Effects of Pulmonary Rehabilitation on Gait Characteristics in Patients with COPD
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Study Design
2.3. Experimental Setup
2.4. Data Analyses
2.5. Statistics
3. Results
3.1. Baseline Subject Characteristics
3.2. Changes after Pulmonary Rehabilitation
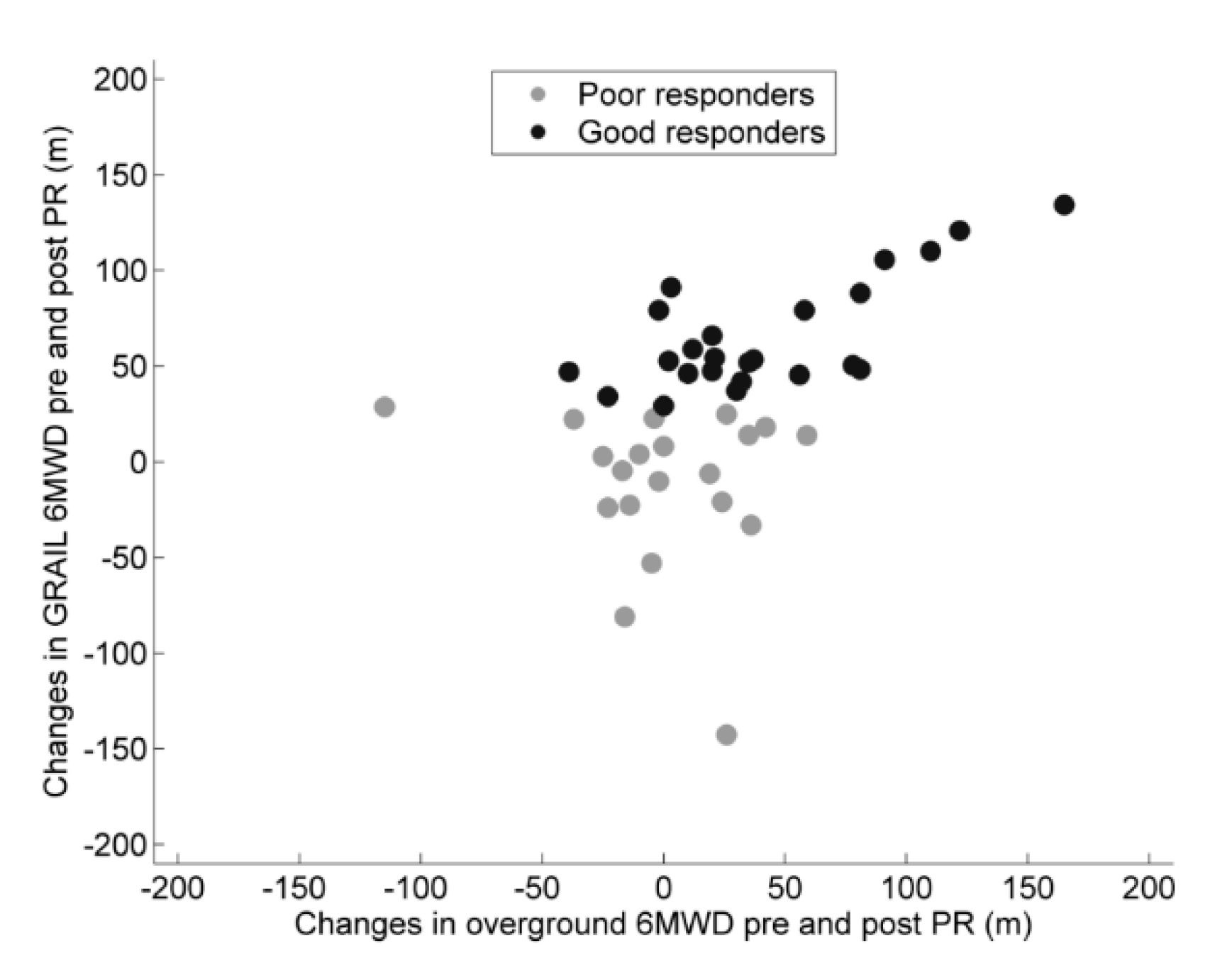
3.3. Good versus Poor Responders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Annegarn, J.; Meijer, K.; Passos, V.L.; Stute, K.; Wiechert, J.; Savelberg, H.H.; Schols, A.M.; Wouters, E.F.; Spruit, M.A. Problematic activities of daily life are weakly associated with clinical characteristics in COPD. J. Am. Med. Dir. Assoc. 2012, 13, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Nakken, N.; Janssen, D.J.; Bootsma, G.P.; Gronenschild, M.H.; Delbressine, J.M.; Muris, J.W.; Wouters, E.F.; Bogaart, E.H.V.D.; Van Vliet, M.; De Vries, G.J.; et al. Patient versus proxy-reported problematic activities of daily life in patients with COPD. Respirology 2016, 22, 307–314. [Google Scholar] [CrossRef]
- Annegarn, J.; Spruit, M.A.; Savelberg, H.H.C.M.; Willems, P.J.B.; Van De Bool, C.; Schols, A.M.W.J.; Wouters, E.F.M.; Meijer, K. Differences in walking pattern during 6-min walk test between patients with copd and healthy subjects. PLoS ONE 2012, 7, e37329. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Y.; Spruit, M.A.; Delbressine, J.M.; Willems, P.J.; Franssen, F.M.E.; Wouters, E.F.M.; Meijer, K. Spatiotemporal gait characteristics in patients with COPD during the gait real-time analysis interactive lab-based 6-minute walk test. PLoS ONE 2017, 12, e0190099. [Google Scholar] [CrossRef]
- Nantsupawat, N.; Lane, P.; Siangpraipunt, O.; Gadwala, S.; Nugent, K. Gait characteristics in patients with chronic obstructive pulmonary disease. J. Prim. Care Health 2015, 6, 222–226. [Google Scholar] [CrossRef]
- Yentes, J.M.; Rennard, S.I.; Schmid, K.K.; Blanke, D.; Stergiou, N. Patients with chronic obstructive pulmonary disease walk with altered step time and step width variability as compared with healthy control subjects. Ann. Am. Thorac. Soc. 2017, 14, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Zago, M.; Sforza, C.; Bonardi, D.R.; Guffanti, E.E.; Galli, M. Gait analysis in patients with chronic obstructive pulmonary disease: A systematic review. Gait Posture 2018, 61, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Mitchell, S.L.; Firtion, R.; Peng, C.K.; Cudkowicz, M.E.; Wei, J.Y.; Goldberger, A.L. Altered fractal dynamics of gait: Reduced stride-interval correlations with aging and Huntington’s disease. J. Appl. Physiol. 1997, 82, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.A.; Johanning, J.M.; Stergiou, N.; Celis, R.I.; Robinson, L.; Pipinos, I.I. Gait variability is altered in patients with peripheral arterial disease. J. Vasc. Surg. 2009, 49, 924–931.e1. [Google Scholar] [CrossRef] [PubMed]
- Buzzi, U.H.; Stergiou, N.; Kurz, M.J.; Hageman, P.; Heidel, J. Nonlinear dynamics indicates aging affects variability during gait. Clin. Biomech. 2003, 18, 435–443. [Google Scholar]
- Maki, B.E. Gait changes in older adults: Predictors of falls or indicators of fear? J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Moe-Nilssen, R.; Helbostad, J.L. Interstride trunk acceleration variability but not step width variability can differentiate between fit and frail older adults. Gait Posture 2005, 21, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, N.; Harbourne, R.; Cavanaugh, J. Optimal movement variability: A new theoretical perspective for neurologic physical therapy. J. Neurol. Phys. Ther. 2006, 30, 120–129. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Cudkowicz, M.E.; Firtion, R.; Wei, J.Y.; Goldberger, A.L. Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov. Disord. 1998, 13, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Eng, J.J.; Road, J.D.; Reid, W.D. Falls in patients with chronic obstructive pulmonary disease: A call for further research. Respir. Med. 2009, 103, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Valentine, R.J.; Evans, E.M.; Sosnoff, J.J. Lower extremity muscle quality and gait variability in older adults. Age Ageing 2012, 41, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Marques, N.R.; Laroche, D.P.; Hallal, C.Z.; Crozara, L.F.; Morcelli, M.H.; Karuka, A.H.; Navega, M.T.; Gonçalves, M. Association between energy cost of walking, muscle activation, and biomechanical parameters in older female fallers and non-fallers. Clin. Biomech. 2013, 28, 330–336. [Google Scholar] [CrossRef]
- Lord, S.R.; Lloyd, D.G.; Nirui, M.; Raymond, J.; Williams, P.; Stewart, R.A. The effect of exercise on gait patterns in older women: A randomized controlled trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1996, 51, M64–M70. [Google Scholar] [CrossRef]
- Mathur, S.; Brooks, D.; Carvalho, C.R.F. Structural alterations of skeletal muscle in COPD. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- De Brandt, J.; Spruit, M.A.; Derave, W.; Hansen, D.; Vanfleteren, L.E.; Burtin, C. Changes in structural and metabolic muscle characteristics following exercise-based interventions in patients with COPD: A systematic review. Rev. Respir. Med. 2016, 10, 521–545. [Google Scholar] [CrossRef] [PubMed]
- De Brandt, J.; A Spruit, M.; Hansen, D.; Franssen, F.M.; Derave, W.; Sillen, M.J.; Burtin, C. Changes in lower limb muscle function and muscle mass following exercise-based interventions in patients with chronic obstructive pulmonary disease: A review of the English-language literature. Respir. Dis. 2017, 15, 182–219. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Augustin, I.M.L.; Wouters, E.F.M.; Houben-Wilke, S.; Gaffron, S.; Janssen, D.J.A.; Franssen, F.M.E.; Spruit, M.A. Comprehensive lung function assessment does not allow to infer response to pulmonary rehabilitation in patients with COPD. J. Clin. Med. 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Augustin, I.M.; Vanfleteren, L.E.; Janssen, D.J.; Gaffron, S.; Pennings, H.-J.; Smeenk, F.; Pieters, W.; Bergh, J.J.V.D.; Michels, A.-J.; et al. Differential response to pulmonary rehabilitation in COPD: Multidimensional profiling. Eur. Respir. J. 2015, 46, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Y.; Meijer, K.; Delbressine, J.M.; Willems, P.J.; Franssen, F.M.E.; Wouters, E.F.M.; Spruit, M.A. Reproducibility and validity of the 6-minute walk test using the gait real-time analysis interactive lab in patients with COPD and healthy elderly. PLoS ONE 2016, 11, e0162444. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- A Spruit, M.; Vanderhoven-Augustin, I.; Janssen, P.P.; Wouters, E.F. Integration of pulmonary rehabilitation in COPD. Lancet 2008, 371, 12–13. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; LaMonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Van De Bool, C.; Mattijssen-Verdonschot, C.; Van Melick, P.P.M.J.; A Spruit, M.; E Franssen, F.M.; Wouters, E.F.M.; Schols, A.M.W.J.; A Rutten, E.P. Quality of dietary intake in relation to body composition in patients with chronic obstructive pulmonary disease eligible for pulmonary rehabilitation. Eur. J. Clin. Nutr. 2013, 68, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Makrides, L.; Hernandez, P. Test-retest reliability of isometric and isokinetic torque in patients with chronic obstructive pulmonary disease. Physiother. Can. 2004, 56, 94. [Google Scholar] [CrossRef]
- Bogert, A.J.V.D.; Geijtenbeek, T.; Even-Zohar, O.; Steenbrink, F.; Hardin, E.C.; Bogert, A.V.D. A real-time system for biomechanical analysis of human movement and muscle function. Med. Boil. Eng. Comput. 2013, 51, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Bruijn, S.M.; Meijer, O.G.; Beek, P.J.; Van Dieen, J.H.; Bruijn, S. Assessing the stability of human locomotion: A review of current measures. J. R. Soc. 2013, 10, 20120999. [Google Scholar] [CrossRef]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2012, 41, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, C.; Cao, Z.; Sun, B.; Lo, I.L.; Liu, T.-M.; Zheng, J.; Sun, S.; Shi, Y.; Zhang, X.D. Entropy change of biological dynamics in COPD. Int. J. Obstr. Pulm. Dis. 2017, 12, 2997–3005. [Google Scholar] [CrossRef] [PubMed]
- Polkey, M.I.; Spruit, M.A.; Edwards, L.D.; Watkins, M.L.; Pinto-Plata, V.; Vestbo, J.; Calverley, P.M.A.; Tal-Singer, R.; Agusti, A.; Bakke, P.S.; et al. Six-minute-walk test in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 382–386. [Google Scholar] [CrossRef] [PubMed]
- McClellan, R.; Amiri, H.M.; Limsuwat, C.; Nugent, K.M. pulmonary rehabilitation increases gait speed in patients with chronic lung diseases. Health Serv. Res. Manag. Epidemiol. 2014, 1. [Google Scholar] [CrossRef]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, L.A. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA 1992, 267, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Dingwell, J.B.; Cusumano, J.P. Nonlinear time series analysis of normal and pathological human walking. Chaos Interdiscip. J. Sci. 2000, 10, 848. [Google Scholar] [CrossRef]
- Beauchet, O.; Launay, C.; Annweiler, C.; Fantino, B.; Allali, G.; De Decker, L. Physical training-related changes in gait variability while single and dual tasking in older adults: Magnitude of gait variability at baseline matters. Eur. J. Phys. Rehabil. Med. 2013, 49, 857–864. [Google Scholar] [PubMed]
- Wang, R.-Y.; Wang, Y.-L.; Cheng, F.-Y.; Chao, Y.-H.; Chen, C.-L.; Yang, Y.-R. Effects of combined exercise on gait variability in community-dwelling older adults. AGE 2015, 37, 9780. [Google Scholar] [CrossRef] [PubMed]
- Nici, L.; Donner, C.; Wouters, E.; ZuWallack, R.; Ambrosino, N.; Bourbeau, J.; Carone, M.; Celli, B.; Engelen, M.; Fahy, B.; et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2006, 173, 1390–1413. [Google Scholar] [CrossRef] [PubMed]
- Mador, M.J.; Bozkanat, E.; Aggarwal, A.; Shaffer, M.; Kufel, T.J. Endurance and strength training in patients with COPD. Chest 2004, 125, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Brach, J.S.; Van Swearingen, J.M. Interventions to improve walking in older adults. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013, 2, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Sloot, L.; Van Der Krogt, M.; Harlaar, J. Self-paced versus fixed speed treadmill walking. Gait Posture 2014, 39, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Azrad, T.; Bondi, M.; Bahat, Y.; Gimmon, Y.; Zeilig, G.; Inzelberg, R.; Siev-Ner, I. Self-selected gait speed—Over ground versus self-paced treadmill walking, a solution for a paradox. J. Neuroeng. Rehabil. 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.M.; Spruit, M.A.; Hopkinson, N.S.; Sathyapala, S.; Man, W.D.-C.; Jackson, A.; Gosker, H.R.; Schols, A.M.W.J.; Moxham, J.; Polkey, M.I.; et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur. Respir. J. 2009, 36, 81–88. [Google Scholar] [CrossRef]
- Gagnon, P.; Maltais, F.; Bouyer, L.; Ribeiro, F.; Coats, V.; Brouillard, C.; Noël, M.; Rousseau-Gagnon, M.; Saey, D. Distal leg muscle function in patients with COPD. COPD J. Obstr. Pulm. Dis. 2013, 10, 235–242. [Google Scholar] [CrossRef] [PubMed]
- McCrum, C.; Gerards, M.H.G.; Karamanidis, K.; Zijlstra, W.; Meijer, K. A systematic review of gait perturbation paradigms for improving reactive stepping responses and falls risk among healthy older adults. Eur. Rev. Phys. Act. 2017, 14, 1224. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Rochester, L.; Reelick, M.; Nieuwhof, F.; Pelosin, E.; Abbruzzese, G.; Dockx, K.; Nieuwboer, A.; Hausdorff, J.M. V-TIME: A treadmill training program augmented by virtual reality to decrease fall risk in older adults: Study design of a randomized controlled trial. BMC Neurol. 2013, 13, 15. [Google Scholar] [CrossRef] [PubMed]
| Variable | Pre-Pulmonary Rehabilitation (n = 44) |
|---|---|
| Age, years | 62.2 (7.5) |
| Gender M/F | 25/19 |
| Weight, kg | 76.5 (16.6) |
| Height, m | 1.68 (0.18) |
| BMI, kg/m2 | 26.9 (5.2) |
| FEV1/FVC | 0.42 (0.12) |
| FEV1% predicted | 55.88 (19.73) |
| GOLD stage 1 | 6 (13.6%) |
| GOLD stage 2 | 18 (40.9%) |
| GOLD stage 3 | 17 (38.6%) |
| GOLD stage 4 | 3 (6.8%) |
| Never smoker | 1 (2.3%) |
| Former smoker | 41 (93.2%) |
| Current smoker | 2 (4.6%) |
| Pack years | 41.55 (20.6) |
| Pre-Pulmonary Rehabilitation | Post-Pulmonary Rehabilitation | |||||
|---|---|---|---|---|---|---|
| Test | Parameter | n | value | n | value | p-value |
| Body composition | Total FM, kg | 43 | 27.3 (9.2) | 43 | 27.1 (8.6) | 0.745 |
| Total FFM, kg | 43 | 47.8 (9.5) * | 43 | 48.7 (9.7) | <0.001 † | |
| FFMI, kg/m2 | 43 | 16.8 (2.4) | 43 | 17.1 (2.4) | <0.001 | |
| Quadriceps muscle function | Peak torque, N/m | 38 | 106 (33) | 38 | 114 (35) | <0.001 |
| Peak torque, % predicted | 38 | 71 (14) | 38 | 77 (14) | <0.001 | |
| Total work, J | 38 | 1978 (625) | 38 | 2220 (669) * | <0.001 † | |
| Work fatigue, % | 38 | 43 (11) | 38 | 39 (9) * | 0.048 † | |
| TUG test | Time, s | 44 | 8.8 (1.3) | 44 | 8.7 (1.1) | 0.653 |
| Overground 6MWT | 6MWD, m | 44 | 512 (67) | 44 | 535 (71) | 0.003 |
| Walking speed, m/s | 44 | 1.42 (0.18) | 44 | 1.5 (0.2) | 0.003 | |
| SpO2 rest, % | 44 | 94 (2) | 44 | 95 (2) * | 0.760 † | |
| SpO2 end, % | 44 | 88 (6) * | 44 | 87 (6) | 0.055 † | |
| Heart rate rest, bpm | 44 | 85 (14) | 44 | 80 (14) | 0.014 | |
| Heart rate end, bpm | 44 | 115 (17) | 44 | 117 (17) | 0.318 | |
| Dyspnea rest | 44 | 1.2 (1.1) * | 44 | 1.0 (1.0) | 0.243 † | |
| Dyspnea end | 44 | 5.6 (2.0) * | 44 | 5.1 (2.2) | 0.108 † | |
| Fatigue rest | 44 | 1.3 (1.5) * | 44 | 1.1 (1.2) * | 0.172 † | |
| Fatigue end | 44 | 5.0 (2.5) | 44 | 4.3 (2.4) | 0.030 | |
| Pre-Pulmonary Rehabilitation | Post-Pulmonary Rehabilitation | |||||
|---|---|---|---|---|---|---|
| Test | Parameter | n | Value | n | value | p-value |
| 6MWT | 6MWD, m | 44 | 506 (75) | 44 | 537 (82) | <0.001 |
| Walking speed, m/s | 44 | 1.43 (0.19) | 44 | 1.48 (0.23) | 0.056 | |
| SpO2 rest, % | 44 | 96 (2) | 44 | 95 (2) | 0.357 | |
| SpO2 end, % | 44 | 92 (5) * | 44 | 93 (4) * | 0.773 † | |
| Heart rate rest, bpm | 44 | 82 (16) | 44 | 80 (15) | 0.252 | |
| Heart rate end, bpm | 44 | 104 (20) | 44 | 103 (21) | 0.599 | |
| Dyspnea rest | 44 | 1.2 (1.1) * | 44 | 1.0 (0.9) * | 0.120 † | |
| Dyspnea end | 44 | 5.3 (2.2) * | 44 | 4.6 (2.2) * | 0.019 † | |
| Fatigue rest | 44 | 1.2 (1.2) * | 44 | 1.1 (1.1) * | 0.487 † | |
| Fatigue end | 44 | 4.6 (2.3) | 44 | 4.4 (2.4) | 0.494 | |
| Gait | Mean stride time, s | 44 | 1.02 (0.08) | 44 | 1.00 (0.08) | 0.001 |
| Mean stride length, m | 44 | 1.45 (0.19) | 44 | 1.48 (0.18) * | 0.037 † | |
| Mean step width, m | 44 | 0.18 (0.05) | 44 | 0.18 (0.05) | 0.101 † | |
| SD stride time, s | 44 | 0.02 (0.01) | 44 | 0.02 (0.01) | 0.599 † | |
| SD stride length, m | 44 | 0.05 (0.02) | 44 | 0.04 (0.03) | 0.036 † | |
| SD step width, m | 44 | 0.02 (0.01) | 444 | 0.02 (0.001) | 0.916 † | |
| CoV stride time | 44 | 0.02 (0.01) * | 44 | 0.02 (0.01) * | 0.889 † | |
| CoV stride length | 44 | 0.03 (0.02) * | 44 | 0.03 (0.03) * | 0.024 † | |
| CoV step width | 44 | 0.15 (0.07) * | 44 | 0.15 (0.05) * | 0.363 † | |
| Sample entropy stride length | 44 | 1.17 (0.17) * | 44 | 1.21 (0.17) * | 0.037 † | |
| Sample entropy step width | 44 | 1.43 (0.04) * | 44 | 1.43 (0.05) * | 0.825 † | |
| LDE CoMvel-ML | 44 | 2.83 (0.17) | 44 | 2.77 (0.19) * | 0.031 † | |
| LDE CoMvel-V | 44 | 2.78 (0.14) | 44 | 2.79 (0.14) | 0.828 | |
| LDE CoMvel-AP | 44 | 2.75 (0.15) * | 44 | 2.70 (0.15) | 0.018 † | |
| Pre-Pulmonary Rehabilitation | Changes Following Pulmonary Rehabilitation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Good Responders | Poor Responders | Good Responders | Poor Responders | |||||||
| Parameter | n | value | n | value | p-value | n | value | n | value | p-value |
| Age, years | 24 | 61 (8) | 20 | 63 (7) | 0.331 | 24 | 0 (1) | 20 | 0 (2) | 0.611 |
| Gender M, % | 12 | 50 | 13 | 85 | ||||||
| BMI, kg/m2 | 24 | 28 (6) | 20 | 26 (5) | 0.172 | 24 | 0 (1) | 20 | 0 (2) | 0.611 |
| FEV1/FVC | 24 | 0.4 (0.1) | 20 | 0.4 (0.1) | 0.299 | 24 | 0.0 (0.1) * | 20 | 0.0 (0.1) * | 0.706 |
| FEV1% predicted | 24 | 60.2 (20.8) | 20 | 50.7 (17.5) | 0.075 | 24 | −2.1 (4.4) | 20 | −2.4 (5.2) | 0.852 |
| GOLD stage 1, % | 4 | 17 | 2 | 10 | 3 | 13 | 2 | 10 | ||
| GOLD stage 2, % | 10 | 42 | 8 | 40 | 13 | 54 | 7 | 35 | ||
| GOLD stage 3, % | 10 | 42 | 7 | 35 | 8 | 33 | 9 | 45 | ||
| GOLD stage 4, % | 0 | 0 | 3 | 15 | 0 | 0 | 2 | 10 | ||
| Total FFM, kg | 23 | 48.0 (10.9) | 20 | 47.6 (7.8) | 0.906 | 23 | 1.0 (1.6) | 20 | 0.7 (1.2) | 0.586 |
| Peak torque, N/m | 23 | 105 (39) * | 18 | 111 (31) | 0.358 † | 21 | 8 (12) | 17 | 8 (12) | 0.887 |
| Peak torque, % predicted | 23 | 72 (16) | 18 | 72 (14) | 0.916 | 21 | 7 (9) | 17 | 5 (7) | 0.544 |
| Total work, J | 23 | 1929 (641) | 18 | 2055 (613) | 0.526 | 21 | 279 (264) | 17 | 196 (193) | 0.289 |
| Work fatigue, % | 23 | 46 (11) | 18 | 41 (11) | 0.125 | 21 | −6 (11) | 17 | −1 (11) | 0.122 |
| TUG test, s | 18 | 8.6 (1.5) | 16 | 9.0 (1.2) | 0.366 | 18 | −0.3 (1.1) * | 16 | 0.2 (1.1) | 0.427 † |
| Overground 6MWD, m | 24 | 520 (61) | 20 | 503 (71) * | 0.137 † | 24 | 42 (49) | 20 | 0 (37) * | 0.005 † |
| Speed, m/s | 24 | 1.44 (0.17) | 20 | 1.40 (0.20) * | 0.137 † | 24 | 0.12 (0.14) | 20 | 0.00 (0.10) * | 0.005 † |
| SpO2 rest, % | 24 | 94 (2) | 20 | 95 (3) | 0.546 | 24 | 1 (2) | 20 | −1 (3) | 0.140 |
| SpO2 end, % | 24 | 88 (7) * | 20 | 87 (5) | 0.370 † | 24 | −2 (2) * | 20 | 0 (4) | 0.067 † |
| Heart rate rest, bpm | 24 | 83 (15) | 20 | 87 (12) | 0.358 | 24 | −4 (14) | 20 | −6 (12) * | 0.777 † |
| Heart rate end, bpm | 24 | 113 (19) | 20 | 116 (14) | 0.632 | 24 | 6 (16) | 20 | −3 (14) | 0.057 |
| Dyspnea rest | 24 | 1.0 (1.0) * | 20 | 1.4 (1.2) * | 0.289 † | 24 | −0.5 (0.9) | 20 | 0.2 (1.3) | 0.028 |
| Dyspnea end | 24 | 5.9 (1.8) | 20 | 5.3 (2.2) | 0.303 | 24 | −0.8 (1.9) | 20 | −0.2 (2.1) | 0.306 |
| Fatigue rest | 24 | 1.0 (1.1) * | 20 | 1.7 (1.8) * | 0.242 † | 24 | −0.3 (1.2) | 20 | −0.2 (1.8) | 0.884 |
| Fatigue end | 24 | 4.9 (2.4) | 20 | 5.1 (2.7) | 0.774 † | 24 | −0.7 (1.7) | 20 | −0.6 (2.2) | 0.797 |
| Pre-Pulmonary Rehabilitation | Changes Following Pulmonary Rehabilitation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Good Responders | Poor Responders | Good Responders | Poor Responders | ||||||||
| Test | Parameter | n | value | n | value | p-value | n | value | n | value | p-value |
| 6MWT | 6MWD, m | 24 | 505 (60) | 20 | 508 (91) | 0.901 | 24 | 66 (29) * | 20 | −12 (42) * | <0.001 † |
| Speed, m/s | 24 | 1.43 (0.15) | 20 | 1.42 (0.24) | 0.896 | 24 | 0.14 (0.19) * | 20 | −0.04 (0.15) * | <0.001 † | |
| SpO2 rest, % | 24 | 95 (2) | 20 | 96 (2) | 0.634 | 24 | 0 (2) | 20 | 0 (2) | 0.769 | |
| SpO2 end, % | 24 | 93 (6) * | 20 | 92 (4) | 0.203 † | 24 | 0 (5) | 20 | 1 (3) | 0.443 | |
| Heart rate rest, bpm | 24 | 84 (14) | 20 | 82 (16) | 0.858 | 24 | −4 (12) | 20 | −1 (15) | 0.461 | |
| Heart rate end, bpm | 24 | 104 (22) | 20 | 104 (17) | 0.960 | 24 | 0 (19) | 20 | −3 (12) * | 0.550 † | |
| Dyspnea rest | 24 | 1.0 (0.9) * | 20 | 1.5 (1.2) * | 0.347 † | 24 | −0.4 (1.0) | 20 | −0.1 (1.3) | 0.356 | |
| Dyspnea end | 24 | 5.5 (2.1) | 20 | 5.1 (2.4) | 0.538 | 24 | −0.7 (1.8) | 20 | −0.6 (2.4) | 0.896 | |
| Fatigue rest | 24 | 1.0 (0.9) * | 20 | 1.5 (1.4) * | 0.561 † | 24 | −0.4 (1.0) | 20 | 0.2 (1.4) | 0.128 | |
| Fatigue end | 24 | 4.6 (2.0) | 20 | 4.5 (2.6) | 0.910 | 24 | −0.2 (1.8) | 20 | −0.2 (2.2) * | 0.792 † | |
| Gait | Mean stride time, s | 24 | 1.00 (0.06) | 20 | 1.04 (0.09) | 0.105 | 24 | −0.05 (0.02) | 20 | 0.01 (0.04) | <0.001 † |
| Mean stride length, m | 24 | 1.43 (0.15) * | 20 | 1.48 (0.22) | 0.621 | 24 | 0.09 (0.08) | 20 | −0.04 (0.08) * | <0.001 † | |
| Mean step width, m | 24 | 0.17 (0.05) | 20 | 0.19 (0.04) * | <0.001 † | 24 | 0.00 (0.02) | 20 | −0.01 (0.03) | 0.136 | |
| SD stride time, s | 24 | 0.02 (0.01) | 20 | 0.02 (0.01) | 0.203 † | 24 | 0.00 (0.01) | 20 | 0.00 (0.00) | 0.193 | |
| SD stride length, m | 24 | 0.05 (0.02) | 20 | 0.05 (0.01) | 0.759 † | 24 | 0.01 (0.02) | 20 | 0.00 (0.03) | 0.109 † | |
| SD step width, m | 24 | 0.02 (0.01) | 20 | 0.03 (0.01) | 0.155 † | 24 | 0.00 (0.01) | 20 | 0.00 (0.01) | 0.346 † | |
| CoV stride time | 24 | 0.02 (0.01) * | 20 | 0.02 (0.01) * | 0.203 † | 24 | 0.00 (0.01) | 20 | 0.00 (0.00) | 0.424 | |
| CoV stride length | 24 | 0.04 (0.02) * | 20 | 0.03 (0.01) * | 0.588 † | 24 | −0.01 (0.02) * | 20 | 0.01 (0.04) * | 0.017 † | |
| CoV step width | 24 | 0.15 (0.08) * | 20 | 0.14 (0.05) | 0.541 † | 24 | 0.00 (0.04) | 20 | 0.00 (0.05) | 0.744 | |
| Sample entropy stride length | 24 | 1.14 (0.19) | 20 | 1.20 (0.13) | 0.243 | 24 | 0.09 (0.19) | 20 | −0.01 (0.16) * | 0.131 † | |
| Sample entropy step width | 24 | 1.43 (0.05) | 20 | 1.43 (0.03) * | 0.540 † | 24 | 0.01 (0.07) | 20 | −0.02 (0.06) * | 0.144 † | |
| LDE CoMvel-ML | 24 | 2.84 (0.18) | 20 | 2.82 (0.15) | 0.734 | 24 | −0.06 (0.16) | 20 | −0.06 (0.18) * | 0.869 † | |
| LDE CoMvel-V | 24 | 2.80 (0.14) | 20 | 2.76 (0.14) | 0.417 | 24 | 0.04 (0.17) | 20 | −0.04 (0.14) | 0.126 | |
| LDE CoMvel-AP | 24 | 2.76 (0.15) * | 20 | 2.74 (0.15) | 0.671 † | 24 | −0.07 (0.13) * | 20 | −0.02 (0.13) | 0.195 † | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-Y.; Meijer, K.; Delbressine, J.M.; Willems, P.J.; Wouters, E.F.M.; Spruit, M.A. Effects of Pulmonary Rehabilitation on Gait Characteristics in Patients with COPD. J. Clin. Med. 2019, 8, 459. https://doi.org/10.3390/jcm8040459
Liu W-Y, Meijer K, Delbressine JM, Willems PJ, Wouters EFM, Spruit MA. Effects of Pulmonary Rehabilitation on Gait Characteristics in Patients with COPD. Journal of Clinical Medicine. 2019; 8(4):459. https://doi.org/10.3390/jcm8040459
Chicago/Turabian StyleLiu, Wai-Yan, Kenneth Meijer, Jeannet M. Delbressine, Paul J. Willems, Emiel F. M. Wouters, and Martijn A. Spruit. 2019. "Effects of Pulmonary Rehabilitation on Gait Characteristics in Patients with COPD" Journal of Clinical Medicine 8, no. 4: 459. https://doi.org/10.3390/jcm8040459
APA StyleLiu, W.-Y., Meijer, K., Delbressine, J. M., Willems, P. J., Wouters, E. F. M., & Spruit, M. A. (2019). Effects of Pulmonary Rehabilitation on Gait Characteristics in Patients with COPD. Journal of Clinical Medicine, 8(4), 459. https://doi.org/10.3390/jcm8040459





