Rational Use of CT-Scan for the Diagnosis of Pneumonia: Comparative Accuracy of Different Strategies
Abstract
:1. Introduction
2. Experimental Section
2.1. Setting and Patients
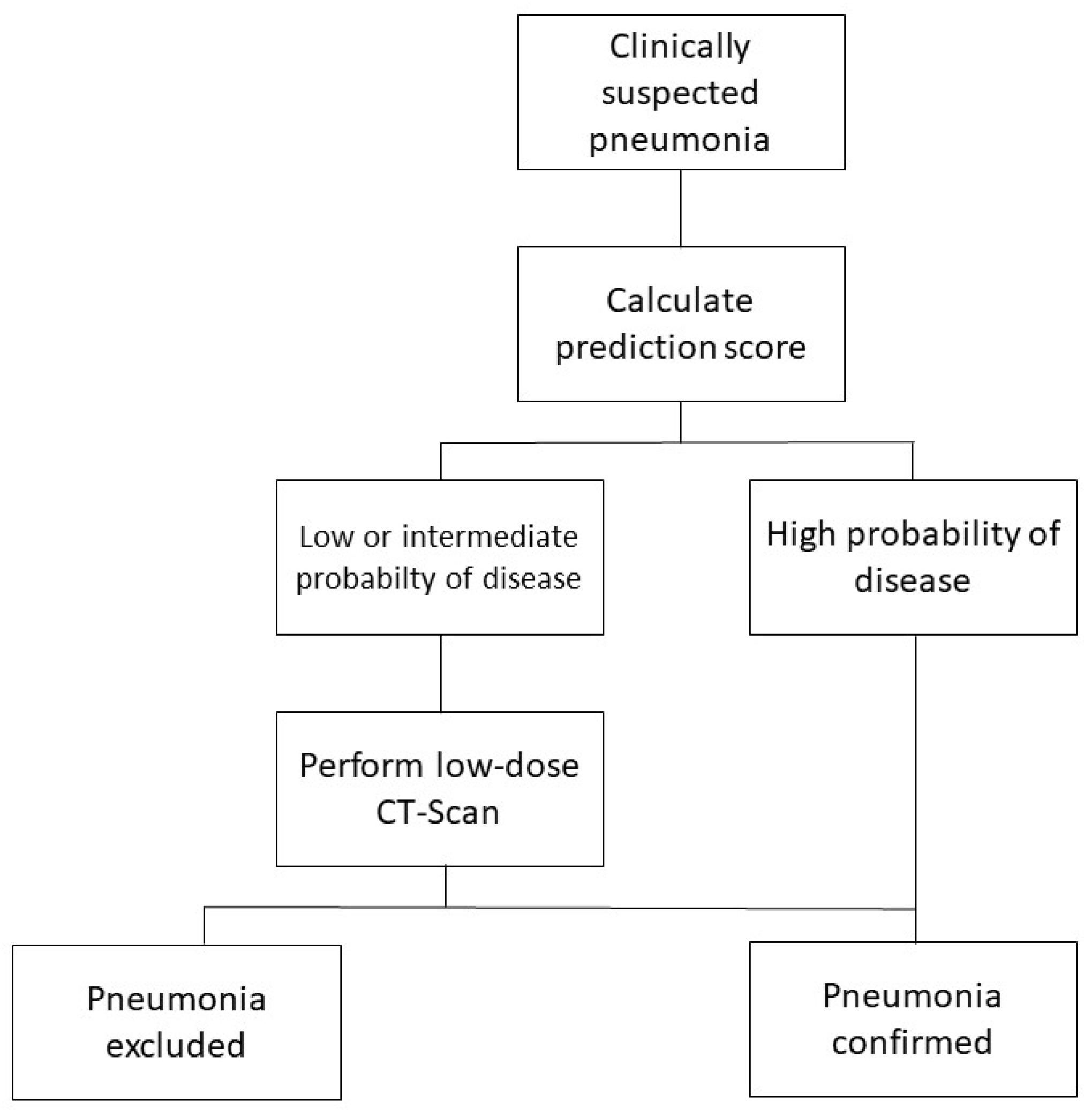
2.2. Diagnostic Criteria
2.3. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections—Full version. Clin. Microbiol. Infect. 2011, 17, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Kapoor, W.N.; Fine, M.J. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997, 278, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Fine, M.J. Testing strategies in the initial management of patients with community-acquired pneumonia. Ann. Intern. Med. 2003, 138, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Courtney, D.M.; McNaughton, C.D.; Wunderink, R.G.; Kline, J.A. High discordance of chest X-ray and computed tomography for detection of pulmonary opacities in ED patients: Implications for diagnosing pneumonia. Am. J. Emerg. Med. 2013, 31, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Claessens, Y.E.; Debray, M.P.; Tubach, F.; Brun, A.L.; Rammaert, B.; Hausfater, P.; Naccache, J.M.; Ray, P.; Choquet, C.; Carette, M.F.; et al. Early Chest Computed Tomography Scan to Assist Diagnosis and Guide Treatment Decision for Suspected Community-acquired Pneumonia. Am. J. Respir. Crit. Care Med. 2015, 192, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Hopstaken, R.M.; Witbraad, T.; van Engelshoven, J.M.; Dinant, G.J. Inter-observer variation in the interpretation of chest radiographs for pneumonia in community-acquired lower respiratory tract infections. Clin. Radiol. 2004, 59, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Janssens, J.P.; Krause, K.H. Pneumonia in the very old. Lancet Infect. Dis. 2004, 4, 112–124. [Google Scholar] [CrossRef]
- Van Vugt, S.F.; Broekhuizen, B.D.; Lammens, C.; Zuithoff, N.P.; de Jong, P.A.; Coenen, S.; Ieven, M.; Butler, C.C.; Goossens, H.; Little, P.; et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: Diagnostic study. BMJ 2013, 346, f2450. [Google Scholar] [CrossRef] [PubMed]
- Minnaard, M.C.; de Groot, J.A.; Hopstaken, R.M.; Schierenberg, A.; de Wit, N.J.; Reitsma, J.B.; Broekhuizen, B.D.; van Vugt, S.F.; Knuistingh Neven, A.; Graffelman, A.W.; et al. The added value of C-reactive protein measurement in diagnosing pneumonia in primary care: A meta-analysis of individual patient data. CMAJ 2017, 189, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Le Bel, J.; Hausfater, P.; Chenevier-Gobeaux, C.; Blanc, F.X.; Benjoar, M.; Ficko, C.; Ray, P.; Choquet, C.; Duval, X.; Claessens, Y.E.; et al. Diagnostic accuracy of C-reactive protein and procalcitonin in suspected community-acquired pneumonia adults visiting emergency department and having a systematic thoracic CT scan. Crit. Care 2015, 19, 366. [Google Scholar] [CrossRef] [PubMed]
- Schierenberg, A.; Minnaard, M.C.; Hopstaken, R.M.; van de Pol, A.C.; Broekhuizen, B.D.; de Wit, N.J.; Reitsma, J.B.; van Vugt, S.F.; Graffelman, A.W.; Melbye, H.; et al. External Validation of Prediction Models for Pneumonia in Primary Care Patients with Lower Respiratory Tract Infection: An Individual Patient Data Meta-Analysis. PLoS ONE 2016, 11, e0149895. [Google Scholar] [CrossRef] [PubMed]
- Prendki, V.; Scheffler, M.; Huttner, B.; Garin, N.; Herrmann, F.; Janssens, J.P.; Marti, C.; Carballo, S.; Roux, X.; Serratrice, C.; et al. Low-dose CT for the diagnosis of pneumonia in elderly patients: A prospective, interventional cohort study. Eur. Respir. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Oren, O.; Kebebew, E.; Ioannidis, J.P.A. Curbing Unnecessary and Wasted Diagnostic Imaging. JAMA 2019, 321, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Ewig, S.; Birkner, N.; Strauss, R.; Schaefer, E.; Pauletzki, J.; Bischoff, H.; Schraeder, P.; Welte, T.; Hoeffken, G. New perspectives on community-acquired pneumonia in 388,406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax 2009, 64, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, J.; Grams, M.E.; Chang, A.R.; Carrero, J.J.; Coresh, J.; Matsushita, K. CKD and Risk for Hospitalization with Infection: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. 2017, 69, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Hash, R.B.; Stephens, J.L.; Laurens, M.B.; Vogel, R.L. The relationship between volume status, hydration, and radiographic findings in the diagnosis of community-acquired pneumonia. J. Fam. Pract. 2000, 49, 833–837. [Google Scholar] [PubMed]
- Hagaman, J.T.; Rouan, G.W.; Shipley, R.T.; Panos, R.J. Admission chest radiograph lacks sensitivity in the diagnosis of community-acquired pneumonia. Am. J. Med. Sci. 2009, 337, 236–240. [Google Scholar] [CrossRef] [PubMed]
- McClester Brown, M.; Sloane, P.D.; Kistler, C.E.; Reed, D.; Ward, K.; Weber, D.; Zimmerman, S. Evaluation and Management of the Nursing Home Resident with Respiratory Symptoms and an Equivocal Chest X-Ray Report. J. Am. Med. Dir. Assoc. 2016, 17, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Gatt, M.E.; Spectre, G.; Paltiel, O.; Hiller, N.; Stalnikowicz, R. Chest radiographs in the emergency department: Is the radiologist really necessary? Postgrad. Med. J. 2003, 79, 214–217. [Google Scholar] [CrossRef] [PubMed]
| Variable | Pneumonia (N = 133) | No Pneumonia (N = 67) | p-Value |
|---|---|---|---|
| Demographic Characteristics | |||
| Age | 83 (78–89) | 86 (80–92) | 0.03 |
| Male gender | 71 (53) | 28 (42) | 0.18 |
| Ambulatory setting (vs. nursing home or other) | 117 (88) | 55 (82) | 0.28 |
| Symptoms | |||
| Acute cough | 120 (90) | 50 (75) | <0.01 |
| Rales | 114 (86) | 57 (85) | 1 |
| Sputum production | 49 (37) | 25 (37) | 1 |
| Dyspnea | 95 (71) | 50 (75) | 0.74 |
| Chest pain | 26 (20) | 9 (15) | 0.33 |
| Confusion | 60 (45) | 32 (48) | 0.6 |
| Signs | |||
| Heart rate | 90 (78–104) | 89 (77–101) | 0.53 |
| Respiratory rate | 24 (20–28) | 22 (15–24) | 0.08 |
| Temperature (°C) | 38.0 (37.4–38.7) | 37.7 (37.1–38.4) | 0.07 |
| Systolic blood pressure | 131 (113–148) | 138 (116–156) | 0.2 |
| Diastolic blood pressure | 72 (62–83) | 73 (67–85) | 0.24 |
| Hypoxemia (PaO2 < 8 kPa or SaO2 < 90%) | 72 (54) | 30 (45) | 0.22 |
| Ancillary Tests | |||
| C-reactive protein (mg/L) | 101 (59–135) | 63 (38–108) | <0.01 |
| Leucocytes (G/L) | 12.0 (5.6) | 10.7 (4.0) | 0.09 |
| Procalcitonin µg/L | 0.36 (0.14–1.93) | 0.25 (0.11–0.66) | 0.04 |
| Urea (mmol/L) | 7.7 (5.7–10.8) | 8.3 (6.2–12.9) | 0.04 |
| Creatinine (µg/L) | 92 (69–125) | 101 (83–141) | 0.03 |
| Probability of pneumonia on CXR (according to physician) | <0.01 | ||
| Low | 31 (23) | 26 (39) | |
| Intermediate | 36 (27) | 23 (34) | |
| High | 66 (50) | 18 (27) | |
| Variable | Odd Ratio (95% CI) | p-Value |
|---|---|---|
| Male gender | 2.23 (1.12–4.44) | 0.022 |
| Acute cough | 3.77 (1.51–9.40) | 0.004 |
| C-reactive protein (mg/dL) | 1.01 (1.00–1.01) 1 | <0.001 |
| Urea (mmol/L) | 0.92 (0.86–0.98) 2 | 0.007 |
| Number of Points | Number of Patients with Suspected Pneumonia (%) 1 N = 200 | Number and Prevalence of Confirmed Pneumonia (%) |
|---|---|---|
| 0 | 3 (2) | 1/3 (33) |
| 1 | 29 (15) | 10/29 (35) |
| 2 | 76 (38) | 49/76 (65) |
| 3 | 77 (39) | 60/77 (78) |
| 4 | 15 (8) | 13/15 (87) |
| Physician in Charge without LDCT | Score-Based Algorithm (Derivation Cohort) | Score-Based Algorithm (Validation Cohort) | Physician in Charge with LDCT | |
|---|---|---|---|---|
| Proportion of CT scan (%) | 0 | 54 | 59 | 100 |
| Sensitivity | 95 | 95 | 80 | 92 |
| Specificity | 15 | 48 | 57 | 69 |
| Positive predictive value | 69 | 78 | 66 | 85 |
| Negative predictive value | 59 | 82 | 74 | 81 |
| Positive likelihood ratio | 1.1 | 1.8 | 2.4 | 2.9 |
| Negative likelihood ratio | 0.4 | 0.1 | 0.3 | 0.1 |
| Diagnostic odd ratio | 3 | 18 | 8 | 29 |
| AUROC | 0.55 (0.46–0.64) | 0.71 (0.63–0.80) | 0.69 (0.64–0.74) | 0.80 (0.73–0.87) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garin, N.; Marti, C.; Carballo, S.; Darbellay Farhoumand, P.; Montet, X.; Roux, X.; Scheffler, M.; Serratrice, C.; Serratrice, J.; Claessens, Y.-E.; et al. Rational Use of CT-Scan for the Diagnosis of Pneumonia: Comparative Accuracy of Different Strategies. J. Clin. Med. 2019, 8, 514. https://doi.org/10.3390/jcm8040514
Garin N, Marti C, Carballo S, Darbellay Farhoumand P, Montet X, Roux X, Scheffler M, Serratrice C, Serratrice J, Claessens Y-E, et al. Rational Use of CT-Scan for the Diagnosis of Pneumonia: Comparative Accuracy of Different Strategies. Journal of Clinical Medicine. 2019; 8(4):514. https://doi.org/10.3390/jcm8040514
Chicago/Turabian StyleGarin, Nicolas, Christophe Marti, Sebastian Carballo, Pauline Darbellay Farhoumand, Xavier Montet, Xavier Roux, Max Scheffler, Christine Serratrice, Jacques Serratrice, Yann-Erick Claessens, and et al. 2019. "Rational Use of CT-Scan for the Diagnosis of Pneumonia: Comparative Accuracy of Different Strategies" Journal of Clinical Medicine 8, no. 4: 514. https://doi.org/10.3390/jcm8040514
APA StyleGarin, N., Marti, C., Carballo, S., Darbellay Farhoumand, P., Montet, X., Roux, X., Scheffler, M., Serratrice, C., Serratrice, J., Claessens, Y.-E., Duval, X., Loubet, P., Stirnemann, J., & Prendki, V. (2019). Rational Use of CT-Scan for the Diagnosis of Pneumonia: Comparative Accuracy of Different Strategies. Journal of Clinical Medicine, 8(4), 514. https://doi.org/10.3390/jcm8040514





