Paraoxonase 1 Activity, Polymorphism and Atherosclerosis Risk Factors in Patients Undergoing Coronary Artery Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. DNA Extraction and Analysis
2.2. Paraoxonase Activity
2.3. Lipid Profile
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
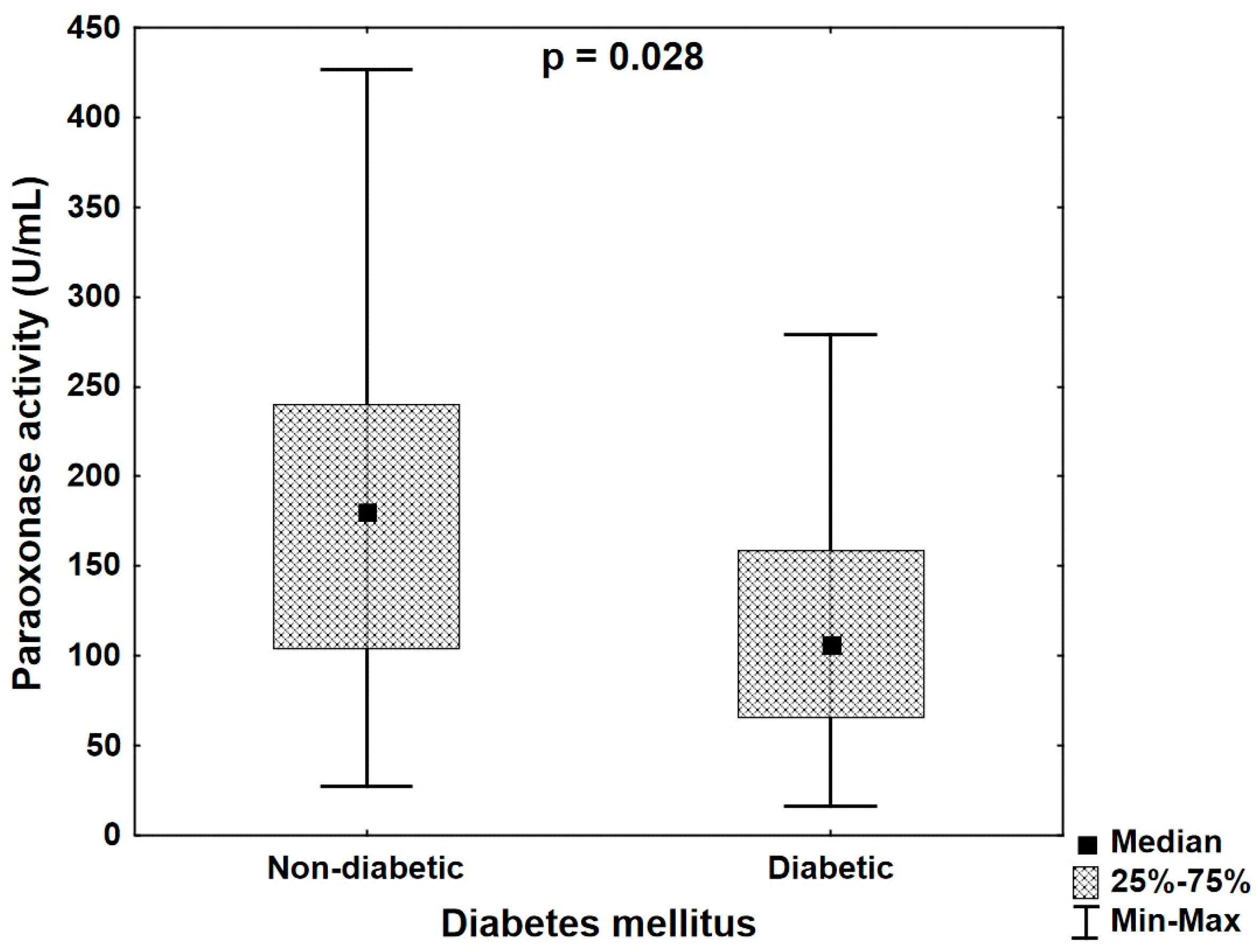
- In diabetic patients with CHD, paraoxonase activity is lower than in normoglycemic patients despite similar lipid profiles.
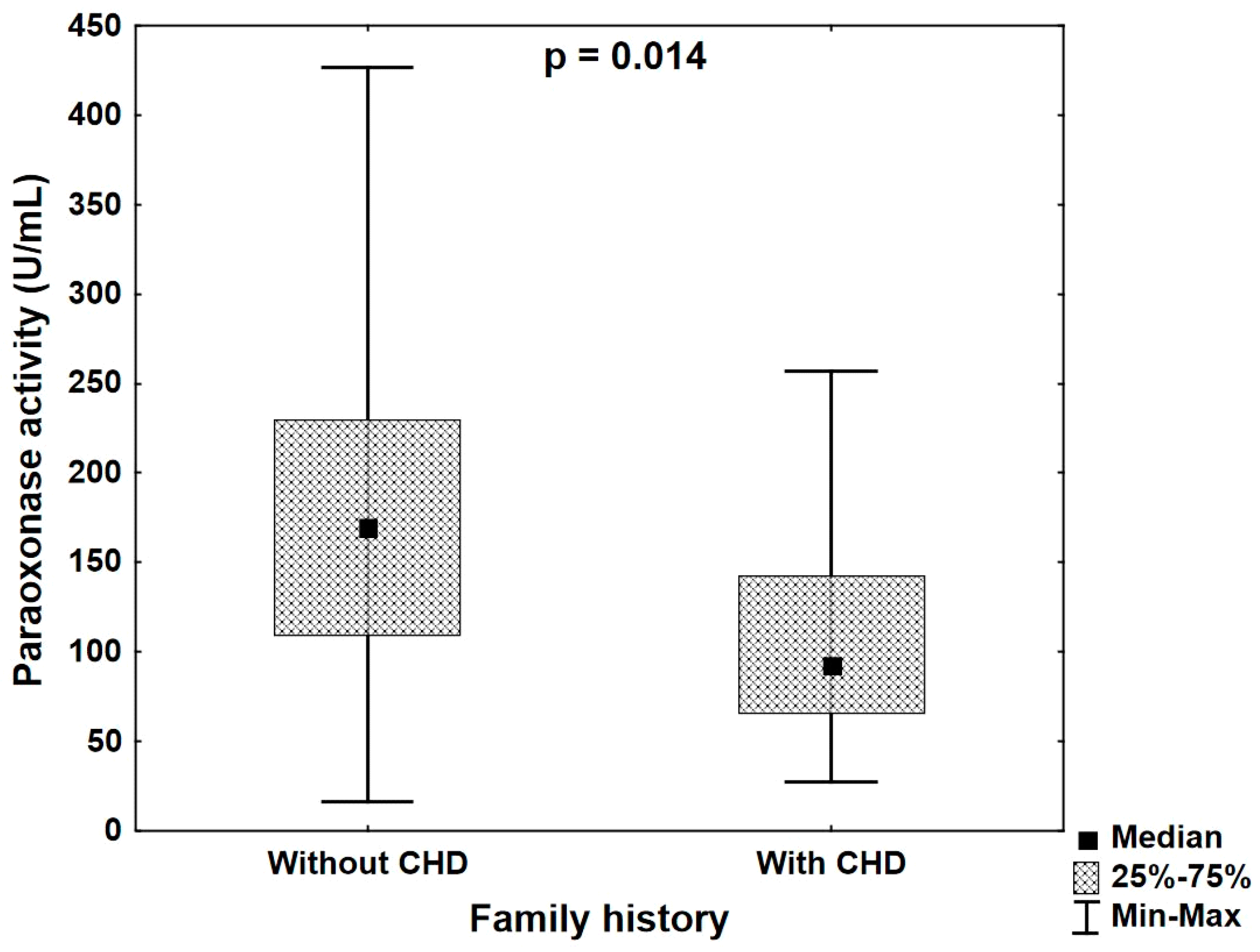
- Diabetes and positive family history in patients with overt CHD are associated with the serum PON1 activity, which might be an additional factor helpful in evaluating cardiovascular risk in this group of patients.
Author Contributions
Funding
Conflicts of Interest
References
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and other Societies on Cardiovascular Disease Prevention in Clinical Practic (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention& Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of coronary heart disease and lipoprotein cholesterol levels: The Framingham Study. JAMA 1986, 256, 2835–2838. [Google Scholar] [CrossRef] [PubMed]
- Rye, K.A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–169. [Google Scholar] [CrossRef]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease; atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011, 8, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high- density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Primo-Parma, S.L.; Sorenson, R.C.; Teiber, J.; La Du, B.N. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics 1996, 33, 498–509. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S.; Primo-Parmo, S.L.; La Du, B.N. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Investig. 1998, 101, 1581–1590. [Google Scholar] [CrossRef]
- Draganov, D.J.; Teiber, J.F.; Speelman, A.; Osawa, Y.; Sunhara, R.; La Du, B.N. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specifies. J. Lip. Res. 2005, 46, 1239–1247. [Google Scholar] [CrossRef]
- Yildiz, A.; Gur, M.; Yilmaz, R.; Demirbag, R.; Polat, M.; Selek, S. Association of paraoxonase activity and coronary blood flow. Atherosclerosis 2008, 197, 257–263. [Google Scholar] [CrossRef]
- Tang, W.H.; Hartiala, J.; Fan, Y.; Wu, Y.; Stewart, A.F.; Erdmann, J. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler. ThrombVasc. Biol. 2012, 32, 2803–2812. [Google Scholar] [CrossRef]
- Ding, J.; Chen, Q.; Zhuang, X.; Feng, Z.; Xu, L.; Chen, F. Low Paraoxonase 1 Arylesterase Activity and High von Willebrand Factor Levels are Associated with Severe Coronary Atherosclerosis in Patients with Non-Diabetic Stable Coronary Artery Disease. Med. SciMonit. 2014, 20, 2421–2429. [Google Scholar]
- Wysocka, A.; Cybulski, M.; Berbeć, H.; Wysokiński, A.; Stążka, J.; Zapolski, T. Prognostic value of paraoxonase 1 in patients undergoing coronary artery bypass grafting surgery. Med. Sci. Monit. 2014, 20, 594–600. [Google Scholar]
- Sanghera, D.K.; Aston, C.E.; Saha, N.; Kamboh, M.I. DNA polymorphisms in two paraoxonase genes (PON1 and PON2) are associated with the risk of coronary heart disease. Am. J. Hum. Genet. 1998, 62, 36–44. [Google Scholar] [CrossRef]
- Sanghera, D.K.; Saha, N.; Aston, C.E.; Kamboh, M.I. Genetic polymorphism of paraoxonase and the risk of coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1067–1073. [Google Scholar] [CrossRef]
- Antikainen, M.; Murtomki, S.; Syvnne, M.; Pahlman, R.; Tahvanainen, E.; Jauhiainen, M.; Frick, M.H.; Ehnholm, C. The Gln-Arg polymorphism of human paraoxonase gene (HUMPONA) is not associated with the risk of coronary artery disease in Finns. J. Clin. Investig. 1996, 191, 883–885. [Google Scholar] [CrossRef]
- Ombres, D.; Pannitteri, G.; Moutali, A.; Candeloro, A.; Seccareccia, F.; Campagna, F.; Cantini, R.; Campa, P.P.; Ricci, G.; Arca, M. The Gln-Arg 192 polymorphism of the human paraoxonase gene is not associated with coronary artery disease in Italian patients. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1611–1616. [Google Scholar] [CrossRef]
- Schmidt, H.; Schmidt, R.; Niederkorn, K.; Gradert, G.A.; Schumacher, M.; Watzinger, N.; Hartung, H.P.; Kostner, G.M. Paraoxonase PON1 polymorphism Leu-Met54 is associated with carotid atherosclerosis: Results of the Austrian Stroke Prevention Study. Stroke 1998, 29, 2043–2048. [Google Scholar] [CrossRef]
- Watzinger, N.; Schmidt, H.; Schumacher, M.; Schmidt, R.; Eber, B.; Fruhwald, F.M.; Zweiker, R.; Kostner, G.M.; Klein, W. Human paraoxonase 1 gene polymorphisms and the risk of coronary heart disease: A community-based study. Cardiology 2002, 98, 116–122. [Google Scholar] [CrossRef]
- Sanghera, D.K.; Saha, N.; Kamboh, M.I. The codon 55 polymorphism of the paraoxonase 1 gene is not associated with risk of coronary heart disease in Asian Indians and Chinese. Atherosclerosis 1998, 136, 217–223. [Google Scholar] [CrossRef]
- Chen, M.L.; Zhao, H.; Liao, N.; Xie, Z.F. Association between paraoxonase 2 Ser311 Cys polymorphism and Coronary Heart Disease Risk: A meta-analysis. Med. Sci. Monit. 2016, 22, 3196–3201. [Google Scholar] [CrossRef]
- Wheeler, J.G.; Keavney, B.D.; Watkins, H.; Collins, R.; Danesh, J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: Meta-analysis of 43 studies. Lancet 2004, 363, 689–695. [Google Scholar] [CrossRef]
- Robertson, K.S.; Hawe, E.; Miller, G.J.; Talmud, P.J.; Humphries, S.E. Human paraoxonase gene cluster polymorphism as a predictors of coronary heart disease risk in the prospective Northwick Park Heart Study, I.I. Biochim. Biophys. (BBA)/Mol. Basis Dis. 2003, 1639, 203–212. [Google Scholar] [CrossRef]
- Mackness, B.; Turkie, W.; Mackness, M. Paraoxonase-1 (PON1) promoter region polymorphisms serum PON1 status and coronary heart disease. Arch. Med. Sci. 2013, 9, 8–13. [Google Scholar] [CrossRef]
- Mackness, B.; Davies, G.K.; Turkie, W.; Lee, E.; Roberts, D.H.; Hill, E.; Roberts, C.; Durrington, P.N.; Mackness, M.I. Paraoxonase status in coronary heart disease: Are activity and concentration more important than genotype? Arterioscler. ThrombVasc. Biol. 2001, 21, 1451–1457. [Google Scholar] [CrossRef]
- Brophy, V.H.; Jampsa, R.L.; Clendening, J.B.; McKinstry, L.A.; Jarvik, G.P.; Furlong, C.E. Effects of 5′ regulatory-region polymorphisms on paraoxonase-gene (PON1) expression. Am. J. Hum. Genet. 2001, 68, 1428–1436. [Google Scholar] [CrossRef]
- Eckerson, H.; Romson, W.J.; Wyte, C.; La Du, B. The human serum paraoxonase polymorphism: Identification of phenothypes by their response to salts. Am. J. Hum. Genet. 1983, 35, 214–217. [Google Scholar]
- Fuhrman, B.; Volkova, N.; Aviram, M. Paraoxonase 1 (PON1) is present in postprandial chylomicrons. Atherosclerosis 2005, 180, 55–61. [Google Scholar] [CrossRef]
- Saha, N.; Roy, A.C.; Teo, S.H.; Tay, J.S.; Ratnam, S.S. Influence of serum paraoxonase polymorphism on serum lipids and apolipoproteins. Clin. Genet. 1991, 40, 277–282. [Google Scholar] [CrossRef]
- Rivera-Mancia, S.; Jimenez-Osorio, A.S.; Medina-Campos, O.N.; Colin-Ramirez, E.; Vallejo, M.; Alcantara-Gaspar, A.; Cartas-Rosado, R.; Vargas-Barron, J.; Perdraza-Chaveri, J. Acitivity of antioxidant enzymes and their association with lipid profile in Mexican people without cardiovascular disease: An analysis of interactions. Int. J. Environ. Res. Public Health 2018, 15, 2687. [Google Scholar] [CrossRef]
- Karakaya, A.; Ibis, S.; Kural, T.; Kose, S.K.; Karakaya, A.E. Serum paraoxonase activity and phenotype distribution in Turkish subjects with coronary heart disease and its relationship to serum lipids and lipoproteins. Chem. Biol. Interact. 1999, 118, 193–200. [Google Scholar] [CrossRef]
- Kabaroglu, C.; Mutaf, I.; Boydak, B.; Ozmen, D.; Habif, S.; Erdener, D.; Parildar, Z.; Bayindir, O. Association between serum paraoxonase activity and oxidative stress in acute coronary syndromes. Acta Cardiol. 2004, 59, 606–611. [Google Scholar] [CrossRef]
- Tomas, M.; Senti, M.; Garcia-Faria, F.; Vila, J.; Torrents, A.; Covas, M.J. Effect of simvastatin therapy on paraoxonase activity and related lipoproteins in familial hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2113–2119. [Google Scholar] [CrossRef]
- Fuhrman, B.; Koren, L.; Volkova, N.; Keidar, S.; Hayek, T.; Aviram, M. Atorvastatin therapy in hypercholesterolemic patients suppresses cellular uptake of oxidized-LDL by differentiating monocytes. Atherosclerosis 2002, 164, 179–185. [Google Scholar] [CrossRef]
- Gouedard, C.; Koum-Besson, N.; Barouki, R.; Morel, Y. Opposite regulation of the human paraoxonase-1 gene PON-1 by fenofibrate and statins. Mol. Pharmacol. 2003, 63, 945–956. [Google Scholar] [CrossRef]
- Ferreti, G.; Bacchetti, T.; Sahebkar, A. Effect of statin theray on paraoxonase -1 status: A systematic review and meta-analysis of 25 clinical trials. Prog. Lipid Res. 2015, 60, 50–57. [Google Scholar] [CrossRef]
- Abd Elgwad, E.R.; Behiry, E.G.; Swailem, F.M.; Ameen, S.G.; Abdelhasib, D.M.; Abd Elhamid, R.O. Association between Q192R polymorphism in the PON1 gene and statin responses in cardiac patients. Ann. Med. Surg. (Lond.) 2018, 31, 1–5. [Google Scholar] [CrossRef]
- Latellier, C.; Durou, M.R.; Jouanolle, A.M.; Le Gall, J.Y.; Poitrier, J.Y.; Ruelland, A. Serum paraoxonase activity and paraoxonase gene polymorphism in type 2 diabetic patients with or without vascular complications. Diabetes Metab. 2002, 28, 297–304. [Google Scholar]
- Shen, Y.; Ding, F.H.; Sun, J.T.; Zhang, R.Y.; Zhang, Q.; Chen, Q.J.; Shen, W.F.; Lu, L. Association of elevated apoA- I glycation and reduced HDL—Associated paraoxonase 1,3 activity, and their interaction with angiographic severity of coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2015, 14, 52. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Thorpe, S.R.; Fu, M.X.; Harper, C.M.; Yoo, J.; Kim, S.M.; Wong, H.; Peters, L. Glycation impairs high density lipoprotein function. Diabetologia 2000, 43, 312–320. [Google Scholar] [CrossRef]
- Leview, I.; James, R.W. Promoter polymorphism of human paraoxonase PON1 gene and serum paraoxonase activities and concentrations. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 516–521. [Google Scholar] [CrossRef]
- Lu, J.Q.; Ren, H.; Liu, M.Z.; Fang, P.F.; Xiang, D.X. European versus Asian differences for the associatcion between paraoxonase-1 genetic polymorphisms and susceptibility to type 2 diabetes mellitus. J. Cell. Mol. Med. 2018, 22, 1720–1732. [Google Scholar] [CrossRef]
- Aviram, M.; Billecke, S.; Sorenson, R.; Bisaiger, C.; Newton, R.; Rosenblat, M.; Erogul, J.; Hsu, C.; Dunlop, C.; LaDu, B. Paraoxonase active site is required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities. Selective action of human arylesterase/paraoxonase activities. Arteriocler. Thromb. Vasc. Biol. 1998, 18, 1617–1624. [Google Scholar] [CrossRef]
- Boemi, M.; Leviev, I.; Sirolla, C.; Pieri, C.; Marra, M.; James, R.W. Serum paraoxonase is reduced in type 1 diabetic patients compared to non-diabetic, first degree relatives; influence on the ability of HDL to protect LDL from oxidation. Atherosclerosis 2001, 155, 229–235. [Google Scholar] [CrossRef]
- Passaro, A.; Vigna, G.B.; Romani, A.; Sanz, J.M.; Cavvichio, C.; Bonaccorsi, G.; Valacchi, G.; Cervello, C. Distribution of paraoxonase (PON-1) and lipoprotein Phospholipase A2(Lp-PLA2) across lipoprotein subclasses in Subjects with type 2 diabetes. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Haffner, S.M.; Solomon, C.G.; Willet, W.C.; Manson, J.E. Elevated risk of cardiovascular disease prior to clinical diagnosis of type. Diabetes Care 2002, 25, 1129–3436. [Google Scholar] [CrossRef]
- Uzun, H.; Zengin, K.; Taskin, M.; Aydin, S.; Simsek, G.; Dariyerli, N. Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Obes. Surg. 2004, 14, 659–665. [Google Scholar] [CrossRef]
- Bełtowski, J.; Wojcicka, G.; Jamroz, A. Leptindecreasesplasmaparaoxonase 1 (PON1) activity and inducesoxidativestress: The possiblenovelmechanism for proatherogeniceffect of chronichyperleptinemia. Atherosclerosis 2003, 170, 21–29. [Google Scholar]
- Deakin, S.; Leviev, I.; BrulhartMeynet, M.C. Paraoxonase-1 promoter haplotypes and serum paraoxonase: A predominant role in vivo for polymorphic position-107 implicating the transcription factor Sp1. Bioch. J. 2003, 372, 643–649. [Google Scholar] [CrossRef]
- Li, Y.; Liang, G.; Shi, L.; Liang, X.; Long, B.; Qin, J.; Zhang, Z. Paraoxonase-1 (PON1) rs662 Polymorphism and Its Association with Serum Lipid Levels and Longevity in the Bama Zhuang Population. Med. Sci. Monit. 2016, 22, 5154–5162. [Google Scholar] [CrossRef] [PubMed]
| Group of Patients (n = 71) | |
|---|---|
| Men (n/%) | 52/73.24 |
| Women (n/%) | 19/26.76 |
| Age (years ± SD) | 61.09 ± 8.98 |
| Total cholesterol level (mg/dL ± SD) | 197.86 ± 47.29 |
| LDL cholesterol (mg/dL ± SD) | 122.02 ± 39.43 |
| HDL cholesterol (mg/dL ± SD) | 45.48 ± 13.62 |
| Triglycerides (mg/dL ± SD) | 155.95 ± 107.18 |
| Body mass index (±SD) | 27.96 ± 4.39 |
| Smoking (n/%) | 19/26.76 |
| Hypertension (n/%) | 46/64.79 |
| Previous myocardial infarct (n/%) | 36/50.70 |
| Coronary heart disease in family history (n/%) | 10/14.08 |
| Diabetes (n/%) | 14/19.72 |
| Lipid lowering drugs | 51/71.08 |
| - statins | 49/69.01 |
| - fibrates | 2/2.82 |
| R/p | Age | Diabetes Mellitus | Smoking | Family History | BMI | |
|---|---|---|---|---|---|---|
| Paraoxonase activity (U/mL) | R | 0.020 | −0.264 | −0.174 | −0.293 | −0.100 |
| p | 0.875 | 0.026 | 0.147 | 0.013 | 0.416 | |
| Arylesterase activity (U/mL) | R | 0.087 | 0.150 | −0.144 | −0.020 | 0.125 |
| p | 0.484 | 0.211 | 0.230 | 0.870 | 0.300 |
| R/p | Total Cholesterol Level (mg/dL) | HDL Level (mg/dL) | LDL Level (mg/dL) | Triglycerides Level (mg/dL) | Total Cholesterol/HDL Index | |
|---|---|---|---|---|---|---|
| Paraoxonase activity (U/mL) | R | −0.130 | −0.054 | −0.130 | 0.110 | −0.042 |
| p | 0.281 | 0.657 | 0.281 | 0.361 | 0.728 | |
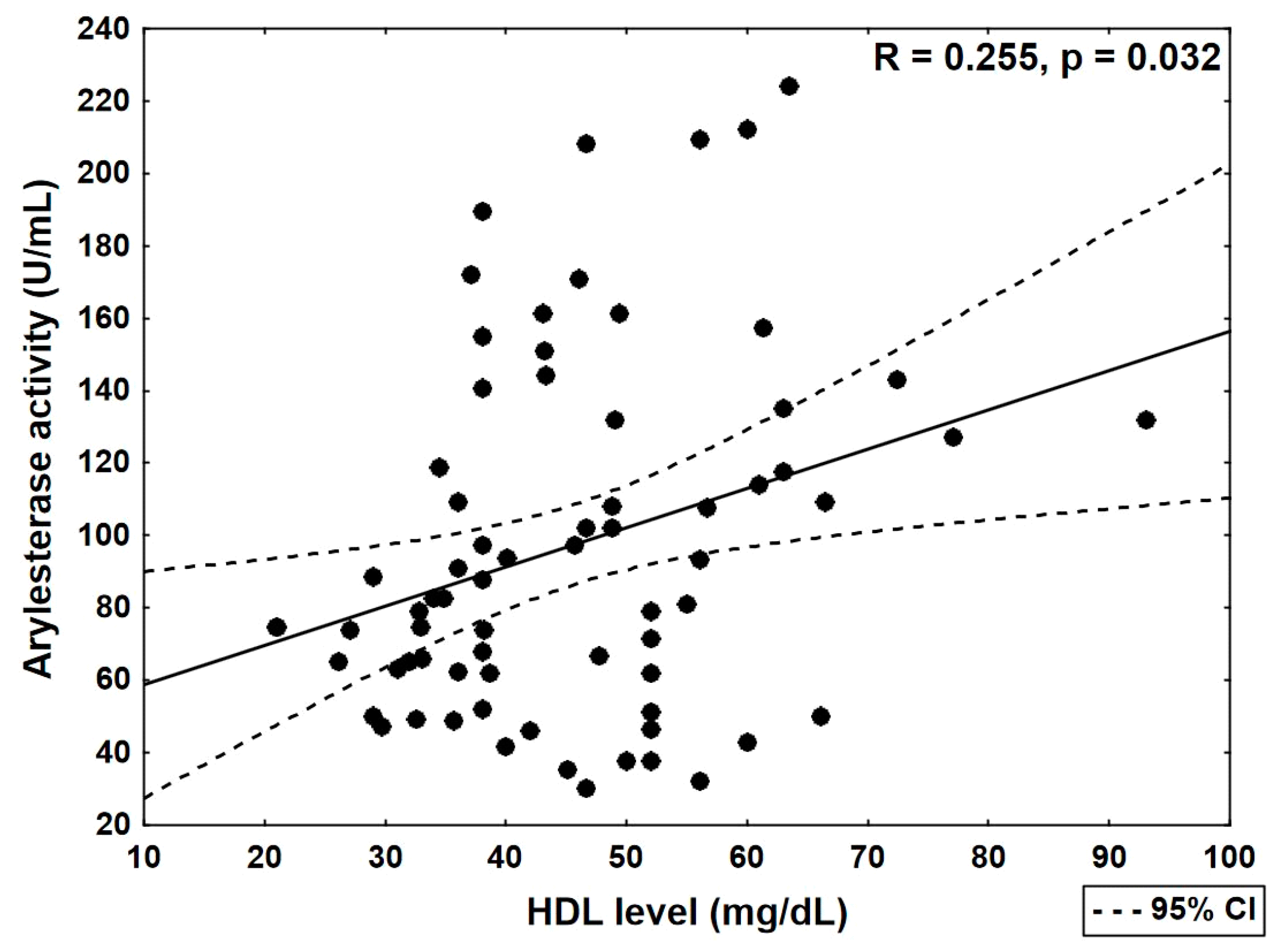
| Arylesterase activity (U/mL) | R | 0.171 | 0.255 | 0.161 | −0.016 | −0.118 |
| p | 0.153 | 0.032 | 0.180 | 0.895 | 0.328 |
| Patients Treated with Statins (n = 49) | Patients Untreated with Statins (n = 22) | p | |
|---|---|---|---|
| Total cholesterol (mg/dL ± SD) | 195,265 ± 49,211 | 201,227 ± 41,300 | 0.546 |
| HDL (mg/dL ± SD) | 46,133 ± 13,393 | 43,745 ± 13,788 | 0.398 |
| LDL (mg/dL) | 115,461 ± 35,270 | 135,518 ± 43,545 | 0.069 |
| Triglycerides (mg/dL ± SD) | 174,071 ± 117,520 | 109,818 ± 48,735 | 0.008 |
| Total cholesterol/HDL index ± SD | 4525 ± 1646 | 5036 ± 1924 | 0.317 |
| PON1 paraoxonase activity (U/mL ± SD) | 183,980 ± 90,846 | 134,443 ± 76,330 | 0.033 |
| PON1 arylesterase activity (U/mL ± SD) | 99,026 ± 54,013 | 93,122 ± 34,562 | 0.917 |
| p | Odds Ratio | |
|---|---|---|
| HDL-C level | 0.825 | 0.993 |
| LDL-C level | 0.058 | 0.978 |
| TG level | 0.884 | 1.000 |
| PON1 paraoxonase activity | 0.024 | 0.985 |
| PON1 arylesterase activity | 0.348 | 1.008 |
| Family history of cardiac incidents | 0.988 | 1.210 |
| CC + CT genotype | 0.887 | 1.246 |
| TT genotype | 0.904 | 1.112 |
| Previous myocardial infarction | 0.031 | 5.314 |
| Sex | 0.871 | 0.850 |
| p | Odds Ratio | |
|---|---|---|
| HDL-C level | 0.056 | 1,027 |
| LDL-C level | 0.129 | 0.981 |
| TG level | 0.689 | 1.001 |
| PON1 araoxonase activity | 0.027 | 0.983 |
| PON1 arylesterase activity | 0.428 | 0.992 |
| Diabetes mellitus | 0.397 | 2.597 |
| CC + CT genotype | 0.999 | 0.000 |
| TT genotype | 0.521 | 1.899 |
| Previous myocardial infarct | 0.805 | 1.263 |
| Sex | 0.864 | 0.850 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocka, A.; Cybulski, M.; Wysokiński, A.P.; Berbeć, H.; Stążka, J.; Zapolski, T. Paraoxonase 1 Activity, Polymorphism and Atherosclerosis Risk Factors in Patients Undergoing Coronary Artery Surgery. J. Clin. Med. 2019, 8, 441. https://doi.org/10.3390/jcm8040441
Wysocka A, Cybulski M, Wysokiński AP, Berbeć H, Stążka J, Zapolski T. Paraoxonase 1 Activity, Polymorphism and Atherosclerosis Risk Factors in Patients Undergoing Coronary Artery Surgery. Journal of Clinical Medicine. 2019; 8(4):441. https://doi.org/10.3390/jcm8040441
Chicago/Turabian StyleWysocka, Anna, Marek Cybulski, Andrzej P. Wysokiński, Henryk Berbeć, Janusz Stążka, and Tomasz Zapolski. 2019. "Paraoxonase 1 Activity, Polymorphism and Atherosclerosis Risk Factors in Patients Undergoing Coronary Artery Surgery" Journal of Clinical Medicine 8, no. 4: 441. https://doi.org/10.3390/jcm8040441
APA StyleWysocka, A., Cybulski, M., Wysokiński, A. P., Berbeć, H., Stążka, J., & Zapolski, T. (2019). Paraoxonase 1 Activity, Polymorphism and Atherosclerosis Risk Factors in Patients Undergoing Coronary Artery Surgery. Journal of Clinical Medicine, 8(4), 441. https://doi.org/10.3390/jcm8040441





