpes zoster in older adults in a us community population. Mayo Clin. Proc. 2016, 91, 33–44. [Google Scholar] [CrossRef]
- Langan, S.M.; Minassian, C.; Smeeth, L.; Thomas, S.L. Risk of stroke following herpes zoster: A self-controlled case-series study. Clin. Infect. Dis. 2014, 58, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Schink, T.; Behr, S.; Thone, K.; Bricout, H.; Garbe, E. Risk of stroke after herpes zoster - evidence from a german self-controlled case-series study. PLoS ONE 2016, 11, e0166554. [Google Scholar] [CrossRef] [PubMed]
- Hosamirudsari, H.; Rashed, P.; Afsari, F.; Akbarpour, S.; Bagherzadeh, A. Correlation between herpes zoster and stroke-a case-control study. J. Med. Virol. 2018, 90, 1370–1374. [Google Scholar] [CrossRef]
- Kim, M.C.; Yun, S.C.; Lee, H.B.; Lee, P.H.; Lee, S.W.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H.; Kwon, S.U. Herpes zoster increases the risk of stroke and myocardial infarction. J. Am. Coll. Cardiol. 2017, 70, 295–296. [Google Scholar] [CrossRef]
- Minassian, C.; Thomas, S.L.; Smeeth, L.; Douglas, I.; Brauer, R.; Langan, S.M. Acute cardiovascular events after herpes zoster: A self-controlled case series analysis in vaccinated and unvaccinated older residents of the united states. PLoS Med. 2015, 12, e1001919. [Google Scholar] [CrossRef]
- Seo, H.M.; Cha, M.J.; Han, J.H.; Han, K.; Park, S.H.; Bang, C.H.; Lee, J.H.; Lee, J.Y.; Choi, E.K.; Park, Y.M. Reciprocal relationship between herpes zoster and cardiovascular diseases: A nationwide population-based case-control study in korea. J. Dermatol. 2018, 45, 1312–1318. [Google Scholar] [CrossRef]
- Kwon, S.U.; Yun, S.C.; Kim, M.C.; Kim, B.J.; Lee, S.H.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Risk of stroke and transient ischaemic attack after herpes zoster. Clin. Microbiol. Infect. 2016, 22, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, N.; Basit, S.; Wohlfahrt, J.; Pasternak, B.; Munch, T.N.; Nielsen, L.P.; Melbye, M. The short-and long-term risk of stroke after herpes zoster-a nationwide population-based cohort study. PLoS ONE 2013, 8, e69156. [Google Scholar] [CrossRef]
- Wang, C.C.; Lin, C.L.; Chang, Y.J.; Wang, G.J.; Sung, F.C.; Kao, C.H. Herpes zoster infection associated with acute coronary syndrome: A population-based retrospective cohort study. Br. J. Dermatol. 2014, 170, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Lin, C.L.; Sung, F.C.; Chou, T.C.; Lee, Y.T. Increased risk of cardiovascular events in patients with herpes zoster: A population-based study. J. Med. Virol. 2014, 86, 772–777. [Google Scholar] [CrossRef]
- Lin, H.C.; Chien, C.W.; Ho, J.D. Herpes zoster ophthalmicus and the risk of stroke: A population-based follow-up study. Neurology 2010, 74, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guan, Y.; Hou, L.; Huang, H.; Liu, H.; Li, C.; Zhu, Y.; Tao, X.; Wang, Q. The short- and long-term risk of stroke after herpes zoster: A meta-analysis. PLoS ONE 2016, 11, e0165203. [Google Scholar] [CrossRef]
- Lian, Y.; Zhu, Y.; Tang, F.; Yang, B.; Duan, R. Herpes zoster and the risk of ischemic and hemorrhagic stroke: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0171182. [Google Scholar] [CrossRef]
- Yang, S.Y.; Li, H.X.; Yi, X.H.; Han, G.L.; Zong, Q.; Wang, M.X.; Peng, X.X. Risk of stroke in patients with herpes zoster: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 301–307. [Google Scholar] [CrossRef]
- Marra, F.; Ruckenstein, J.; Richardson, K. A meta-analysis of stroke risk following herpes zoster infection. BMC Infect. Dis. 2017, 17, 198. [Google Scholar] [CrossRef]
- Erskine, N.; Tran, H.; Levin, L.; Ulbricht, C.; Fingeroth, J.; Kiefe, C.; Goldberg, R.J.; Singh, S. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS ONE 2017, 12, e0181565. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, G.; Huang, Y.; Yu, Q.; Wang, L.; Li, K. Risk of stroke/transient ischemic attack or myocardial infarction with herpes zoster: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 1807–1816. [Google Scholar] [CrossRef]
- Patterson, B.; Rausch, D.A.; Irwin, D.E.; Liang, M.; Yan, S.; Yawn, B. Risk of myocardial infarction and composite vascular events in patients with herpes zoster: 2007-2014 united states claims data analysis. J. Am. Coll. Cardiol. 2018, 71, A177. [Google Scholar] [CrossRef]
- Patterson, B.; Rausch, D.; Irwin, D.; Liang, M.; Yan, S.; Yawn, B. Risk of transient ischemic attack and stroke in patients with herpes zoster: 2007–2014 us claims data analysis (s15.008). Neurology 2018, 90, S15.008. [Google Scholar]
- Nagel, M.A.; Traktinskiy, I.; Azarkh, Y.; Kleinschmidt-DeMasters, B.; Hedley-Whyte, T.; Russman, A.; VanEgmond, E.M.; Stenmark, K.; Frid, M.; Mahalingam, R.; et al. Varicella zoster virus vasculopathy: Analysis of virus-infected arteries. Neurology 2011, 77, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Cohrs, R.J.; Mahalingam, R.; Wellish, M.C.; Forghani, B.; Schiller, A.; Safdieh, J.E.; Kamenkovich, E.; Ostrow, L.W.; Levy, M.; et al. The varicella zoster virus vasculopathies: Clinical, csf, imaging, and virologic features. Neurology 2008, 70, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.; Cohrs, R.J.; Mahalingam, R.; Nagel, M.A. Varicella zoster virus vasculopathies: Diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009, 8, 731–740. [Google Scholar] [CrossRef]
- Amlie-Lefond, C.; Gilden, D. Varicella zoster virus: A common cause of stroke in children and adults. J. Stroke Cerebrovasc. Dis. 2016, 25, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Uthman, I.; Taher, A.; Khalil, I. Hughes syndrome associated with varicella infection. Rheumatol. Int. 2001, 20, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Bodensteiner, J.B.; Hille, M.R.; Riggs, J.E. Clinical features of vascular thrombosis following varicella. Am. J. Dis. Child. 1992, 146, 100–102. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. Complications of varicella zoster virus reactivation. Curr. Treat Options Neurol. 2013, 15, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Yeager, M.E.; El Kasmi, K.C.; Nozik-Grayck, E.; Gerasimovskaya, E.V.; Li, M.; Riddle, S.R.; Frid, M.G. The adventitia: Essential regulator of vascular wall structure and function. Annu. Rev. Physiol. 2013, 75, 23–47. [Google Scholar] [CrossRef]
- Gilden, D.H.; Lipton, H.L.; Wolf, J.S.; Akenbrandt, W.; Smith, J.E.; Mahalingam, R.; Forghani, B. Two patients with unusual forms of varicella-zoster virus vasculopathy. N. Engl. J. Med. 2002, 347, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Gilden, D. Neurological complications of varicella zoster virus reactivation. Curr. Opin. Neurol. 2014, 27, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Miravet, E.; Danchaivijitr, N.; Basu, H.; Saunders, D.E.; Ganesan, V. Clinical and radiological features of childhood cerebral infarction following varicella zoster virus infection. Dev. Med. Child. Neurol. 2007, 49, 417–422. [Google Scholar] [CrossRef]
- Grose, C.; Adams, H.P. Reassessing the link between herpes zoster ophthalmicus and stroke. Expert Rev. Anti. Infect. Ther. 2014, 12, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Franco-Paredes, C.; Bellehemeur, T.; Merchant, A.; Sanghi, P.; DiazGranados, C.; Rimland, D. Aseptic meningitis and optic neuritis preceding varicella-zoster progressive outer retinal necrosis in a patient with aids. AIDS 2002, 16, 1045–1049. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. The challenging patient with varicella-zoster virus disease. Neurol. Clin. Pract. 2013, 3, 109–117. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. The relationship between herpes zoster and stroke. Curr. Neurol. Neurosci. Rep. 2015, 15, 16. [Google Scholar] [CrossRef]
- Gilden, D.; Nagel, M.A.; Cohrs, R.J. Persistence of varicella zoster virus DNA in saliva after herpes zoster. J. Infect. Dis. 2012, 205, 1178–1179. [Google Scholar] [CrossRef]
- Hayman, M.; Hendson, G.; Poskitt, K.J.; Connolly, M.B. Postvaricella angiopathy: Report of a case with pathologic correlation. Pediatr. Neurol. 2001, 24, 387–389. [Google Scholar] [CrossRef]
- Josephson, C.; Nuss, R.; Jacobson, L.; Hacker, M.R.; Murphy, J.; Weinberg, A.; Manco-Johnson, M.J. The varicella-autoantibody syndrome. Pediatr. Res. 2001, 50, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Emsley, H.C.; Tyrrell, P.J. Inflammation and infection in clinical stroke. J. Cereb. Blood Flow. Metab. 2002, 22, 1399–1419. [Google Scholar] [CrossRef]
- Vila, N.; Reverter, J.C.; Yague, J.; Chamorro, A. Interaction between interleukin-6 and the natural anticoagulant system in acute stroke. J. Interferon Cytokine Res. 2000, 20, 325–329. [Google Scholar] [CrossRef]
- Nagel, M.A.; Traktinskiy, I.; Stenmark, K.R.; Frid, M.G.; Choe, A.; Gilden, D. Varicella-zoster virus vasculopathy: Immune characteristics of virus-infected arteries. Neurology 2013, 80, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R.; Messaoudi, I.; Gilden, D. Simian varicella virus pathogenesis. Curr. Top. Microbiol. Immunol. 2010, 342, 309–321. [Google Scholar] [PubMed]
- Mahalingam, R.; Traina-Dorge, V.; Wellish, M.; Deharo, E.; Singletary, M.L.; Ribka, E.P.; Sanford, R.; Gilden, D. Latent simian varicella virus reactivates in monkeys treated with tacrolimus with or without exposure to irradiation. J. Neurovirol. 2010, 16, 342–354. [Google Scholar] [CrossRef]
- Nagel, M.A.; Traktinskiy, I.; Choe, A.; Rempel, A.; Gilden, D. Varicella-zoster virus expression in the cerebral arteries of diabetic subjects. Arch. Neurol. 2012, 69, 142–144. [Google Scholar] [CrossRef] [PubMed]
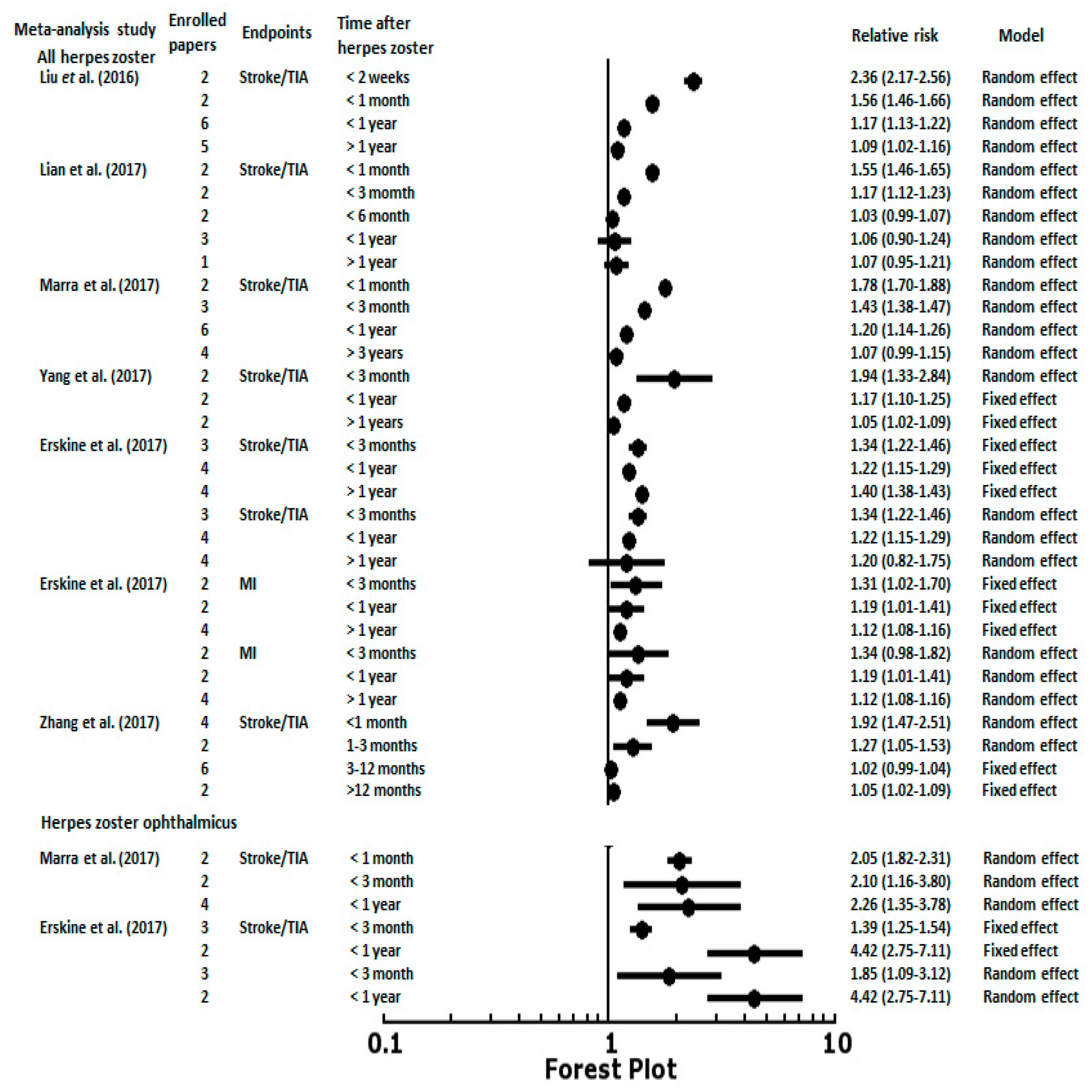
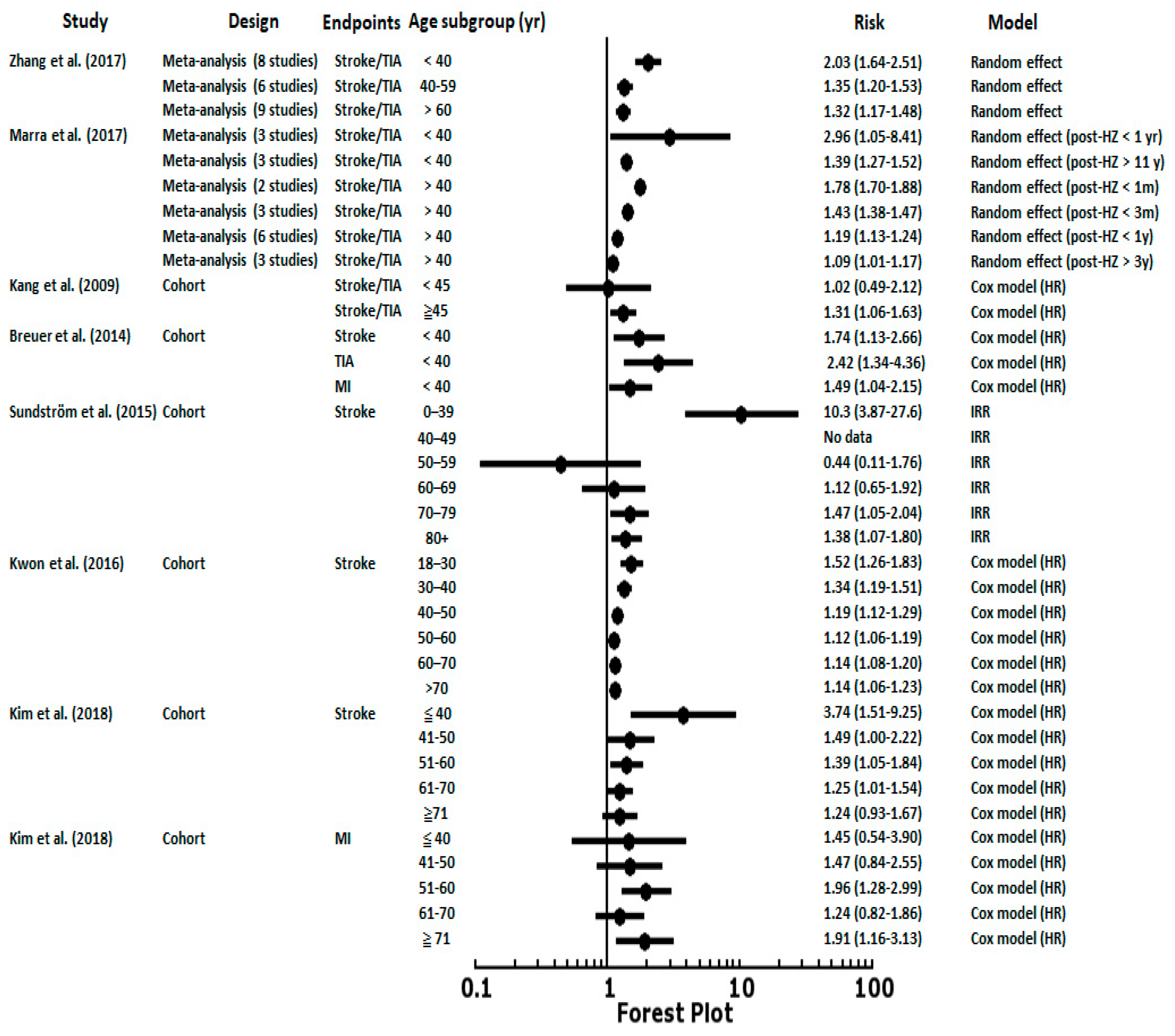
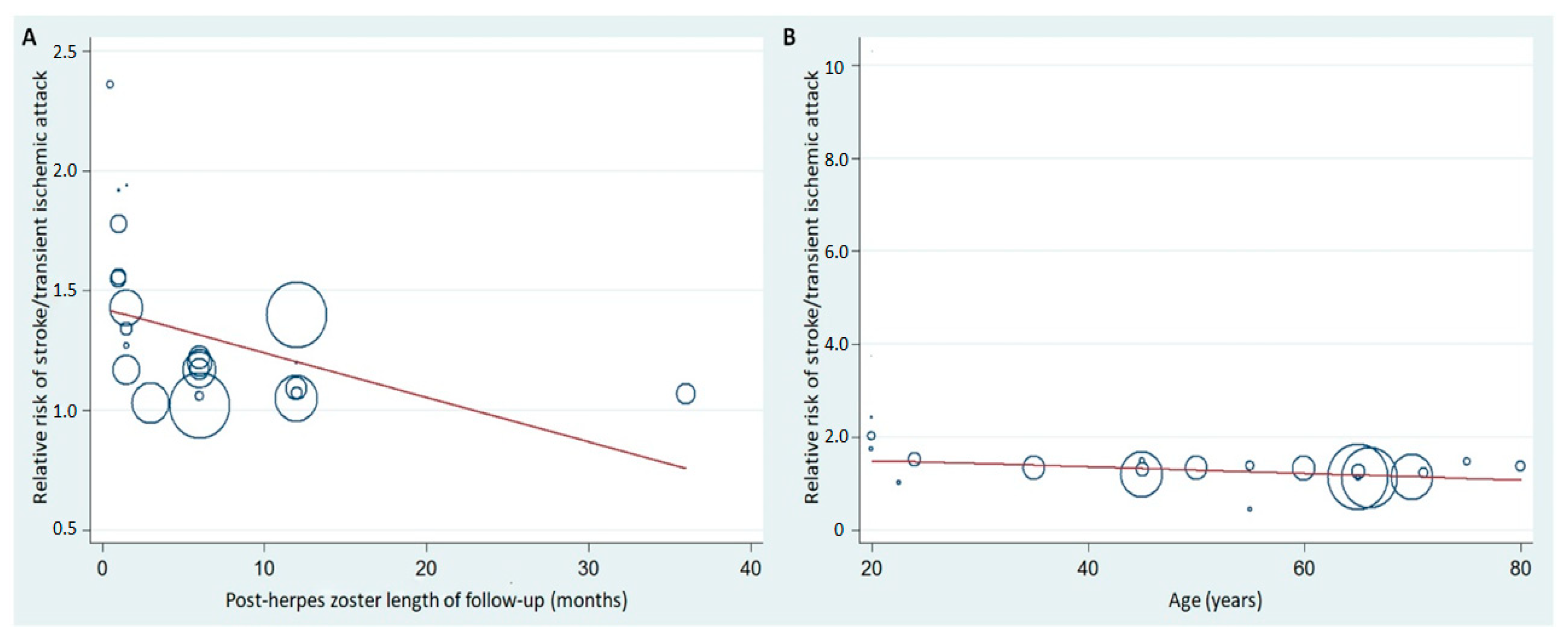
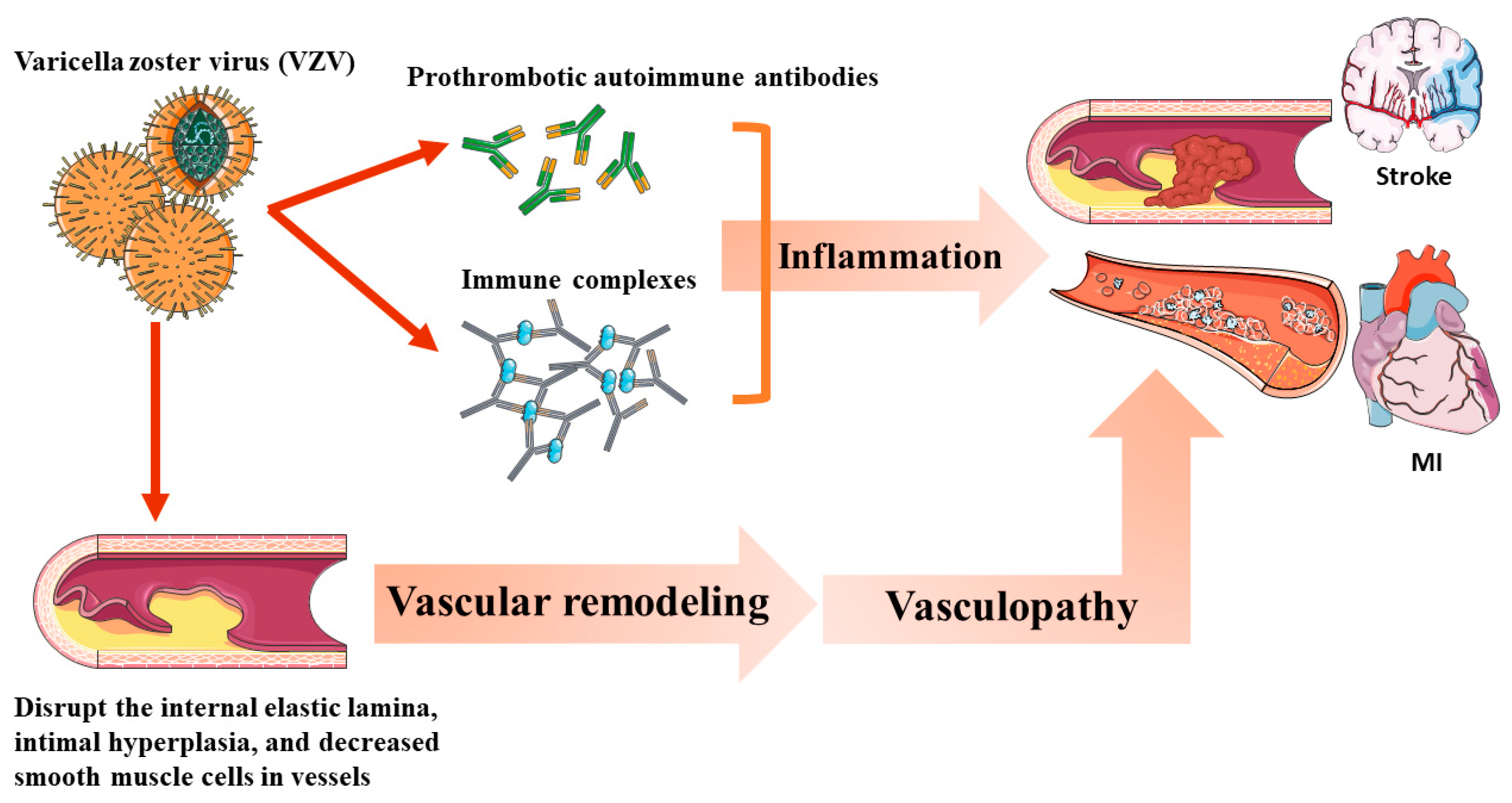
| Author | Year | Country | Study Design | Study Period | Age (y) | Follow-up (y) | Sample Size | Controlled Factors |
|---|---|---|---|---|---|---|---|---|
| Kang et al. | 2009 | Taiwan | Retrospective matched cohort (age and sex matched) | 1997–2001 | ≧18 (mean:46.7) | 1 | 31,040 | Age, sex, hypertension, diabetes, coronary heart disease, hyperlipidemia, renal disease, atrial fibrillation, heart failure, heart valve/myocardium disease, carotid/peripheral vascular disease, monthly income, urbanization level, and geographical region |
| Sreenivasan et al. | 2013 | Denmark | Retrospective cohort | 1995–2008 | ≧18 | 13 | 4,620,980 | Age, sex, calendar period, acute MI, atrial fibrillation, education, cancer, medications (antihypertensives, drugs used to treat dyslipidemia and atrial fibrillation, and immunosuppressive drugs) |
| Breuer et al. | 2014 | UK | Retrospective matched cohort (age and sex matched) | 2002–2010 | ≧18 (mean:57.8) | 23.7 (median:6.3) | 319,803 | Age, sex, obesity, smoking, high cholesterol recording, hypertension, diabetes, ischemic heart disease, atrial fibrillation, intermittent arterial claudication, carotid stenosis, and valvular heart disease |
| Langan et al. | 2014 | UK | Self-controlled case series | 1987–2012 | ≧18 (median:77) | 25 (median:12.5) | 6584 | Confounders are implicitly controlled for due to the study design |
| Sundström et al. | 2015 | Sweden | Retrospective cohort | 2008–2010 | ≧0 | 1 | 1.5 million | Age and sex |
| Minassian et al. | 2015 | USA | Self-controlled case series | 2006–2011 | ≧65 (median:81.1) | 5 (median) | 42,954 | Confounders are implicitly controlled for due to the study design |
| Kwon et al. | 2016 | Korea | Retrospective matched cohort (age matched) | 2003–2013 | ≧18 (mean:41.4) | 11 | 766,179 | Age, male gender, hypertension, hyperlipidemia, ischemic heart disease, diabetes, heart failure, peripheral vascular disease, arterial fibrillation or atrial flutter, renal disease, and valvular heart disease |
| Yawn et al. | 2016 | USA | Retrospective matched cohort (age and sex matched) | 1986–2011 | ≧50 (mean:68.2) | 28.6 (mean:7.1) | 24,295 | Age, sex, hypertension, dyslipidemia, coronary artery disease (including MI), arrhythmias, congestive heart failure, diabetes, depression, chronic obstructive pulmonary disorder, vasculopathies, stroke, and anxiety |
| Schink et al. | 2016 | Germany | Self-controlled case series | 2004–2011 | ≧0 (mean:71.3) | 1 | 124,462 | Confounders are implicitly controlled for due to the study design |
| Hosamirudsari et al. | 2018 | Iran | Case-control | 2015–2017 | 66.99 (mean) | 0.5 | 210 | Age, sex, and hypertension |
| Kim et al. | 2018 | Korea | Retrospective propensity-matched cohort | 2003–2013 | Not reported | 11 | 519,880 | Age, sex, body mass index, obesity, smoking, drinking, exercise, economic class, hypertension, diabetes, dyslipidemia, angina pectoris, transient ischemic attack, heart failure, atrial fibrillation/flutter, valvular heart disease, chronic renal disease, carotid stenosis, peripheral vascular disease, chronic liver disease, rheumatoid disease, inflammatory bowel disease, malignancy, transplantation, HIV, and depression |
| Seo et al. | 2018 | Korea | Retrospective matched cohort (age and sex matched) | 2006–2013 | ≧40 (mean:63.1 in hospitalized cases and 58 in non-hospitalized cases | 8 | 104,191 | Age, sex, income, diabetes mellitus, hypertension, and dyslipidemia |
| Author | Year | Country | Study Design | Study Period | Age (y) | Follow-up (y) | Sample Size | Controlled Factors |
|---|---|---|---|---|---|---|---|---|
| Wang et al. | 2014 | Taiwan | Retrospective matched cohort | 1999–2010 | ≧0 | 12 | 289,790 | Age, sex, urbanization, monthly income, occupation, frequency of medical visits, hypertension, diabetes mellitus, hyperlipidemia, cerebral vascular disease, chronic obstructive pulmonary disease, renal function, cancer, and medication |
| Breuer et al. | 2014 | UK | Retrospective matched cohort | 2002–2010 | ≧18 (mean:57.8) | 24 (median:6.3) | 319,803 | Age, sex, obesity, smoking, high cholesterol recording, hypertension, diabetes, ischemic heart disease, atrial fibrillation, intermittent arterial claudication, carotid stenosis, and valvular heart disease |
| Wu et al. | 2015 | Taiwan | Retrospective matched cohort | 1998–2010 | ≧20 (mean:46.4) | 10 | 97,415 | Age, sex, diabetes, hypertension, and hyperlipidemia |
| Minassian et al. | 2015 | USA | Self-controlled case series | 2006–2011 | ≧65 (median:80.3) | 5 (median) | 24,237 | Confounders are implicitly controlled for due to the study design |
| Yawn et al. | 2016 | USA | Retrospective matched cohort (age and sex matched) | 1986–2011 | ≧50 (mean:68.2) | 28 (mean:7) | 24,295 | Age, sex, hypertension, dyslipidemia, coronary artery disease (including MI), arrhythmias, congestive heart failure, diabetes, depression, chronic obstructive pulmonary disorder, vasculopathies, stroke, and anxiety |
| Kim et al. | 2018 | Korea | Retrospective propensity-matched cohort | 2003–2013 | Not reported | 11 | 519,880 | Age, sex, body mass index, obesity, smoking, drinking, exercise, economic class, hypertension, diabetes, dyslipidemia, angina pectoris, transient ischemic attack, heart failure, atrial fibrillation/flutter, valvular heart disease, chronic renal disease, carotid stenosis, peripheral vascular disease, chronic liver disease, rheumatoid disease, inflammatory bowel disease, malignancy, transplantation, HIV, and depression |
| Seo et al. | 2018 | Korea | Retrospective case–control study (age and sex matched) | 2006–2013 | ≧40 (mean:63.1 in hospitalized cases and 58 in non-hospitalized cases | 8 | 104,191 | Age, sex, income, diabetes mellitus, hypertension, and dyslipidemia |
| Author | Year | Country | Study Design | Study Period | Age (y) | Follow-up (y) | Sample Size | Endpoints | Confounders (Adjusted for) |
|---|---|---|---|---|---|---|---|---|---|
| Kang et al. | 2009 | Taiwan | Retrospective matched cohort (age and sex matched) | 1997–2001 | ≧18 (mean:46.7) | 1 | 31,040 | Stroke | Age, sex, hypertension, diabetes, coronary heart disease, hyperlipidemia, renal disease, atrial fibrillation, heart failure, heart valve/myocardium disease, carotid/peripheral vascular disease, monthly income, urbanization level, and geographical region |
| Lin et al. | 2010 | Taiwan | Retrospective matched cohort (age and sex matched) | 2003–2005 | ≧18 (mean:56.9) | 1 | 2632 | Stroke/TIA | Age, sex, hypertension, diabetes, hyperlipidemia, coronary heart disease, chronic rheumatic heart disease, other forms of heart disease, and medication habits |
| Minassian et al. | 2015 | USA | Self-controlled case series | 2006–2011 | 80 (median) | 6 (median:5) | 6971 | Stroke | Confounders are implicitly controlled for due to the study design |
| 3946 | MI | Confounders are implicitly controlled for due to the study design | |||||||
| Breuer et al. | 2014 | UK | Cohort | 2002–2010 | ≧18 (mean:57.8) | 24 (median:6.3) | 2324 | Stroke | Age, sex, obesity, smoking, high cholesterol recording, hypertension, diabetes, ischemic heart disease, atrial fibrillation, intermittent arterial claudication, carotid stenosis, and valvular heart disease |
| Langan et al. | 2014 | UK | Self-controlled case series | 1987–2012 | ≧0 (median:77) | 25 (median:12.5) | 6584 | Stroke | Confounders are implicitly controlled for due to the study design |
| Schink et al. | 2016 | Germany | Self-controlled case series | 2004–2011 | ≧ 0 | 1 | 124,462 | Stroke | Confounders are implicitly controlled for due to the study design |
| Study | Year | Herpes Zoster Type | Enrolled Papers | Endpoints | Relative Risk (95% CI) | Model |
|---|---|---|---|---|---|---|
| Yang et at. | 2017 | All herpes zoster | 6 | Stroke/TIA | 1.36 (1.10–1.67) | Random effect |
| Herpes zoster ophthalmicus | 3 | Stroke/TIA | 2.62 (0.85–8.06) | Random effect | ||
| Zhang et al. | 2017 | All herpes zoster | 5 | Stroke/TIA | 1.30 (1.17–1.46) | Random effect |
| All herpes zoster | 6 | MI | 1.18 (1.07–1.30) | Random effect | ||
| Herpes zoster ophthalmicus | 8 | Stroke/TIA | 1.91 (1.32–2.76) | Random effect |
| # Time | Langan et al. | Schink et al. | Minassian et al. | Minassian et al. |
|---|---|---|---|---|
| Stroke/TIA | Stroke/TIA | Stroke/TIA | Myocardial Infarction | |
| 1 week | 2.37 (2.17–2.59) * | 1.68 (1.47–1.92) * | ||
| 2 weeks | 1.30 (1.00–1.68) * | |||
| 3–4 weeks | 1.63 (1.32–2.02) * | 1.52 (1.20–1.91 ) * | 1.55 (1.46–1.66) * | 1.25 (1.14–1.37) * |
| 5–12 weeks | 1.42 (1.21–1.68) * | 1.24 (1.08–1.42) * | 1.17 (1.11–1.22) * | 1.07 (1.00–1.14) * |
| 13–26 weeks | 1.23 (1.07–1.42) * | 1.09 (0.97–1.24) | 1.03 (0.99–1.07) | 1.02 (0.96–1.07) |
| 27–52 weeks | 0.99 (0.88–1.12) | 0.96 (0.87–1.06) | 1.00 (0.96–1.03) | 1.02 (0.98–1.07) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-H.; Chuang, Y.-S.; Lin, Y.-T. Does Herpes Zoster Increase the Risk of Stroke and Myocardial Infarction? A Comprehensive Review. J. Clin. Med. 2019, 8, 547. https://doi.org/10.3390/jcm8040547
Wu P-H, Chuang Y-S, Lin Y-T. Does Herpes Zoster Increase the Risk of Stroke and Myocardial Infarction? A Comprehensive Review. Journal of Clinical Medicine. 2019; 8(4):547. https://doi.org/10.3390/jcm8040547
Chicago/Turabian StyleWu, Ping-Hsun, Yun-Shiuan Chuang, and Yi-Ting Lin. 2019. "Does Herpes Zoster Increase the Risk of Stroke and Myocardial Infarction? A Comprehensive Review" Journal of Clinical Medicine 8, no. 4: 547. https://doi.org/10.3390/jcm8040547
APA StyleWu, P.-H., Chuang, Y.-S., & Lin, Y.-T. (2019). Does Herpes Zoster Increase the Risk of Stroke and Myocardial Infarction? A Comprehensive Review. Journal of Clinical Medicine, 8(4), 547. https://doi.org/10.3390/jcm8040547







