Mechanical and Biological Advantages of a Tri-Oval Implant Design
Abstract
1. Introduction
2. Materials and methods
2.1. Implant Design
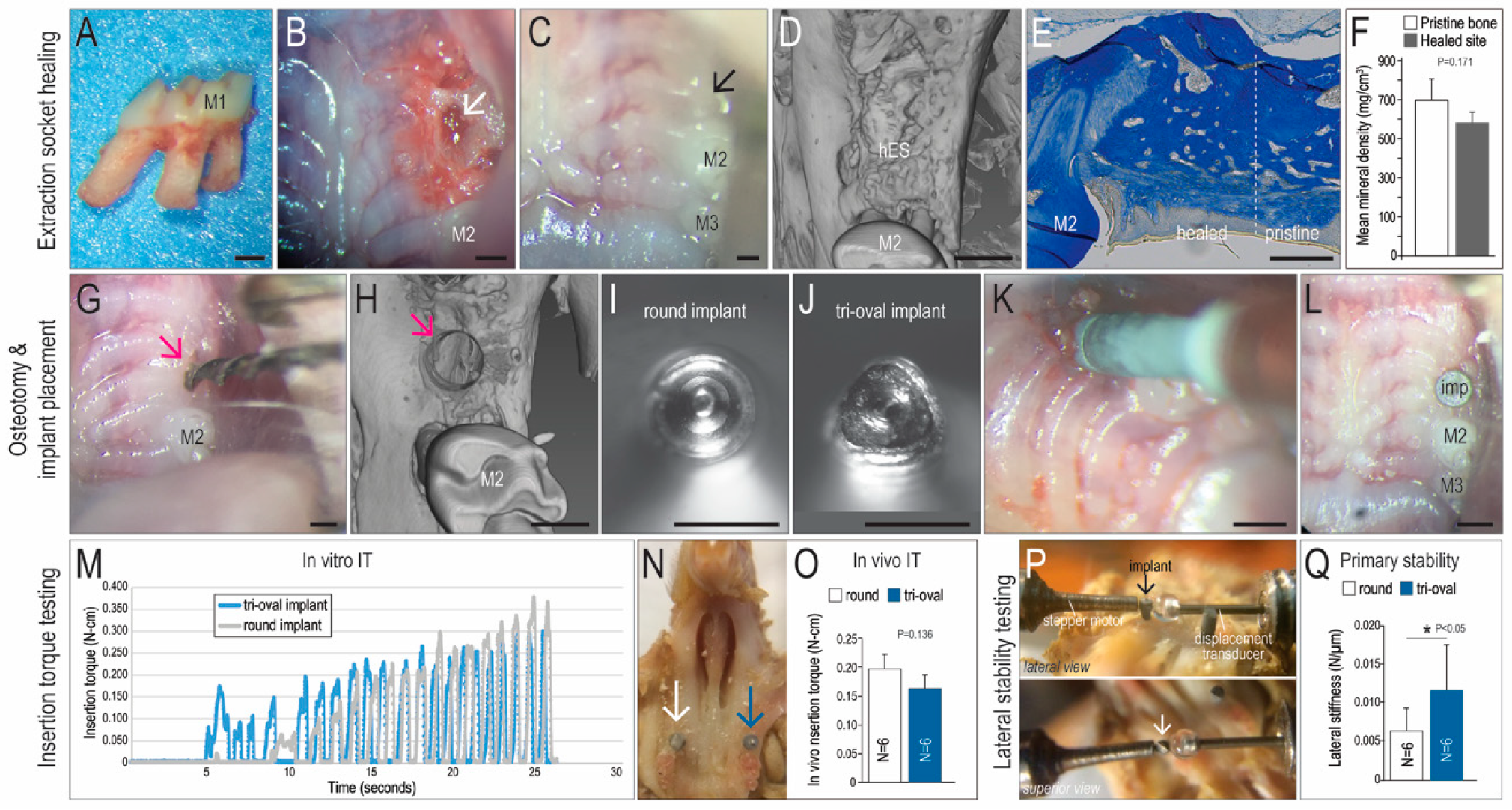
2.2. Animals and Tooth Extraction Surgeries
2.3. Implant Placement, Osteotomy Site Preparation, and Experimental Groups
2.4. Implant Insertion Torque Measurement
2.5. Lateral Stability Testing, Finite Element Modeling, and Calculation of Elastic Modulus
2.6. Calculating Elastic Modulus of Peri-Implant Bone as a Function of Lateral Stability
2.7. Sample Preparation, Tissue Processing, and Histology
2.8. Histomorphometry
2.9. TUNEL Staining, Alkaline Phosphatase Activity, and Tartrate Resistant Acid Phosphatase Activity
2.10. Micro-CT Imaging
2.11. Statistical Analyses
3. Results
3.1. Tri-oval Implants Exhibit Higher Primary Stability Compared to Round Implants
3.2. The Maxima of a Tri-Oval Implant Provide Higher Stability
3.3. Tri-oval Implants Exhibit Less Bone Resorption, which Allows them to Maintain their Stability Over Time
3.4. Tri-Oval Implants Exhibit Superior Osseointegration Compared to Conventional Round Implants
3.5. The Magnitude of Interfacial Strain is a Key Influence on Whether an Implant will Undergo Fibrous Encapsulation or Osseointegration
4. Discussion
4.1. The Maxima of a Tri-Oval Implant aid in Mechanical Stability
4.2. The Minima of a Tri-Oval Implant Create a Pro-Osteogenic Environment
4.3. Clinical Implications of this Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wennerberg, A.; Albrektsson, T.; Chrcanovic, B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018, 11 (Suppl. 1), S123–S136. [Google Scholar]
- Gurzawska, K.; Dirscherl, K.; Jorgensen, B.; Berglundh, T.; Jorgensen, N.R.; Gotfredsen, K. Pectin nanocoating of titanium implant surfaces—An experimental study in rabbits. Clin. Oral Implants Res. 2017, 28, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; de Rycker, J.; Chaudhari, A.; Coutinho, E.; Yoshida, Y.; Van Meerbeek, B.; Mesquita, M.F.; da Silva, W.J.; Yoshihara, K.; Vandamme, K.; et al. Titanium implant functionalization with phosphate-containing polymers may favour in vivo osseointegration. J. Clin. Periodontol. 2017, 44, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; Chaudhari, A.; Yoshihara, K.; Mesquita, M.F.; Yoshida, Y.; Van Meerbeek, B.; Vandamme, K.; Duyck, J. Phosphorylated Pullulan Coating Enhances Titanium Implant Osseointegration in a Pig Model. Int. J. Oral Maxillofac. Implants 2017, 32, 282–290. [Google Scholar] [CrossRef]
- Becker, W.; Hujoel, P.; Becker, B.E.; Wohrle, P. Survival rates and bone level changes around porous oxide-coated implants (TiUnite). Clin. Implants Dent. Relat. Res. 2013, 15, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindstrom, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Pereira, M.D.; Smith, A.A.; Houschyar, K.S.; Yin, X.; Mouraret, S.; Brunski, J.B.; Helms, J.A. Multiscale analyses of the bone-implant interface. J. Dent. Res. 2015, 94, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Perrotti, V.; Strocchi, R.; Piattelli, A.; Iezzi, G. Is insertion torque correlated to bone-implant contact percentage in the early healing period? A histological and histomorphometrical evaluation of 17 human-retrieved dental implants. Clin. Oral Implants Res. 2009, 20, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N.; Book, K.; Friberg, B.; Jemt, T.; Sennerby, L. Resonance frequency measurements of implant stability in vivo. A cross-sectional and longitudinal study of resonance frequency measurements on implants in the edentulous and partially dentate maxilla. Clin. Oral Implants Res. 1997, 8, 226–233. [Google Scholar] [CrossRef]
- Wazen, R.M.; Currey, J.A.; Guo, H.; Brunski, J.B.; Helms, J.A.; Nanci, A. Micromotion-induced strain fields influence early stages of repair at bone-implant interfaces. Acta Biomater. 2013, 9, 6663–6674. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implants Res. 2003, 14, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Futami, T.; Fujii, N.; Ohnishi, H.; Taguchi, N.; Kusakari, H.; Ohshima, H.; Maeda, T. Tissue response to titanium implants in the rat maxilla: Ultrastructural and histochemical observations of the bone-titanium interface. J. Periodontol. 2000, 71, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, P.M.; Cook, S.D.; Spires, W.P.; Kester, M.A. Tissue response to porous-coated implants lacking initial bone apposition. J. Arthroplast. 1988, 3, 337–346. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Perez, K.C.; Hyman, S.; Brunski, J.B.; Tulu, U.; Bao, C.; Salmon, B.; Helms, J.A. Effects of Condensation on Peri-implant Bone Density and Remodeling. J. Dent. Res. 2017, 96, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.R.; Valstar, E.R.; Rozing, P.M.; van Keulen, F. Fracture risk and initial fixation of a cementless glenoid implant: The effect of numbers and types of screws. Proc. Inst. Mech. Eng. H J. Eng. Med. 2013, 227, 1058–1066. [Google Scholar] [CrossRef]
- Carlsson, L.; Engman, F.; Fromell, R.; Jörneus, L. Threaded Implant for Obtaining Reliable Anchoring in Bone. EP 1 030 622 B2, 14 May 2014. [Google Scholar]
- Reams, J.W.; Goodman, R.E.; Rogers, D.P. Reduced Friction Screw-Type Dental Implant. US5902109, 11 May 1999. [Google Scholar]
- Torcasio, A.; Zhang, X.; Van Oosterwyck, H.; Duyck, J.; van Lenthe, G.H. Use of micro-CT-based finite element analysis to accurately quantify peri-implant bone strains: A validation in rat tibiae. Biomech. Model. Mechanobiol. 2012, 11, 743–750. [Google Scholar] [CrossRef]
- Leucht, P.; Kim, J.B.; Wazen, R.; Currey, J.A.; Nanci, A.; Brunski, J.B.; Helms, J.A. Effect of mechanical stimuli on skeletal regeneration around implants. Bone 2007, 40, 919–930. [Google Scholar] [CrossRef]
- Leucht, P.; Monica, S.D.; Temiyasathit, S.; Lenton, K.; Manu, A.; Longaker, M.T.; Jacobs, C.R.; Spilker, R.L.; Guo, H.; Brunski, J.B.; et al. Primary cilia act as mechanosensors during bone healing around an implant. Med. Eng. Phys. 2012, 35, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, X.; Huang, L.; Mouraret, S.; Brunski, J.B.; Cordova, L.; Salmon, B.; Helms, J.A. Relationships among Bone Quality, Implant Osseointegration, and Wnt Signaling. J. Dent. Res. 2017, 96, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wang, L.; Chen, C.; Yuan, X.; Wan, Q.; Helms, J.A. Contribution of the PDL to Osteotomy Repair and Implant Osseointegration. J. Dent. Res. 2017, 96, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Meier, E. The Wood Database 2018-2019. Available online: http://www.wood-database.com/ (accessed on 26 January 2019).
- Seong, W.J.; Kim, U.K.; Swift, J.Q.; Heo, Y.C.; Hodges, J.S.; Ko, C.C. Elastic properties and apparent density of human edentulous maxilla and mandible. Int. J. Oral Maxillofac. Surg. 2009, 38, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Baldi, D.; Lombardi, T.; Colombo, J.; Cervino, G.; Perinetti, G.; Di Lenarda, R.; Stacchi, C. Correlation between Insertion Torque and Implant Stability Quotient in Tapered Implants with Knife-Edge Thread Design. BioMed Res. Int. 2018, 2018, 7201093. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, J.; Salmon, B.; Huang, L.; Lim, W.H.; Liu, B.; Hunter, D.J.; Ransom, R.C.; Singh, G.; Gillette, M.; et al. Wnt Signaling and Its Contribution to Craniofacial Tissue Homeostasis. J. Dent. Res. 2015, 94, 1487–1494. [Google Scholar] [CrossRef]
- Cicciu, M.; Cervino, G.; Milone, D.; Risitano, G. FEM Investigation of the Stress Distribution over Mandibular Bone Due to Screwed Overdenture Positioned on Dental Implants. Materials 2018, 11, 1512. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Min. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Schropp, L.; Isidor, F. Timing of implant placement relative to tooth extraction. J. Oral Rehabil. 2008, 35 (Suppl. 1), 33–43. [Google Scholar] [CrossRef] [PubMed]
- Mouraret, S.; Hunter, D.J.; Bardet, C.; Brunski, J.B.; Bouchard, P.; Helms, J.A. A pre-clinical murine model of oral implant osseointegration. Bone 2014, 58, 177–184. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.; Chen, T.; Mouraret, S.; Dhamdhere, G.; Brunski, J.B.; Zou, S.; Helms, J.A. Rescuing failed oral implants via Wnt activation. J. Clin. Periodontol. 2016, 43, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Pei, X.; Zhao, Y.; Li, Z.; Chen, C.H.; Tulu, U.S.; Liu, B.; Van Brunt, L.A.; Brunski, J.B.; Helms, J.A. Biomechanics of Immediate Postextraction Implant Osseointegration. J. Dent. Res. 2018, 97, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Han, C.; Yunming, L.; Li, D.; Song, Y.; Zhao, Y. Is the osseointegration of immediately and delayed loaded implants the same?—Comparison of the implant stability during a 3-month healing period in a prospective study. Clin. Oral Implants Res. 2009, 20, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- West, J.D.; Oates, T.W. Identification of stability changes for immediately placed dental implants. Int. J. Oral Maxillofac. Implants 2007, 22, 623–630. [Google Scholar] [PubMed]
- Barewal, R.M.; Stanford, C.; Weesner, T.C. A randomized controlled clinical trial comparing the effects of three loading protocols on dental implant stability. Int. J. Oral Maxillofac. Implants 2012, 27, 945–956. [Google Scholar]
- Friberg, B.; Sennerby, L.; Meredith, N.; Lekholm, U. A comparison between cutting torque and resonance frequency measurements of maxillary implants. A 20-month clinical study. Int. J. Oral Maxillofac. Surg. 1999, 28, 297–303. [Google Scholar] [CrossRef]
- Friberg, B.; Sennerby, L.; Linden, B.; Grondahl, K.; Lekholm, U. Stability measurements of one-stage Branemark implants during healing in mandibles. A clinical resonance frequency analysis study. Int. J. Oral Maxillofac. Surg. 1999, 28, 266–272. [Google Scholar] [CrossRef]
- Branemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Li, J.; Hoffmann, W.; Gasser, A.; Brunski, J.B.; Helms, J.A. Mechanical and Biological Advantages of a Tri-Oval Implant Design. J. Clin. Med. 2019, 8, 427. https://doi.org/10.3390/jcm8040427
Yin X, Li J, Hoffmann W, Gasser A, Brunski JB, Helms JA. Mechanical and Biological Advantages of a Tri-Oval Implant Design. Journal of Clinical Medicine. 2019; 8(4):427. https://doi.org/10.3390/jcm8040427
Chicago/Turabian StyleYin, Xing, Jingtao Li, Waldemar Hoffmann, Angelines Gasser, John B. Brunski, and Jill A. Helms. 2019. "Mechanical and Biological Advantages of a Tri-Oval Implant Design" Journal of Clinical Medicine 8, no. 4: 427. https://doi.org/10.3390/jcm8040427
APA StyleYin, X., Li, J., Hoffmann, W., Gasser, A., Brunski, J. B., & Helms, J. A. (2019). Mechanical and Biological Advantages of a Tri-Oval Implant Design. Journal of Clinical Medicine, 8(4), 427. https://doi.org/10.3390/jcm8040427




