Effect of Pre-Hospital Intubation in Patients with Severe Traumatic Brain Injury on Outcome: A Prospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Participating Centers
2.3. Ethical Aspects
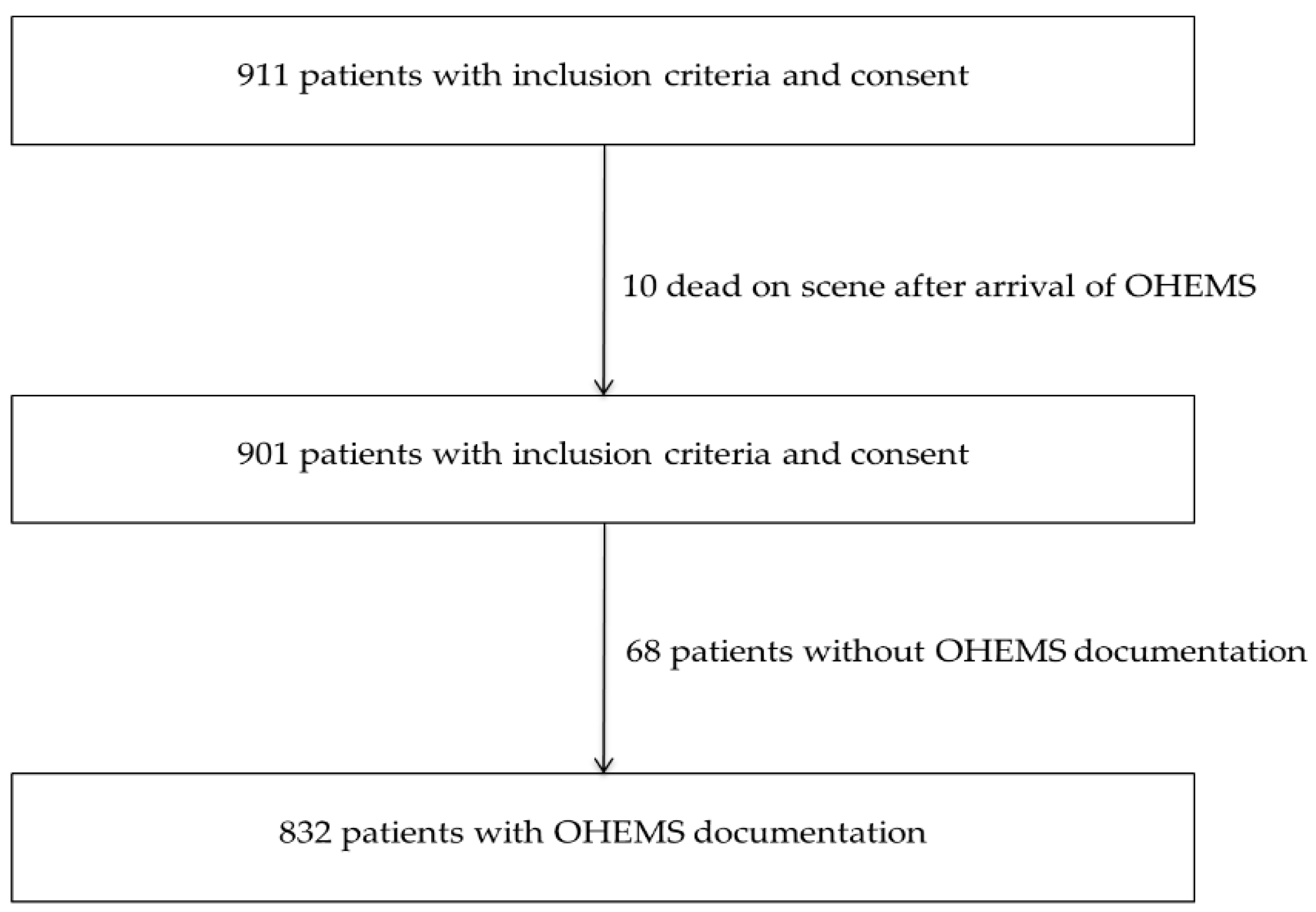
2.4. Included Patients
2.5. Data Collection
2.6. Outcome Measures
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Andelic, N.; Anke, A.; Skandsen, T.; Sigurdardottir, S.; Sandhaug, M.; Ader, T.; Roe, C. Incidence of hospital-admitted severe traumatic brain injury and in-hospital fatality in Norway: A national cohort study. Neuroepidemiology 2012, 38, 259–267. [Google Scholar] [CrossRef]
- Stein, S.C.; Georgoff, P.; Meghan, S.; Mizra, K.; Sonnad, S.S. 150 years of treating severe traumatic brain injury: A systematic review of progress in mortality. J. Neurotrauma 2010, 27, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [PubMed]
- Sitsapesan, H.A.; Lawrence, T.P.; Sweasey, C.; Wester, K. Neurotrauma outside the high-income setting: A review of audit and data-collection strategies. World Neurosurg. 2013, 79, 568–575. [Google Scholar] [CrossRef]
- Stocchetti, N.; Paternò, R.; Citerio, G.; Beretta, L.; Colombo, A. Traumatic brain injury in an aging population. J. Neurotrauma 2012, 29, 1119–1125. [Google Scholar] [CrossRef]
- Walder, B.; Haller, G.; Rebetez, M.M.L.; Delhumeau, C.; Bottequin, E.; Schoettker, P.; Ravussin, P.; Brodmann Maeder, M.; Stover, J.F.; Zürcher, M.; et al. Severe traumatic brain injury in a high-income country: An epidemiological study. J. Neurotrauma 2013, 30, 1934–1942. [Google Scholar] [CrossRef]
- Masson, F.; Thicoipe, M.; Aye, P.; Mokni, T.; Senjean, P.; Schmitt, V.; Dessalles, P.H.; Cazaugade, M.; Labadens, P.; Aquitaine Group for Severe Brain Injuries Study. Epidemiology of severe brain injuries: A prospective population-based study. J. Trauma 2001, 51, 481–489. [Google Scholar]
- Asemota, A.O.; George, B.P.; Bowman, S.M.; Haider, A.H.; Schneider, E.B. Causes and trends in traumatic brain injury for United States adolescents. J. Neurotrauma 2013, 30, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, R.M.; Marshall, L.F.; Klauber, M.R.; Blunt, B.A.; Baldwin, N.; Eisenberg, H.M.; Jane, J.A.; Marmarou, A.; Foulkes, M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 1993, 34, 216–222. [Google Scholar] [CrossRef]
- McHugh, G.S.; Engel, D.C.; Butcher, I.; Steyerberg, E.W.; Lu, J.; Mushkudiani, N.; Hernández, A.V.; Marmarou, A.; Maas, A.I.R.; Murray, G.D. Prognostic value of secondary insults in traumatic brain injury: Results from the IMPACT study. J. Neurotrauma 2007, 24, 287–293. [Google Scholar] [CrossRef]
- Lazaridis, C.; Rusin, C.G.; Robertson, C.S. Secondary brain injury: Predicting and preventing insults. Neuropharmacology 2019, 145, 145–152. [Google Scholar] [CrossRef]
- Tohme, S.; Delhumeau, C.; Zuercher, M.; Haller, G.; Walder, B. Prehospital risk factors of mortality and impaired consciousness after severe traumatic brain injury: An epidemiological study. Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.; Franschman, G.; Loer, S.A. Prehospital management of severe traumatic brain injury: Concepts and ongoing controversies. Curr. Opin. Anaesthesiol. 2012, 25, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Badjatia, N.; Carney, N.; Crocco, T.J.; Fallat, M.E.; Hennes, H.M.A.; Jagoda, A.S.; Jernigan, S.; Letarte, P.B.; Lerner, E.B.; Moriarty, T.M.; et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehospital Emerg. Care Off. J. Natl. Assoc. EMS Physicians Natl. Assoc. State EMS Dir. 2008, 12, S1–S52. [Google Scholar] [CrossRef] [PubMed]
- Winchell, R.J.; Hoyt, D.B. Endotracheal intubation in the field improves survival in patients with severe head injury. Trauma Research and Education Foundation of San Diego. Arch. Surg. Chic. Ill 1960 1997, 132, 592–597. [Google Scholar]
- Baxt, W.G.; Moody, P. The impact of advanced prehospital emergency care on the mortality of severely brain-injured patients. J. Trauma 1987, 27, 365–369. [Google Scholar] [CrossRef]
- Davis, D.P.; Hoyt, D.B.; Ochs, M.; Fortlage, D.; Holbrook, T.; Marshall, L.K.; Rosen, P. The effect of paramedic rapid sequence intubation on outcome in patients with severe traumatic brain injury. J. Trauma 2003, 54, 444–453. [Google Scholar] [CrossRef]
- Von Elm, E.; Schoettker, P.; Henzi, I.; Osterwalder, J.; Walder, B. Pre-hospital tracheal intubation in patients with traumatic brain injury: Systematic review of current evidence. Br. J. Anaesth. 2009, 103, 371–386. [Google Scholar] [CrossRef]
- Reith, F.C.M.; Lingsma, H.F.; Gabbe, B.J.; Lecky, F.E.; Roberts, I.; Maas, A.I.R. Differential effects of the Glasgow Coma Scale Score and its Components: An analysis of 54,069 patients with traumatic brain injury. Injury 2017, 48, 1932–1943. [Google Scholar] [CrossRef]
- Cooke, R.S.; McNicholl, B.P.; Byrnes, D.P. Use of the Injury Severity Score in head injury. Injury 1995, 26, 399–400. [Google Scholar] [CrossRef]
- Demetriades, D.; Kuncir, E.; Murray, J.; Velmahos, G.C.; Rhee, P.; Chan, L. Mortality prediction of head Abbreviated Injury Score and Glasgow Coma Scale: Analysis of 7,764 head injuries. J. Am. Coll. Surg. 2004, 199, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Copes, W.S.; Champion, H.R.; Sacco, W.J.; Lawnick, M.M.; Keast, S.L.; Bain, L.W. The Injury Severity Score revisited. J. Trauma 1988, 28, 69–77. [Google Scholar] [CrossRef]
- Patel, N.Y.; Hoyt, D.B.; Nakaji, P.; Marshall, L.; Holbrook, T.; Coimbra, R.; Winchell, R.J.; Mikulaschek, A.W. Traumatic brain injury: Patterns of failure of nonoperative management. J. Trauma 2000, 48, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Deepika, A.; Prabhuraj, A.R.; Saikia, A.; Shukla, D. Comparison of predictability of Marshall and Rotterdam CT scan scoring system in determining early mortality after traumatic brain injury. Acta Neurochir. (Wien) 2015, 157, 2033–2038. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, M.; Ummenhofer, W.; Baltussen, A.; Walder, B. The use of Glasgow Coma Scale in injury assessment: A critical review. Brain Inj. 2009, 23, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Dick, W.F.; Baskett, P.J.; Grande, C.; Delooz, H.; Kloeck, W.; Lackner, C.; Lipp, M.; Mauritz, W.; Nerlich, M.; Nicholl, J.; et al. Recommendations for uniform reporting of data following major trauma—The Utstein style. An International Trauma Anaesthesia and Critical Care Society (ITACCS) initiative. Br. J. Anaesth. 2000, 84, 818–819. [Google Scholar] [CrossRef] [PubMed]
- Kouloulas, E.J.; Papadeas, A.G.; Michail, X.; Sakas, D.E.; Boviatsis, E.J. Prognostic value of time-related Glasgow coma scale components in severe traumatic brain injury: A prospective evaluation with respect to 1-year survival and functional outcome. Int. J. Rehabil. Res. Int. Z. Rehabil. Rev. Int. Rech. Readaptation 2013, 36, 260–267. [Google Scholar] [CrossRef]
- Bootland, D.; Rose, C.; Barrett, J.W.; Lyon, R.; Kent, Surrey & Sussex Air Ambulance Trust. Pre-hospital anaesthesia and assessment of head injured patients presenting to a UK Helicopter Emergency Medical Service with a high Glasgow Coma Scale: A cohort study. BMJ Open 2019, 9, e023307. [Google Scholar] [PubMed]
- Rubenson Wahlin, R.; Nelson, D.W.; Bellander, B.-M.; Svensson, M.; Helmy, A.; Thelin, E.P. Prehospital Intubation and Outcome in Traumatic Brain Injury-Assessing Intervention Efficacy in a Modern Trauma Cohort. Front. Neurol. 2018, 9, 194. [Google Scholar] [CrossRef]
- Tuma, M.; El-Menyar, A.; Abdelrahman, H.; Al-Thani, H.; Zarour, A.; Parchani, A.; Khoshnaw, S.; Peralta, R.; Latifi, R. Prehospital intubation in patients with isolated severe traumatic brain injury: A 4-year observational study. Crit. Care Res. Pract. 2014, 2014, 135986. [Google Scholar] [CrossRef]
- Haltmeier, T.; Benjamin, E.; Siboni, S.; Dilektasli, E.; Inaba, K.; Demetriades, D. Prehospital intubation for isolated severe blunt traumatic brain injury: Worse outcomes and higher mortality. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2017, 43, 731–739. [Google Scholar] [CrossRef]
- Bossers, S.M.; Schwarte, L.A.; Loer, S.A.; Twisk, J.W.R.; Boer, C.; Schober, P. Experience in Prehospital Endotracheal Intubation Significantly Influences Mortality of Patients with Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0141034. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.A.; Mann, K.P.; Fearnside, M.; Poynter, E.; Gebski, V. The Head Injury Retrieval Trial (HIRT): A single-centre randomised controlled trial of physician prehospital management of severe blunt head injury compared with management by paramedics only. Emerg. Med. J. EMJ 2015, 32, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Mossuti, F.; Fisch, U.; Schoettker, P.; Gugliotta, M.; Morard, M.; Schucht, P.; Schatlo, B.; Levivier, M.; Walder, B.; Fandino, J. Surgical Treatment of Severe Traumatic Brain Injury in Switzerland: Results from a Multicenter Study. J. Neurol. Surg. Part Cent. Eur. Neurosurg. 2016, 77, 36–45. [Google Scholar]
- Hoffmann, M.; Czorlich, P.; Lehmann, W.; Spiro, A.S.; Rueger, J.M.; Lefering, R.; TraumaRegister DGU of the German Trauma Society (DGU). The Impact of Prehospital Intubation With and Without Sedation on Outcome in Trauma Patients With a GCS of 8 or Less. J. Neurosurg. Anesthesiol. 2017, 29, 161–167. [Google Scholar] [CrossRef]
| Patient Characteristics | n Missing | n (%) n = 832 | Survivors n = 577 | Non-Survivors n = 255 | p Value (Univariate Analysis) |
|---|---|---|---|---|---|
| Female | 0 | 220 (26.4%) | 150 (26.0%) | 70 (27.5%) | 0.661 A |
| Age (years; median, IQR) | 0 | 54.3 (32.2–71.3) | 49.8 (28.4–67.5) | 63.3 (41.6–79.5) | <0.0001 Δ |
| GCS on scene (median, IQR) | 11 | 9 (4–14) | 12 (7–14) | 4 (3–9) | <0.0001 Δ |
| Abnormal pupil reaction | 63 | 196 (25.5%) | 69 (13.0%) | 127 (52.9%) | <0.0001 A |
| HAIS 4 | 0 | 357 (42.9%) | 320 (55.5%) | 37 (14.5%) | <0.0001 A |
| HAIS 5 | 0 | 448 (53.9%) | 257 (44.5%) | 191 (74.9%) | |
| HAIS 6 | 0 | 27 (3.3%) | 0 | 27 (10.6%) | |
| ISS (median, IQR) | 0 | 25 (21–34) | 25 (20–30) | 29 (25–41) | <0.0001 Δ |
| Pre-hospital time | 137 | 50 (36–71) | 50 (37–75) | 48 (35–65) | 0.0977 Δ |
| Parameter | n Missing | n (%) n = 832 | Survivors n = 577 | Non-Survivors n = 255 | Odds Ratio Γ (95% CI) | p Value (Univariate Analysis) |
|---|---|---|---|---|---|---|
| Hypoxemia sc/tr | 192 | 100 (15.6%) | 53 (11.7%) | 47 (25.1%) | 2.53 (1.64–3.92) | <0.0001 |
| PHI | 0 | 367 (44.1%) | 203 (35.2%) | 164 (64.3%) | 3.32 (2.44–4.52) | <0.0001 |
| Without PHI | 0 | 465 (55.9%) | 374 (64.8%) | 91 (35.7%) | 0.30 (0.22–0.41) | <0.0001 |
| Hypoxemia ED | 41 | 45 (5.7%) | 12 (2.2%) | 33 (13.8%) | 7.12 (3.61–14.05) | <0.0001 |
| Hypotension ED | 20 | 39 (4.3%) | 8 (1.4%) | 31 (12.7%) | 10.24 (4.64–22.64) | <0.0001 |
| Patient Characteristics | n Missing | n (%) n = 369 | GSC 14/15 n = 267 | GCS ≤ 13 n = 102 | p Value (Univariate Analysis) |
|---|---|---|---|---|---|
| Female | 0 | 95 (25.8%) | 74 (27.7%) | 21 (20.6%) | 0.161A |
| Age (years; median, IQR) | 0 | 50.2 (29.9–66.7) | 52.1 (32.8–69.3) | 45.8 (24.6–63.2) | 0.0261Δ |
| GCS on scene (median, IQR) | 0 | 11 (6–14) | 13 (9–14) | 6 (4–10) | <0.0001Δ |
| Abnormal pupil reaction | 36 | 46 (13.8%) | 21 (8.5%) | 25 (28.7%) | <0.0001A |
| ISS (median, IQR) | 0 | 25 (20–33) | 25 (17–29) | 29 (25–38) | <0.0001Δ |
| HAIS 4 | 0 | 197 (53.4%) | 166 (62.2%) | 31 (30.4%) | <0.0001A |
| HAIS 5 | 0 | 172 | 101 | 71 | <0.0001Δ |
| Parameter | n Missing | n (%) n =369 | GSC 14/15 n = 267 | GCS ≤ 13 n = 102 | Odds Ratio Γ (95% CI) | p Value (Univariate Analysis) |
|---|---|---|---|---|---|---|
| Hypotension sc/tr | 70 | 12 (4.0%) | 7 (3.4%) | 5 (5.6%) | 1.68 (0.52–5.46) | 0.373 |
| Hypoxemia sc/tr | 74 | 34 (11.9%) | 12 (5.9%) | 22 (26.8%) | 5.84 (2.73–12.49) | <0.0001 |
| PHI | 0 | 139 (37.7%) | 66 (24.7%) | 73 (71.6%) | 7.67 (4.59–12.80) | <0.0001 |
| Without PHI | 0 | 230 (62.3%) | 201 (75.3%) | 29 (28.4) | 0.13 (0.08–0.22) | <0.0001 |
| Hypoxemia in ED | 6 | 9 | 6 (2.3%) | 3 (2.9%) | 1.27 (0.31–5.18) | 0.732 |
| Hypotension in ED | 0 | 5 (1.4%) | 4 (1.5%) | 1 (0.98%) | 0.65 (0.07–5.89) | 0.698 |
| Initial Trauma Assessment | Univariate Regression Model (Cox model); Hazard Ratio (95% CI) | p Value | Multivariate Regression Model (Cox Model with BRESLOW Method for Ties); Hazard Ratio Ω (95% CI) | p Value |
|---|---|---|---|---|
| Age (years) | 1.02 (1.01–1.02) | <0.0001 | 1.02 (1.02–1.03) | <0.0001 |
| GCS < 9 on scene | 3.29 (2.48–4.35) | <0.0001 | 2.44 (1.60–3.72) | <0.0001 |
| Abnormal pupil reaction on scene | 3.90 (3.02–5.02) | <0.0001 | 2.54 (1.87–3.47) | <0.0001 |
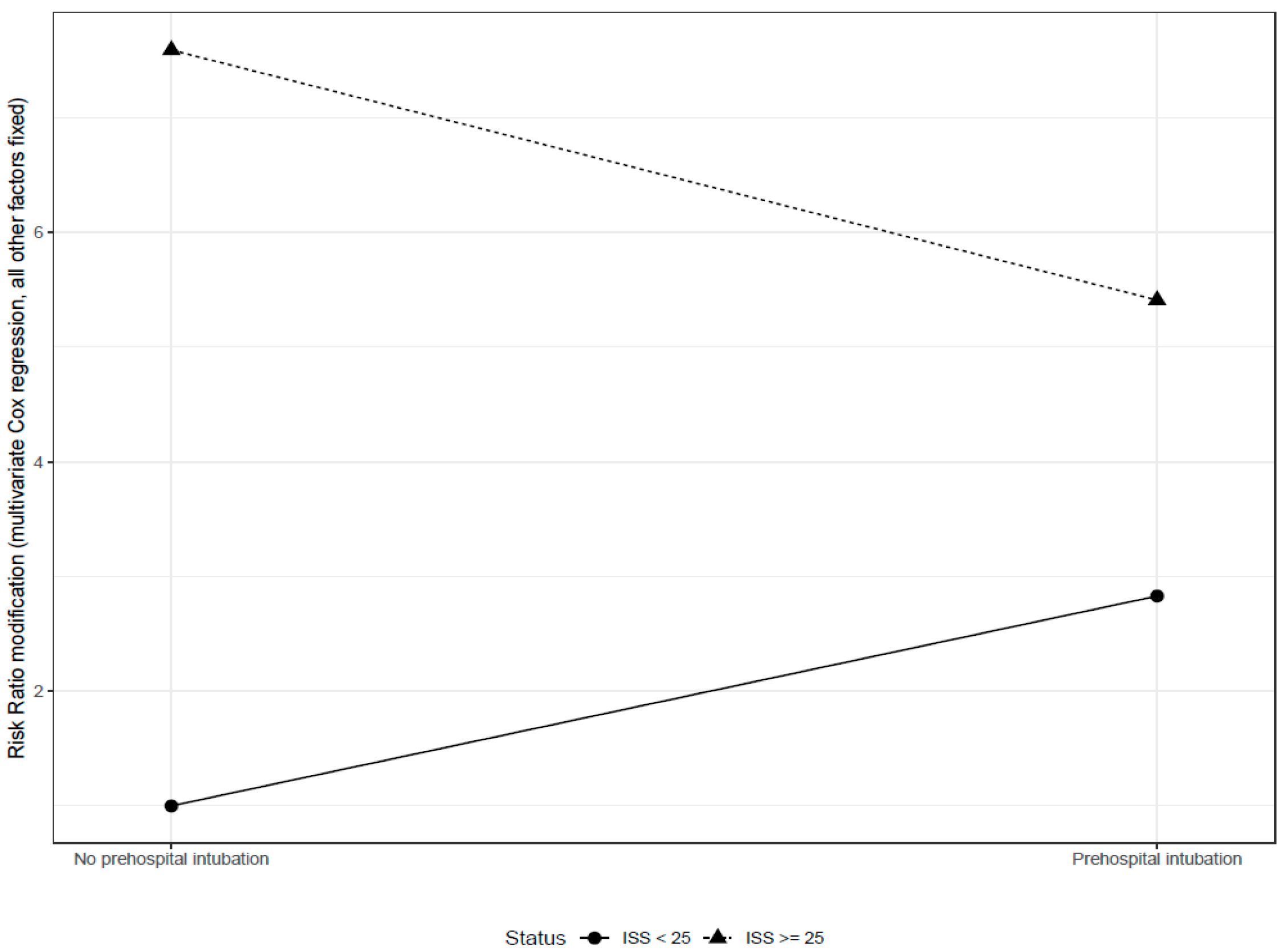
| ISS ≥ 25 | 4.67 (2.96–7.37) | <0.0001 | 7.59 (3.27–17.62) | <0.0001 |
| Pre-hospital procedures | ||||
| Intubation | 2.27 (1.75–2.93) | <0.0001 | 2.83 (0.93–8.56) | 0.066 |
| Interaction: ISS ≥ 25 * Intubation | 0.25 (0.08–0.74) | 0.013 | ||
| Oxygen administration | 0.46 (0.23–0.89) | 0.021 | 0.87 (0.36–2.07) | 0.751 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choffat, C.; Delhumeau, C.; Fournier, N.; Schoettker, P. Effect of Pre-Hospital Intubation in Patients with Severe Traumatic Brain Injury on Outcome: A Prospective Cohort Study. J. Clin. Med. 2019, 8, 470. https://doi.org/10.3390/jcm8040470
Choffat C, Delhumeau C, Fournier N, Schoettker P. Effect of Pre-Hospital Intubation in Patients with Severe Traumatic Brain Injury on Outcome: A Prospective Cohort Study. Journal of Clinical Medicine. 2019; 8(4):470. https://doi.org/10.3390/jcm8040470
Chicago/Turabian StyleChoffat, Caroline, Cecile Delhumeau, Nicolas Fournier, and Patrick Schoettker. 2019. "Effect of Pre-Hospital Intubation in Patients with Severe Traumatic Brain Injury on Outcome: A Prospective Cohort Study" Journal of Clinical Medicine 8, no. 4: 470. https://doi.org/10.3390/jcm8040470
APA StyleChoffat, C., Delhumeau, C., Fournier, N., & Schoettker, P. (2019). Effect of Pre-Hospital Intubation in Patients with Severe Traumatic Brain Injury on Outcome: A Prospective Cohort Study. Journal of Clinical Medicine, 8(4), 470. https://doi.org/10.3390/jcm8040470





