Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Subjects and Procedures
2.3. Cellular Subsets
- Panel 1: Treg cells, CD8+, CD4+, CD3+, CD4+CD45RO+/CD4+, CD4+CD39+/CD4+, NK, NKT cells;
- Panel 2: IFN-γ, TGF-α, TGF-β, IL-1b, IL-6, IL-17, IL-10.
2.3.1. Lymphocytes Isolation
2.3.2. Flow Cytometry
2.3.3. Data Analysis and Absolute Count Determination
- (1)
- Use normal gating strategies to identify the cell population to be enumerated (i.e., FSC/SSC lymphocyte gate CD3+CD4+ gate);
- (2)
- In the same sample, draw a gate on 123 count eBeads in an ungated plot displaying two blue (488 nm) or violet (405 nm) laser excited parameters;
- (3)
- Using the count statistics from these two gates, the concentration of the original cell sample may be determined by the equations:Absolute cell number (cells/μL) = (cell count × eBead volume)/(eBead count × cell volume) × eBead concentration (1000/μL)
2.3.4. Serum Assay
2.4. Statistical Data Analysis
2.5. Ethical Approval
3. Results
3.1. Demography
3.2. Pre- and Post-Liver Resection Modulation of Circulating Immune Cells
3.3. Pre- and Post-Liver Resection Modulation of Circulating Cytokines and Chemokines
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.-M.; et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, L.; Seth, P.; Bian, S.; Li, L.; Csizmadia, E.; Junger, W.G.; Schmelzle, M.; Usheva, A.; Tapper, E.B.; et al. Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/ENTPD1 null mice. Hepatology 2013, 57, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Di Bisceglie, A.M.; Bruix, J.; Kramer, B.S.; Lencioni, R.; Zhu, A.X.; Sherman, M.; Schwartz, M.; Lotze, M.; Talwalkar, J.; et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J. Natl. Cancer Inst. 2008, 100, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Mehrabi, A.; Mollberg, N.M.; Müller, S.; Koch, M.; Büchler, M.W.; Weitz, J. Hepatocellular carcinoma: Current management and perspectives for the future. Ann. Surg. 2011, 253, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.; Schulick, R.D.; Choti, M.A.; Pawlik, T.M. Predictors of survival after resection of early hepatocellular carcinoma. Ann. Surg. 2009, 249, 799–805. [Google Scholar] [CrossRef]
- Roayaie, S.; Obeidat, K.; Sposito, C.; Mariani, L.; Bhoori, S.; Pellegrinelli, A.; Labow, D.; Llovet, J.M.; Schwartz, M.; Mazzaferro, V. Resection of hepatocellular cancer ≤ 2 cm: Results from two Western centers. Hepatology 2013, 57, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Pardee, A.D.; Butterfield, L.H. Immunotherapy of hepatocellular carcinoma: Unique challenges and clinical opportunities. Oncoimmunology 2012, 1, 48–55. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Demma, S.; Ardiri, A.; Proiti, M.; Mangia, A.; Gruttadauria, S.; Toro, A.; Di Carlo, I.; Malaguarnera, G.; Bertino, N.; et al. The immune system in hepatocellular carcinoma and potential new immunotherapeutic strategies. Biomed. Res. Int. 2015, 2015, 731469. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.S.; Gopal, P.; Singal, A.G. The changing landscape of hepatocellular carcinoma: Etiology, genetics, and therapy. Am. J. Pathol. 2014, 184, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Imbeaud, S.; Letouze, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.; Melero, I.; Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014, 59, 1415–1426. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; El-Zamarany, E.A.; Khedr, E.G.; El-Bahrawy, H.A.; El-Feky, O.A. Antigen-loaded dendritic cells triggers a specific cytotoxic T lymphocytes immune response against hepatocellular carcinoma: In vitro study. Clin. Transl. Oncol. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chan, C.J.; Coussens, L. Inflammation and cancer. Immu. Patho. Tumors 2016, 4, 406–415. [Google Scholar]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Karin, M. Obesity, inflammation, and liver cancer. J. Hepatol. 2012, 56, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Oklu, R.; Sheth, R.A. Thermal ablative therapies and immune checkpoint modulation: Can locoregional approaches effect a systemic response? Gastroenterol. Res. Pract. 2016, 2016, 9251375. [Google Scholar] [CrossRef] [PubMed]
- Van den Bijgaart, R.J.E.; Eikelenboom, D.C.; Hoogenboom, M.; Fütterer, J.J.; den Brok, M.H.; Adema, G.J. Thermal and mechanical high-intensity focused ultrasound: Perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol. Immunother. 2017, 66, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Dai, T. Radiation therapy and the abscopal effect: A concept comes of age. Ann. Transl. Med. 2016, 4, 118. [Google Scholar] [CrossRef]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef]
- Den Brok, M.H.M.G.M.; Sutmuller, R.P.M.; Nierkens, S.; Bennink, E.J.; Frielink, C.; Toonen, L.W.J.; Boerman, O.C.; Figdor, C.G.; Ruers, T.J.M.; Adema, G.J. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br. J. Cancer 2006, 95, 896–905. [Google Scholar] [CrossRef]
- De Iongh, F.A.; Rombouts, S.J.E.; Nijkamp, M.W.; Nierkens, S.; Hagendoorn, J.; Kranenburg, O.; Borel Rinkes, I.H.M.; Molenaar, I.Q. Induction of immunomodulatory responses following radiofrequency ablation of solid malignancies: A systematic review. HPB 2016, 18, e747. [Google Scholar] [CrossRef]
- Mazmishvili, K.; Jayant, K.; Janikashvili, N.; Kikodze, N.; Mizandari, M.; Pantsulaia, I.; Paksashvili, N.; Sodergren, M.H.; Reccia, I. Study to evaluate the immunomodulatory effects of radiofrequency ablation compared to surgical resection for liver cancer. J. Cancer 2018, 9, 3187–3195. [Google Scholar] [CrossRef]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-thermal ablations - boosting the anticancer immune response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef]
- Bodey, B.; Siegel, S.E.; Kaiser, H.E. Antigen presentation by dendritic cells and their significance in antineoplastic immunotherapy. In Vivo 2004, 18, 81–100. [Google Scholar]
- Den Brok, M.H.M.G.M.; Sutmuller, R.P.M.; Nierkens, S.; Bennink, E.J.; Toonen, L.W.J.; Figdor, C.G.; Ruers, T.J.M.; Adema, G.J. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006, 66, 7285–7292. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Lee, P.; Kusano, T.; Reccia, I.; Jayant, K.; Habib, N. Impact of cavitron ultrasonic surgical aspirator (CUSA) and bipolar radiofrequency device (Habib-4X) based hepatectomy for hepatocellular carcinoma on tumour recurrence and disease-free survival. Oncotarget 2017, 55, 93644–93654. [Google Scholar] [CrossRef]
- Reccia, I.; Kumar, J.; Kusano, T.; Giakoustidis, A.; Zanellato, A.; Retsas, P.; Habib, N.; Jiao, L.; Spalding, D.; Pai, M. Radiofrequency-assisted liver resection: Technique and results. Surg. Oncol. 2018, 27, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Reccia, I.; Sodergren, M.H.; Jayant, K.; Kurz, E.; Carneiro, A.; Spalding, D.; Pai, M.; Jiao, L.; Habib, N. The journey of radiofrequency-assisted liver resection. Surg. Oncol. 2018, 27, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Lu, W.; Yu, N.; Yang, G.; Li, Y.; Huang, Z.; Li, J.; Li, K.; Xu, H.; Chen, S.; et al. HabibTM 4X-assisted resection versus clamp-crush resection for hepatocellular carcinoma: A propensity-matching study. Oncotarget 2017, 8, 4218–4227. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Spalding, D.; Jiao, L.; Habib, N. Use of bipolar radiofrequency in parenchymal transection of the liver, pancreas and kidney. Dig. Surg. 2012, 29, 43–47. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Ann. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Swann, J.B.; Koebel, C.M.; Schreiber, R.D.; Smyth, M.J. Immune-mediated dormancy: An equilibrium with cancer. J. Leukoc. Biol. 2008, 84, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Khong, H.T.; Restifo, N.P. Natural selection of tumor variants in the generation of ‘tumor escape’ phenotypes. Nat. Immunol. 2002, 3, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor A-chains (CD25). J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Corthay, A. How do regulatory T cells work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Borsellino, G.; Kleinewietfeld, M.; Di Mitri, D.; Sternjak, A.; Diamantini, A.; Giometto, R.; Höpner, S.; Centonze, D.; Bernardi, G.; Dell’Acqua, M.L.; et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 2007, 110, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, Y.; Gao, W.; Enjyoji, K.; Csizmadia, E.; Muller, C.E.; Murakami, T.; Robson, S.C. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 2010, 139, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Hiraoka, N.; Yamagami, W.; Ojima, H.; Kanai, Y.; Kosuge, T.; Nakajima, A.; Hirohashi, S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin. Cancer Res. 2007, 13, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, D.; Liu, Z.; Shi, M.; Zhao, P.; Fu, B.; Zhang, Z.; Yang, H.; Zhang, H.; Zhou, C.; et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007, 132, 2328–2339. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, G.; Liao, W.; Yang, H.; Xu, H.; Du, S.; Zhao, H.; Lu, X.; Sang, X.; Mao, Y. Clinicopathologic and prognostic significance of regulatory T cells in patients with hepatocellular carcinoma: A meta-analysis. Oncotarget 2017, 8, 39658–39672. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Li, W.M.; Lu, Z.Q.; Yao, Y.M. Roles of Tregs in development of hepatocellular carcinoma: A meta-analysis. World J. Gastroenterol. 2014, 20, 7971–7978. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Olive, D.; Kuchroo, V.; Zarour, H.M. CD8+ T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012, 72, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Ohashi, P.S. Clinical blockade of PD1 and LAG3-potential mechanisms of action. Nat. Rev. Immunol. 2015, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Nishikawa, H.; Sugiyama, D.; Ha, D.; Hamaguchi, M.; Saito, T.; Nishioka, M.; Wing, J.B.; Adeegbe, D.; Katayama, I.; et al. Detection of self-reactive CD8+T cells with an anergic phenotype in healthy individuals. Science 2014, 346, 1536–1540. [Google Scholar] [CrossRef]
- Linsley, P.S.; Brady, W.; Urnes, M.; Grosmaire, L.S.; Damle, N.K.; Ledbetter, J.A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991, 174, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.F. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef]
- Schwartz, J.C.D.; Zhang, X.; Fedorov, A.A.; Nathenson, S.G.; Almo, S.C. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature 2001, 410, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Hünig, T.; Beyersdorf, N.; Kerkau, T. CD28 co-stimulation in T-cell homeostasis: A recent perspective. ImmunoTargets Ther. 2015, 4, 111–122. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Saenger, Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 2008, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ashe, S.; Brady, W.A.; Hellström, I.; Hellström, K.E.; Ledbetter, J.A.; McGowan, P.; Linsley, P.S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 1992, 71, 1093–1102. [Google Scholar] [CrossRef]
- Fu, T.; He, Q.; Sharma, P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res. 2011, 71, 5445–5454. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, Z.; Yang, Y.; Jiang, Z.; Gu, Y.; Liu, Y.; Lin, C.; Pan, Z.; Yu, Y.; Jiang, M.; et al. Human CD14+CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 2014, 59, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Kuang, D.-M.; Wu, Y.; Xiao, X.; Li, X.-F.; Li, T.-J.; Zheng, L. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J. Immunol. 2012, 188, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Godin-Ethier, J.; Hanafi, L.A.; Duvignaud, J.B.; Leclerc, D.; Lapointe, R. IDO expression by human B lymphocytes in response to T lymphocyte stimuli and TLR engagement is biologically inactive. Mol. Immunol. 2011, 49, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.-M.; Peng, C.; Zhao, Q.; Wu, Y.; Zhu, L.-Y.; Wang, J.; Yin, X.-Y.; Li, L.; Zheng, L. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J. Immunol. 2010, 185, 1544–1549. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiao, X.; Wu, Y.; Wei, Y.; Zhu, L.Y.; Zhou, J.; Kuang, D.M. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur. J. Immunol. 2011, 41, 2314–2322. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Bonavita, E.; Barajon, I.; Garlanda, C.; Mantovani, A.; Jaillon, S. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013, 218, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.; Brown, I.; Peterson, A.C.; Spiotto, M.; Iwai, Y.; Honjo, T.; Gajewski, T.F. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T Cell Receptor (TCR) transgenic CD8+T Cells. Cancer Res. 2004, 64, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Meng, Y.; Blank, C.; Brown, I.; Kacha, A.; Kline, J.; Harlin, H. Immune resistance orchestrated by the tumor microenvironment. Immunol. Rev. 2006, 213, 131–145. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Rozali, E.N.; Hato, S.V.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. Programmed death ligand 2 in cancer-induced immune suppression. Clin. Dev. Immunol. 2012, 2012, 656340. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Peggs, K.S. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br. J. Cancer 2013, 108, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Takaki, H.; Cornelis, F.; Kako, Y.; Kobayashi, K.; Kamikonya, N.; Yamakado, K. Thermal ablation and immunomodulation: From preclinical experiments to clinical trials. Diag. Int. Imag. 2017, 98, 651–659. [Google Scholar] [CrossRef]
- Qi, X.; Tang, Y.; An, D.; Bai, M.; Shi, X.; Wang, J.; Han, G.; Fan, D. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: A meta-analysis of randomized controlled trials. J. Clin. Gastroenterol. 2014, 48, 450–457. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Li, B.; Xu, D.; Yin, Z.; Xie, F.; Yang, J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010, 10, 78. [Google Scholar] [CrossRef]
- Xu, Q.; Kobayashi, S.; Ye, X.; Meng, X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: A meta-analysis of 16,103 patients. Sci. Rep. 2014, 4, 7252. [Google Scholar] [CrossRef]
- Cho, Y.K.; Kim, J.K.; Kim, W.T.; Chung, J.W. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: A markov model analysis. Hepatology 2010, 51, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Yan, J.; Li, X.; Xia, F.; Ma, K.; Wang, S.; Bie, P.; Dong, J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 2012, 57, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.E.; Shaw, A.K.; Strand, D.W.; Hayward, S.W. Cancer associated fibroblasts in cancer pathogenesis. Semin. Cell Dev. Biol. 2010, 21, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Suriawinata, A.; Xu, R. An update on the molecular genetics of hepatocellular carcinoma. Semin. Liver Dis. 2004, 24, 77–88. [Google Scholar]
- Zhang, Z. Genomic landscape of liver cancer. Nat. Genet. 2012, 44, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Bastianpillai, C.; Petrides, N.; Shah, T.; Guillaumier, S.; Ahmed, H.U.; Arya, M. Harnessing the immunomodulatory effect of thermal and non-thermal ablative therapies for cancer treatment. Tumor Biol. 2015, 36, 9137–9146. [Google Scholar] [CrossRef]
- Fietta, A.M.; Morosini, M.; Passadore, I.; Cascina, A.; Draghi, P.; Dore, R.; Rossi, S.; Pozzi, E.; Meloni, F. Systemic inflammatory response and downmodulation of peripheral CD25+Foxp3+ T-regulatory cells in patients undergoing radiofrequency thermal ablation for lung cancer. Hum. Immunol. 2009, 70, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Nakamoto, Y.; Arai, K.; Yamashita, T.; Sakai, A.; Sakai, Y.; Kagaya, T.; Yamashita, T.; Honda, M.; Kaneko, S. Comparative analysis of various tumor-associated antigen-specific T-cell responses in patients with hepatocellular carcinoma. Hepatology 2011, 53, 1206–1216. [Google Scholar] [CrossRef]
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013, 57, 1448–1457. [Google Scholar] [CrossRef]
- Schietinger, A.; Philip, M.; Krisnawan, V.E.; Chiu, E.Y.; Delrow, J.J.; Basom, R.S.; Lauer, P.; Brockstedt, D.G.; Knoblaugh, S.E.; Hämmerling, G.J.; et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 2016, 45, 389–401. [Google Scholar] [CrossRef]
- Hu, G.; Wang, S. Tumor-infiltrating CD45RO+memory T lymphocytes predict favorable clinical outcome in solid tumors. Sci. Rep. 2017, 7, 10376. [Google Scholar] [CrossRef] [PubMed]
- Woodland, D.L.; Kohlmeier, J.E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 2009, 9, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, S.; Lugade, A.A.; Miller, A.; Iyer, R.; Thanavala, Y. Higher frequencies of GARP+CTLA-4+Foxp3+T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013, 73, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Schultze, J.L. Regulatory T cells: Major players in the tumor microenvironment. Curr. Pharm. Des. 2009, 15, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Kryczek, I.; Wei, S.; Frankel, T.; Zou, W. Regulatory T cells in tumor immunity. Encyclop. Immunobiol. 2016, 4, 451–459. [Google Scholar]
- Tu, J.F.; Ding, Y.H.; Ying, X.H.; Wu, F.Z.; Zhou, X.M.; Zhang, D.K.; Zou, H.; Ji, J.S. Regulatory T cells, especially ICOS+FOXP3+ regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci. Rep. 2016, 11, 35056. [Google Scholar] [CrossRef]
- Huang, X.M.; Liu, X.S.; Lin, X.K.; Yu, H.; Sun, J.Y.; Liu, X.K.; Chen, C.; Jin, H.L.; Zhang, G.E.; Shi, X.X.; et al. Role of plasmacytoid dendritic cells and inducible costimulator-positive regulatory T cells in the immunosuppression microenvironment of gastric cancer. Cancer Sci. 2014, 105, 150–158. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; De Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef]
- Sasada, T.; Kimura, M.; Yoshida, Y.; Kanai, M.; Takabayashi, A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: Possible involvement of regulatory T cells in disease progression. Cancer 2003, 98, 1089–1099. [Google Scholar] [CrossRef]
- Houot, R.; Schultz, L.M.; Marabelle, A.; Kohrt, H. T-cell-based immunotherapy: Adoptive cell transfer and checkpoint inhibition. Cancer Immunol. Res. 2015, 3, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Waitz, R.; Solomon, S.B.; Petre, E.N.; Trumble, A.E.; Fass, M.; Norton, L.; Allison, J.P. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012, 72, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. Cancer Clin. Trials 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.H.; et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 2018, 174, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed]
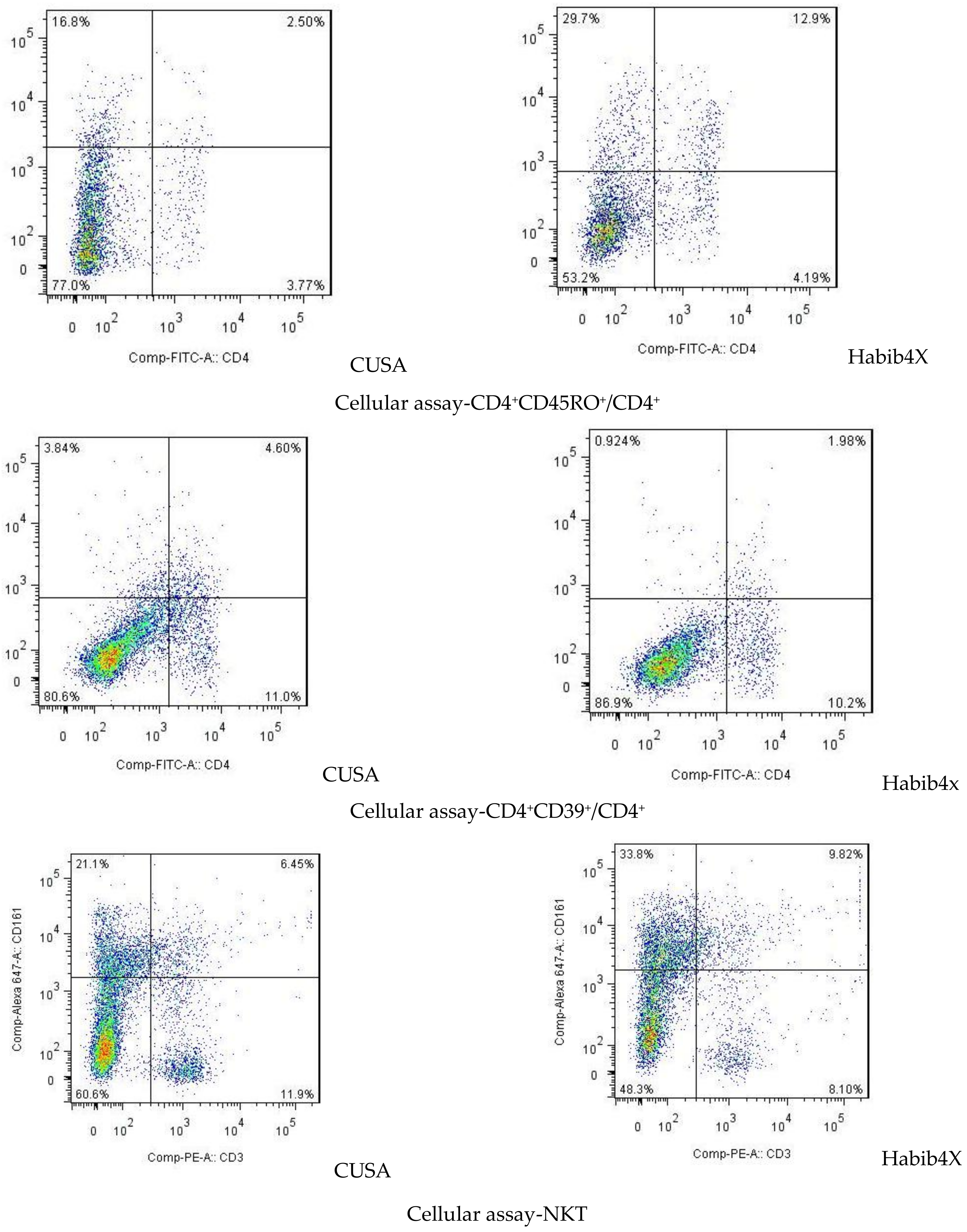

| Attributes | CUSA | HabibTM 4X | p-Value |
|---|---|---|---|
| Number of patients | 5 | 6 | NS # |
| Mean age, Mean ± SD (years) | 66.00 ± 17.00 | 62.00 ± 12.80 | NS $ |
| No. male/female | 1/4 | 5/1 | NS # |
| Albumin, Mean ± SD (g/dL) | 4.45 ± 0.26 | 4.40 ± 0.59 | NS $ |
| Bilirubin, Mean ± SD (mg/dL) | 0.95 ± 0.60 | 1.04 ± 0.30 | NS $ |
| Prothrombin time, Mean ± SD (sec) | 11.5 ± 1.8 | 12.1 ± 2.10 | NS $ |
| Ascites | 0 | 0 | NS # |
| Encephalopathy | 0 | 0 | NS # |
| ICG clearance, Mean ± SD (15 min) | 7.23 ± 3.56 | 11.77 ± 4.04 | NS $ |
| AFP ± SD (ng/mL) | 79.40 ± 151.40 | 52.60 ± 105.30 | NS $ |
| Cirrhosis | 2 | 3 | NS $ |
| HbsAg | 3 | 1 | NS $ |
| HCV | 1 | 5 | NS $ |
| Attributes | CUSA | HabibTM 4X | p-Value |
|---|---|---|---|
| Tumour Numbers | 1–3 | 1–4 | NS $ |
| Tumour Stage | |||
| T1 | 3 | 4 | NS $ |
| T2 | 2 | 2 | NS $ |
| T3 | 0 | 0 | NS $ |
| Tumour Size (cm) | 3.30 ± 2.04 | 3.65 ± 10.60 | NS $ |
| Anatomical resection | 4 | 5 | NS $ |
| Non-anatomical resection | 1 | 1 | NS $ |
| Major resection | 1 | 1 | NS $ |
| Minor resection | 4 | 5 | NS $ |
| Blood loss (mL), Mean ± SD | 300.00 ± 316.00 | 223.00 ± 150.00 | NS $ |
| Major complication | 0 | 0 | NS $ |
| Resection margin | |||
| Free | 2 | 2 | NS $ |
| Free within 1 cm | 3 | 4 | NS $ |
| Involved | 0 | 0 | NS $ |
| Parameters | CUSA | HabibTM 4X | ||||
|---|---|---|---|---|---|---|
| Before Surgery (Mean ± SD) | After 7 Days of Surgery (Mean ± SD) | p-Value | Before Surgery (Mean ± SD) | After 7 Days of Surgery (Mean ± SD) | p-Value | |
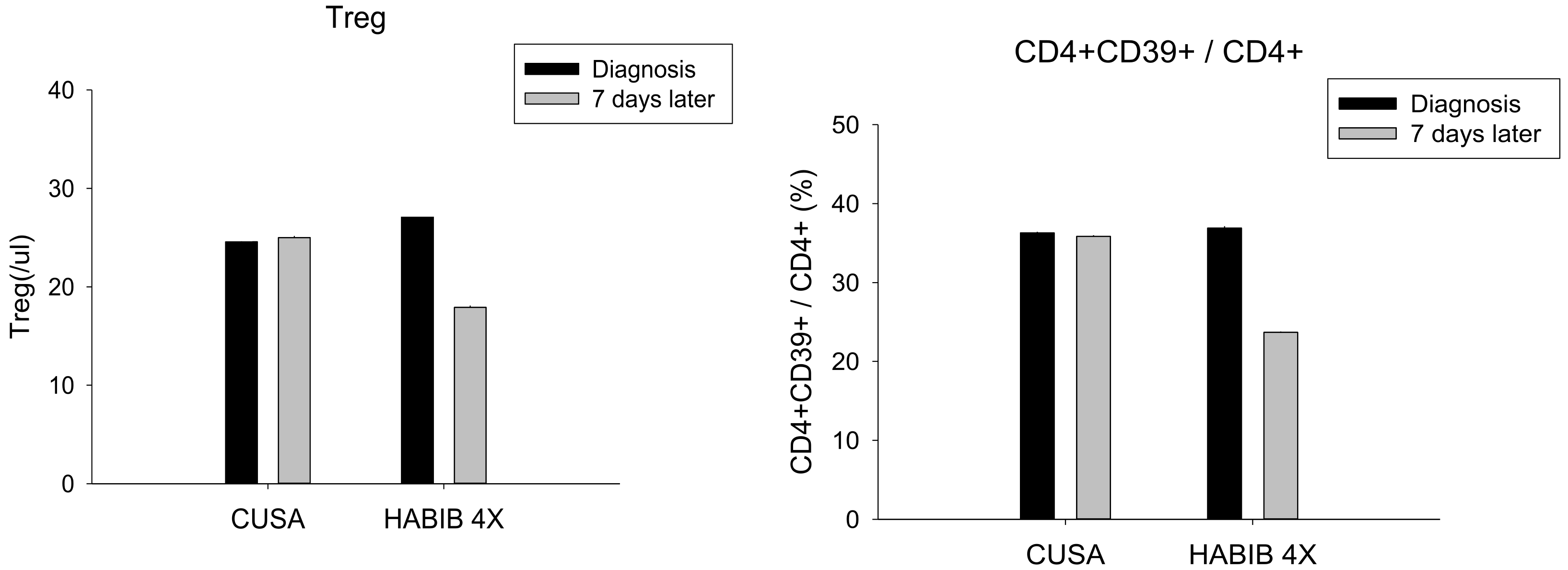
| Treg | 24.57 ± 4.83 | 25.00 ± 3.36 | 0.850 | 27.20 ± 6.17 | 17.90 ± 5.26 | 0.002 * |
| CD3+ | 1681.57 ± 384.25 | 1565.71 ± 459.78 | 0.819 | 1632.00 ± 392.68 | 1700.00 ± 445.35 | 0.721 |
| CD4+ | 1085.71 ± 278.91 | 1095.71 ± 384.48 | 0.956 | 1008.00 ± 283.50 | 1028.00 ± 331.86 | 0.886 |
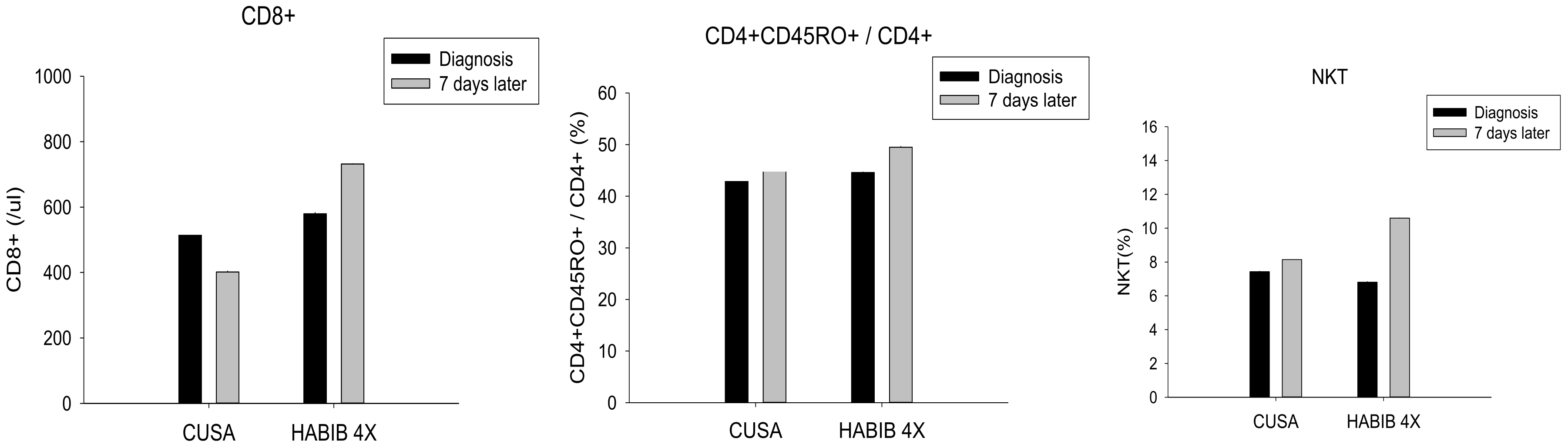
| CD8+ | 515.71 ± 255.46 | 401.42 ± 98.39 | 0.291 | 580.0 ± 216.18 | 732.00 ± 188.31 | 0.050 * |
| CD4+CD45RO+/CD4+ | 44.71 ± 1.98 | 45.00 ± 4.43 | 0.879 | 44.60 ± 1.78 | 49.50 ± 4.03 | 0.002 * |
| CD4+ CD39+/CD4+ | 36.29 ± 4.92 | 35.86 ± 4.38 | 0.866 | 36.90 ± 4.23 | 23.70 ± 8.49 | 0.000 * |
| NK | 11.86 ± 3.02 | 11.57 ± 3.64 | 0.876 | 11.60 ± 2.32 | 10.90 ± 2.51 | 0.526 |
| NKT | 7.43 ± 1.90 | 8.14 ± 2.12 | 0.519 | 6.80 ± 1.62 | 10.60 ± 3.50 | 0.006 * |
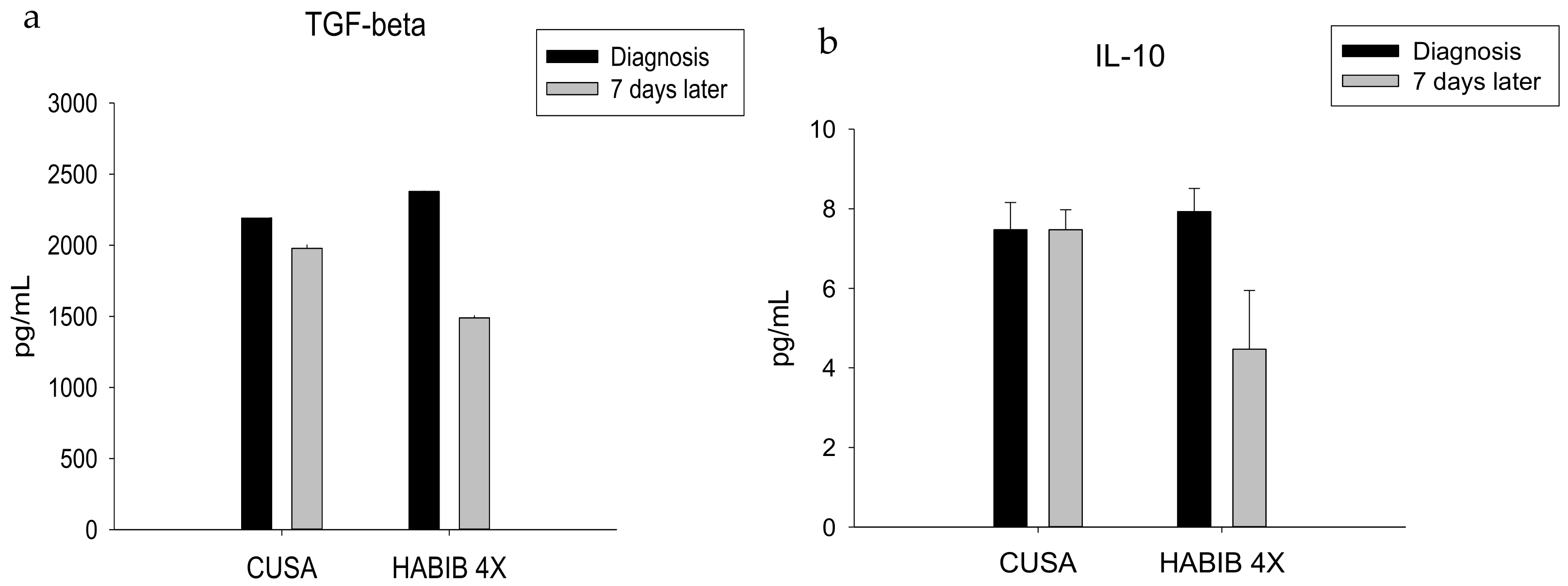
| TGF-ß | 2191.42 ± 400.43 | 1978.57 ± 478.83 | 0.385 | 2378.00 ± 382.35 | 1490.00 ± 366.60 | 0.000 * |
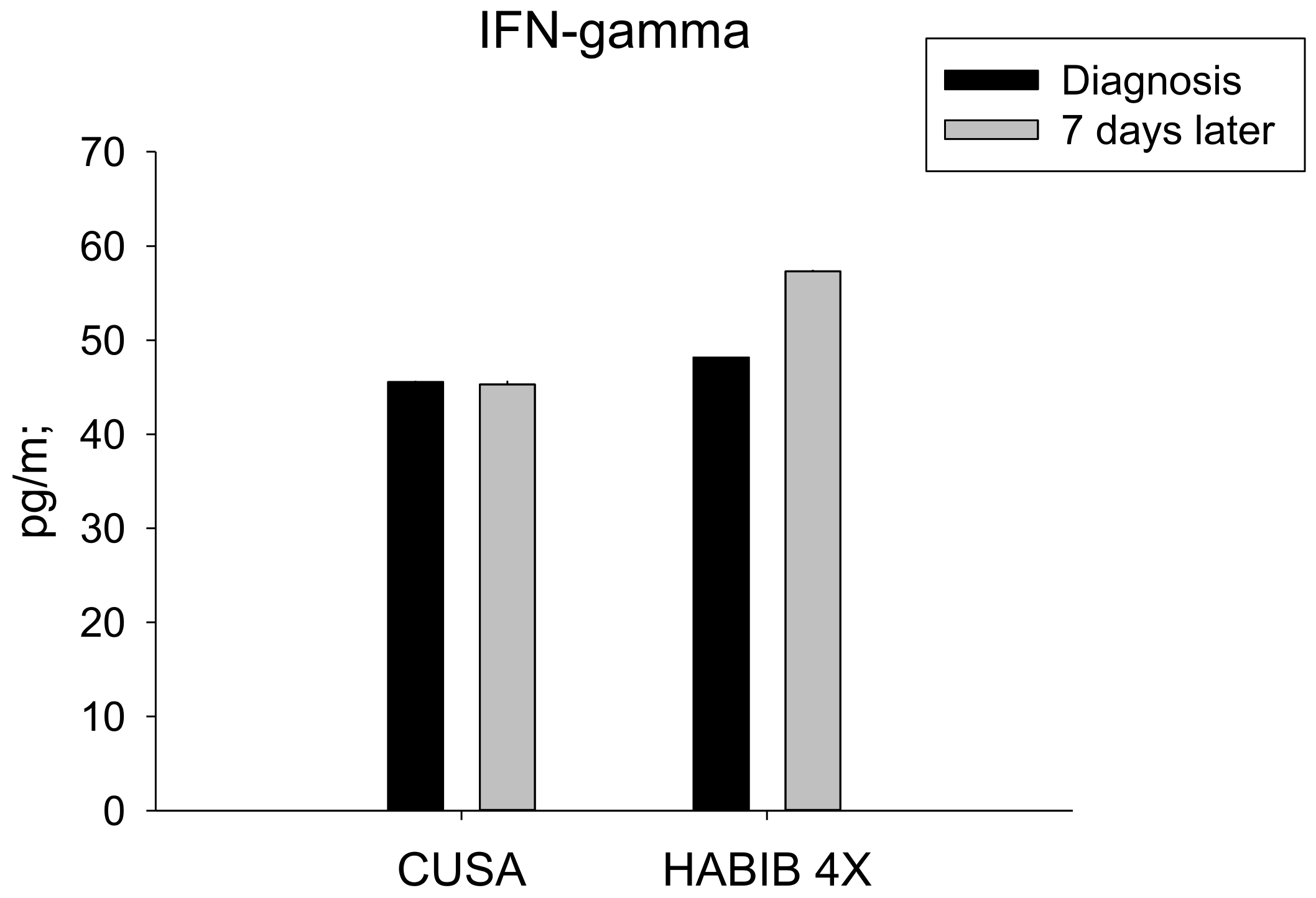
| IFN-γ | 45.57 ± 9.65 | 45.28 ± 10.73 | 0.959 | 48.20 ± 11.82 | 57.30 ± 7.41 | 0.027 * |
| IL-10 | 7.47 ± 0.69 | 7.47 ± 0.50 | 1.000 | 7.93 ± 0.58 | 4.47 ± 1.47 | 0.000 * |
| IL-1b | 7.92 ± 1.47 | 7.90 ± 1.05 | 0.970 | 7.28 ± 1.69 | 9.39 ± 4.51 | 0.180 |
| IL-17 | 58.00 ± 16.54 | 63.00 ± 15.35 | 0.569 | 52.6 ± 13.92 | 36.10 ± 13.55 | 0.010 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.W.; Jayant, K.; Lee, P.-H.; Yang, P.-c.; Hsiao, C.-Y.; Habib, N.; Sodergren, M.H. Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 385. https://doi.org/10.3390/jcm8030385
Huang KW, Jayant K, Lee P-H, Yang P-c, Hsiao C-Y, Habib N, Sodergren MH. Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma. Journal of Clinical Medicine. 2019; 8(3):385. https://doi.org/10.3390/jcm8030385
Chicago/Turabian StyleHuang, Kai Wen, Kumar Jayant, Po-Huang Lee, Po-chih Yang, Chih-Yang Hsiao, Nagy Habib, and Mikael H. Sodergren. 2019. "Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma" Journal of Clinical Medicine 8, no. 3: 385. https://doi.org/10.3390/jcm8030385
APA StyleHuang, K. W., Jayant, K., Lee, P.-H., Yang, P.-c., Hsiao, C.-Y., Habib, N., & Sodergren, M. H. (2019). Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma. Journal of Clinical Medicine, 8(3), 385. https://doi.org/10.3390/jcm8030385






