Role of Mucosal Protrusion Angle in Discriminating between True and False Masses of the Small Bowel on Video Capsule Endoscopy
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Angle Measurement
2.3. SPICE Calculation
2.4. Statistics
3. Results
3.1. Demographics
3.2. Diagnosis
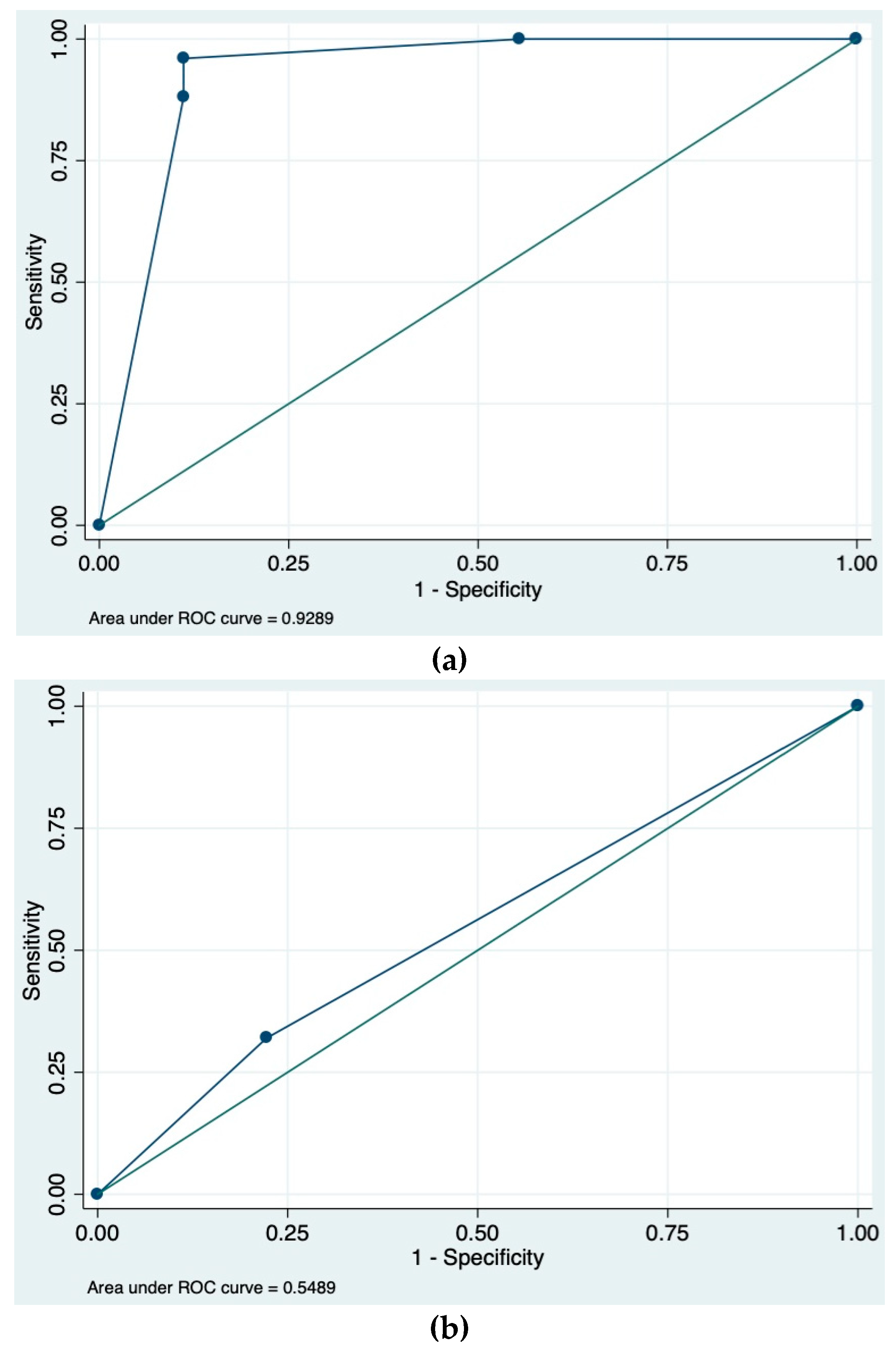
3.3. Protrusion Angle and SPICE Calculations
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Moglia, A.; Menciassi, A.; Dario, P.; Cuschieri, A. Clinical Update: Endoscopy for small-bowel tumours. Lancet 2007, 370, 114–116. [Google Scholar] [CrossRef]
- Cobrin, G.M.; Pittman, R.H.; Lewis, B.S. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer 2006, 107, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.; Eisen, G.; Friedman, S. A pooled analysis to evaluate results of capsule endoscopy trials. Endoscopy 2007, 39, 303–308. [Google Scholar] [CrossRef]
- Mergerner, K.; Ponchon, T.; Gralnek, I.; Pennazio, M.; Gay, G.; Selby, E.G.; Cellier, C.; Murray, J.; de Franchis, R.; Rosch, T.; et al. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy. Endoscopy 2007, 39, 303–308. [Google Scholar]
- Girelli, C.M.; Porta, P.; Colombo, E.; Lesinigo, E.; Bernasconi, G. Development of a novel index to discriminate bulge from mass on small-bowel capsule endoscopy. Gastrointest. Endosc. 2011, 74, 1067–1074. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L.; Talamonti, M.S. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.A.; Debinski, H.S.; Appleyard, M.N.; Remedios, M.L.; Hooper, J.E.; Walsh, A.J.; Selby, W.S. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: A three-center Australian experience. Am. J. Gastroenterol. 2006, 101, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S.F.; Sharma, V.K.; Carey, E.J.; Shiff, A.D.; Heigh, R.I.; Gurudu, S.R.; Erickson, P.J.; Post, J.K.; Hara, A.K.; Fleischer, D.E.; et al. Utility of video capsule endoscopy in the detection of small bowel tumors. A single center experience of 1000 consecutive patients. In Proceedings of the 6th International Conference on Capsule Endoscopy, Madrid, Spain, 8–10 June 2007; p. 45. [Google Scholar]
- Pennazio, M.; Rondonotti, E.; De franchis, R. Capsule endoscopy in neoplastic diseases. World J. Gastroenterol. 2008, 14, 5245–5253. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.S.; Leighton, J.A.; Pasha, S.F. Evaluation and management of small-bowel tumors in the era of deep enteroscopy. GIE 2014, 79, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Shyung, L.-R.; Lin, S.-C.; Shih, S.-C.; Chang, W.-H.; Chu, C.-H.; Wang, T.-E. Proposed scoring system to determine small bowel mass lesions using capsule endoscopy. J. Formos Med. Assoc. 2009, 108, 533–538. [Google Scholar] [CrossRef]
- Barbosa, D.C.; Roupar, D.B.; Ramos, J.C.; Tavares, A.C.; Lima, C.S. Authomatic small bowel tumor diagnosis by using multi-scale wavelet-based analysis in wireless capsule endoscopy images. BioMed. Eng. OnLine 2012, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Kodogiannis, V.; Boulougoura, M.; Wadge, E.; Lygouras, J. The usage of soft-computing methodologies in interpreting capsule endoscopy. Eng. Appl. Artif. Intell. 2007, 20, 539–553. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Pinho, R.; Rodrigues, A.; Silva, J.; Ponte, A.; Sousa, M.; Carvalho, J. Validation of SPICE, a method to differentiate small bowel submucosal lesions from innocent bulges on capsule endoscopy. Rev. Esp. Enferm. Dig. 2017, 109, 106–113. [Google Scholar] [CrossRef]
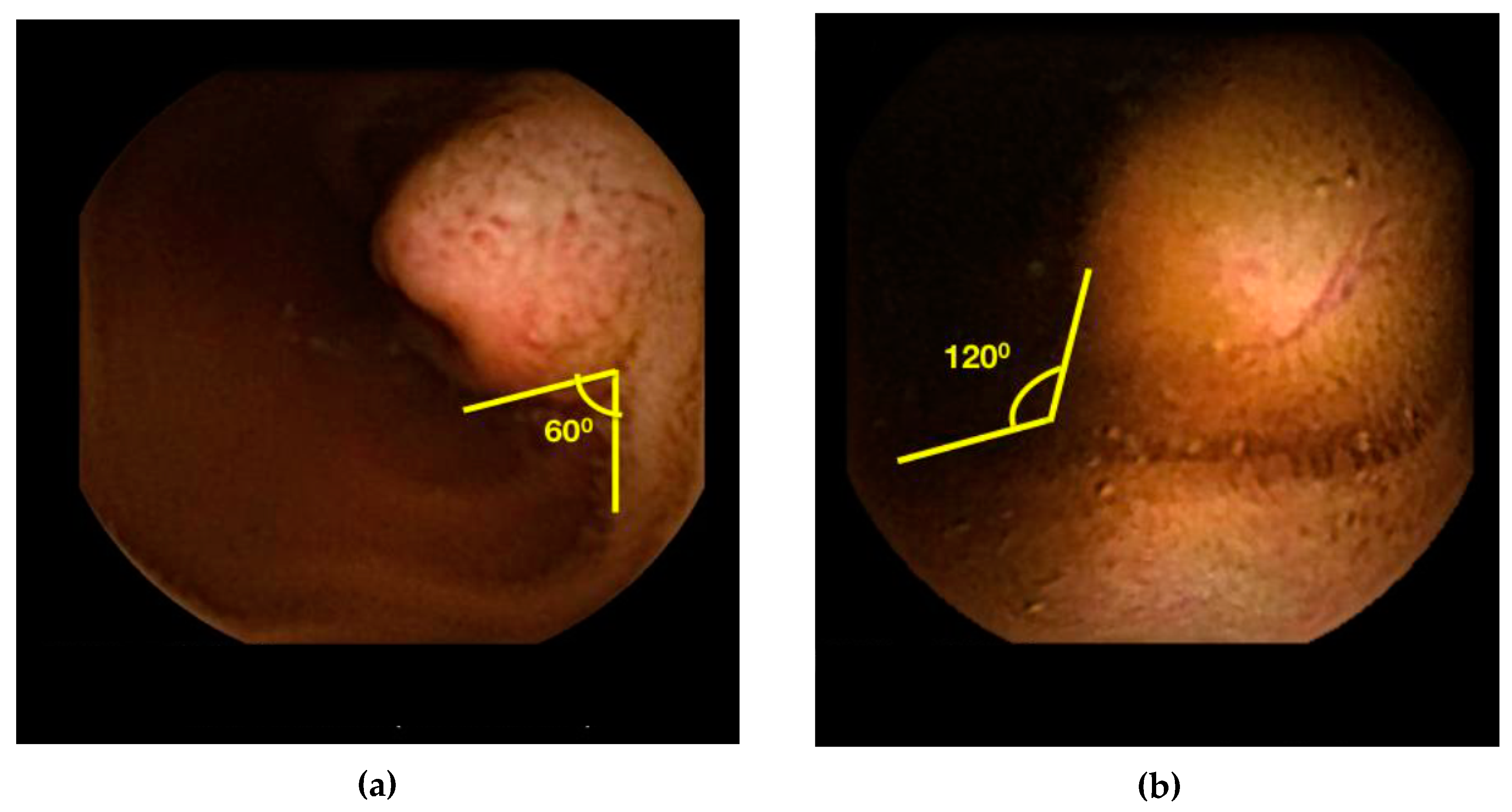
| Gender | Age | Indication | Novice Angle b | Expert Angle | Location | Imaging c | Endoscopy c | Surgery | Final Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| F | 65 | OGB | 42.5 | 20.0 | Jejunum | CTE + | ASBE + | Yes | GIST |
| M | 52 | OGB | 16.0 | 10.0 | Ileum | CT + | ASBE + | Yes | GIST |
| M | 81 | OGB | 50.0 | 30.0 | Ileum | CT − | ASBE − | Yes | Carcinoid |
| F | 56 | Carcinoid a | 20.0 | 10.0 | Ileum | CT ± | Colo + | Yes | Carcinoid |
| F | 77 | IDA | 27.5 | 20.0 | Ileum | CT − | RSBE + | Yes | Carcinoid |
| F | 56 | CD | 55.0 | 50.0 | Ileum | CTE ± | RSBE + | Yes | Carcinoid |
| F | 58 | AP | 10.0 | 20.0 | Ileum | CT ± | Colo + | Yes | Carcinoid |
| F | 62 | AP | 100.0 | 10.0 | Ileum | CT + | Colo − | Yes | Carcinoid |
| M | 38 | AP | 72.5 | 30.0 | Jejunum | CT + | ASBE − | Yes | Inflammatory Polyp |
| F | 73 | OGB | 35.0 | 110.0 | Jejunum | ND | ASBE + | No | Lymphangiectasia |
| M | 53 | OGB | 25.0 | 30.0 | Ileum | CT − | Colo − | Yes | DLBCL |
| F | 30 | Peutz-Jeghers a | 45.0 | 30.0 | Jejunum | ND | RSBE + | Yes | Peutz-Jeghers |
| F | 39 | Peutz-Jeghers a | 47.5 | 30.0 | Ileum | ND | RSBE + | No | Peutz-Jeghers |
| F | 36 | Peutz-Jeghers a | 45.0 | 50.0 | Duodenum | ND | ASBE + | Yes | Peutz-Jeghers |
| M | 37 | IDA | 27.5 | 40.0 | Jejunum | ND | ASBE + | Yes | Peutz-Jeghers |
| F | 49 | OGB | 50.0 | 20.0 | Jejunum | ND | ASBE + | No | Peutz-Jeghers |
| F | 58 | OGB | 52.5 | 15.0 | Jejunum | CT − | ASBE + | No | Inflammatory Polyp |
| M | 37 | Crohn’s a | 65.0 | 20.0 | Jejunum | CT − | ASBE + | No | Inflammatory Polyp |
| F | 76 | OGB | 40.0 | 40.0 | Jejunum | CTE + | ASBE + | Yes | Hamartoma |
| F | 57 | OGB | 45.0 | 10.0 | Duodenum | ND | ASBE + | No | Hamartoma |
| M | 78 | BO | 60.0 | 20.0 | Ileum | MRE ± | ASBE − | Yes | Lipoma |
| F | 83 | AP | 82.5 | >90 | Duodenum | ND | ASBE + | No | Tubular Adenoma |
| F | 41 | OGB | 35.0 | 10.0 | Ileum | ND | Colo − | Yes | Leiomyoma |
| F | 48 | OGB | 47.5 | 10.0 | Jejunum | ND | ASBE − | Yes | Hemangioma |
| M | 47 | AP | 30.0 | 60.0 | Duodenum | CT + | ASBE + | No | Hyperplastic Polyp |
| F | 70 | AP, OGB | 130.0 | 70.0 | Jejunum | CT − | ASBE − | No | Bulge |
| M | 51 | Leukemia a | 95.0 | 50.0 | Jejunum | PET CT + | NA | No | Bulge |
| F | 61 | AP/CD | 115.0 | 20.0 | Duodenum | ND | ND | No | Bulge |
| F | 29 | AP | 105.0 | 110.0 | Ileum | CT − | Colo − | No | Bulge |
| F | 55 | OGB | 75.0 | 20.0 | Jejunum | NA | NA | No | Bulge |
| M | 85 | AP | 122.5 | 130.0 | Ileum | ND | Colo − | No | Bulge |
| M | 29 | AP | 105.0 | 30.0 | Ileum | CT − | Colo − | No | Bulge |
| F | 54 | OGB | 125.0 | 130.0 | Ileum | CT − | Colo − | No | Bulge |
| F | 73 | IDA | 102.5 | 130.0 | Ileum | CT − | ASBE − | No | Bulge |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, M.; Noujaim, M.G.; Green, J.; Schlieve, C.R.; Vaze, A.; Cahan, M.A.; Cave, D.R. Role of Mucosal Protrusion Angle in Discriminating between True and False Masses of the Small Bowel on Video Capsule Endoscopy. J. Clin. Med. 2019, 8, 418. https://doi.org/10.3390/jcm8040418
Min M, Noujaim MG, Green J, Schlieve CR, Vaze A, Cahan MA, Cave DR. Role of Mucosal Protrusion Angle in Discriminating between True and False Masses of the Small Bowel on Video Capsule Endoscopy. Journal of Clinical Medicine. 2019; 8(4):418. https://doi.org/10.3390/jcm8040418
Chicago/Turabian StyleMin, May, Michael G. Noujaim, Jonathan Green, Christopher R. Schlieve, Aditya Vaze, Mitchell A. Cahan, and David R. Cave. 2019. "Role of Mucosal Protrusion Angle in Discriminating between True and False Masses of the Small Bowel on Video Capsule Endoscopy" Journal of Clinical Medicine 8, no. 4: 418. https://doi.org/10.3390/jcm8040418
APA StyleMin, M., Noujaim, M. G., Green, J., Schlieve, C. R., Vaze, A., Cahan, M. A., & Cave, D. R. (2019). Role of Mucosal Protrusion Angle in Discriminating between True and False Masses of the Small Bowel on Video Capsule Endoscopy. Journal of Clinical Medicine, 8(4), 418. https://doi.org/10.3390/jcm8040418





