Effect of Androgen Deprivation Therapy on Other-Cause of Mortality in Elderly Patients with Clinically Localized Prostate Cancer Treated with Modern Radiotherapy: Is There a Negative Impact?
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Treatment Planning
2.2.1. Brachytherapy (BT)
Low-Dose-Rate Interstitial BT (LDR-BT) with or without EBRT
High-Dose-Rate Interstitial Brachytherapy (HDR-BT) Monotherapy
2.2.2. IG-IMRT
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
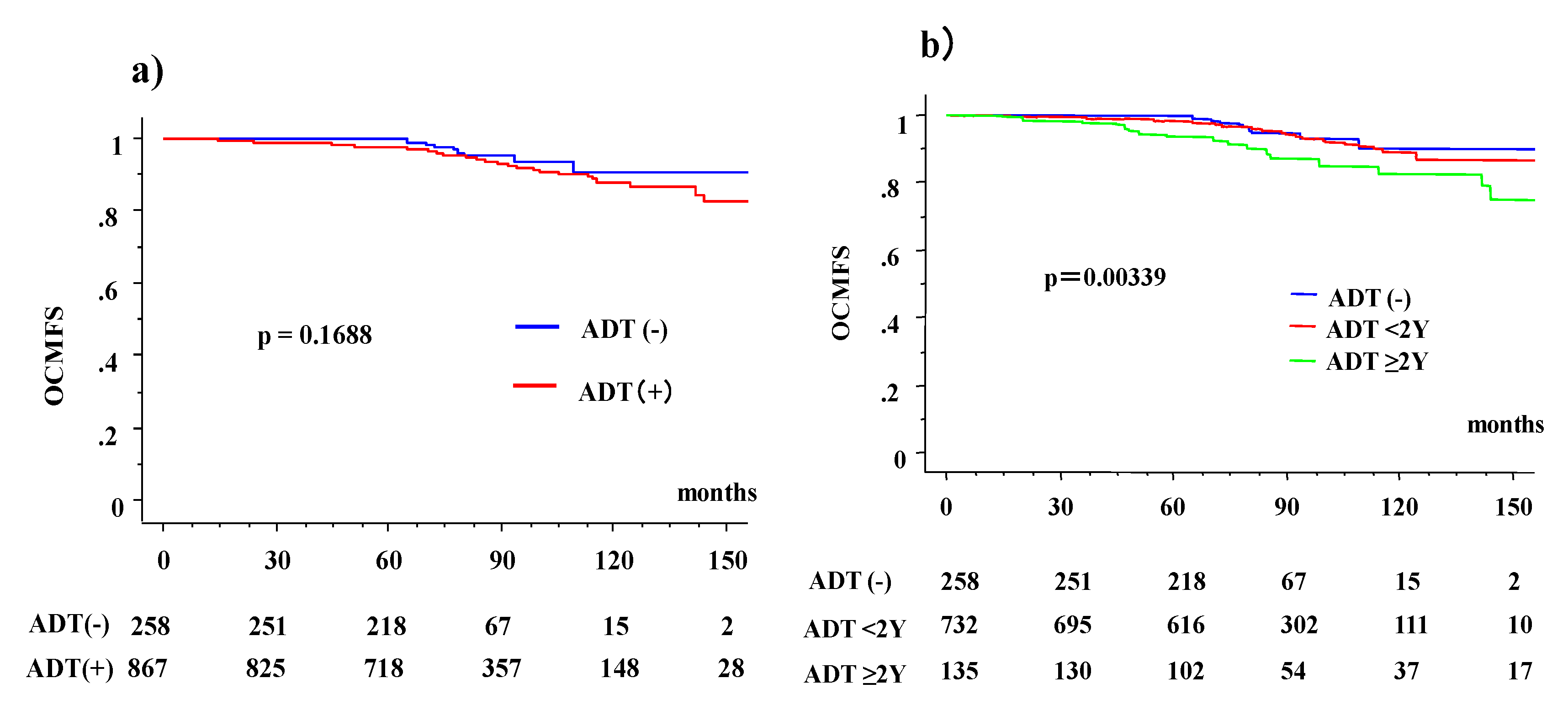
3.2. Overall Survival (OS) and OCM According to ADT Usage
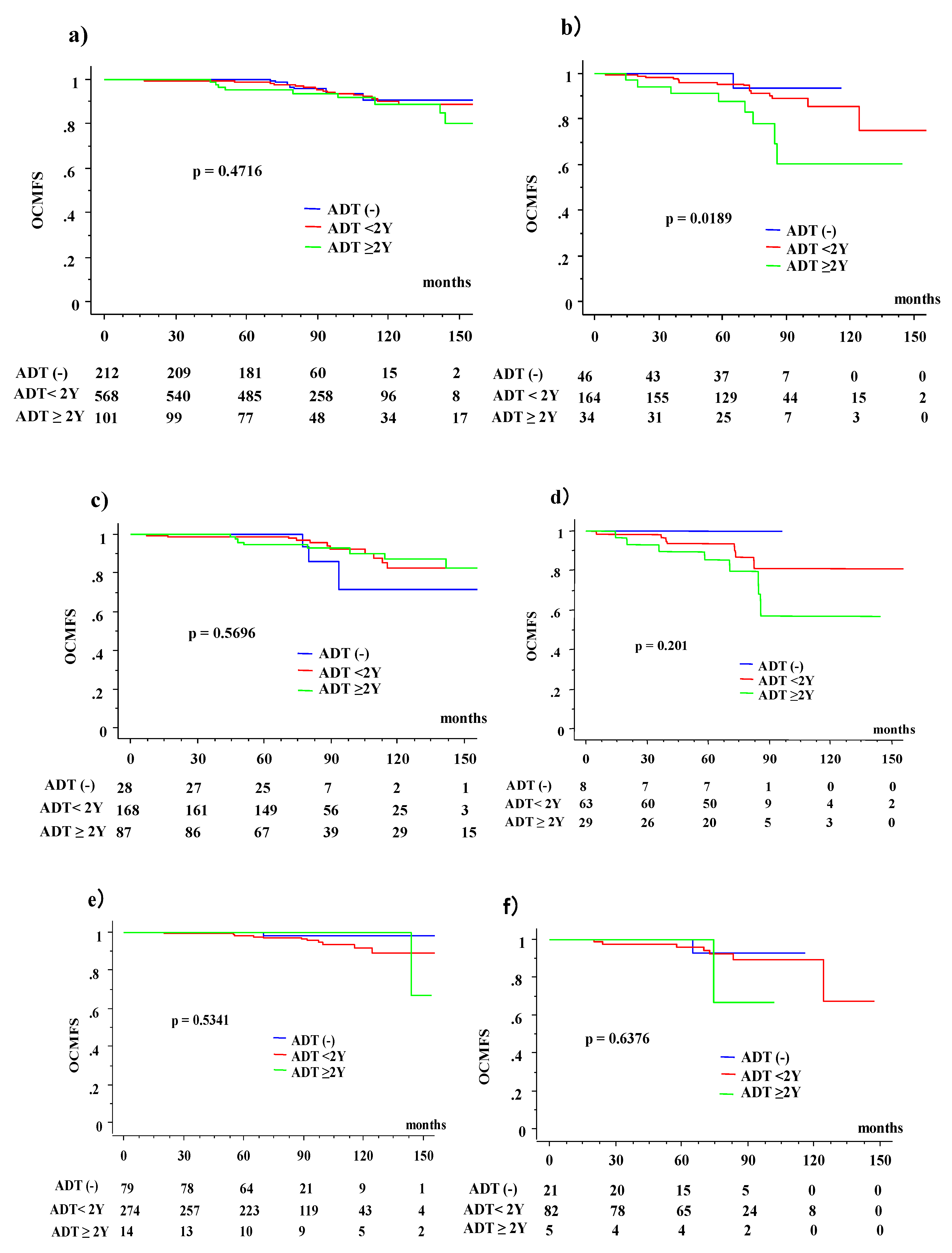
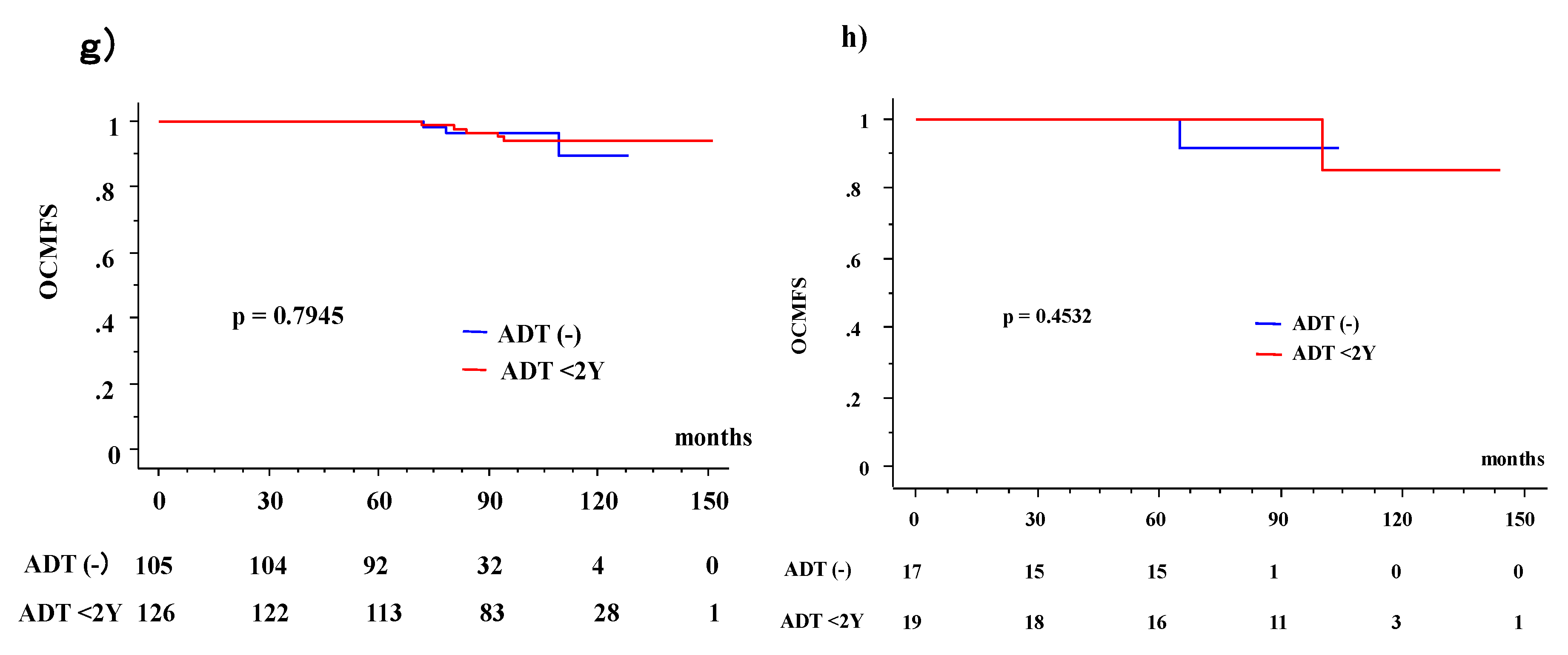
3.3. OS and OCM between ADT (−) and ADT (+) Patients According to Age
3.4. Causes of OCM
4. Discussion
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics 2012. CA. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.K.; Qu, Y.Y.; Ye, D.W. Prostate cancer in East Asia: Evolving trend over the last decade. Asian J. Androl. 2015, 17, 48–57. [Google Scholar] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®); Prostate Cancer 2016 Version 4; National Comprehensive Cancer Network: Fort Washington, PA, USA, 2016. [Google Scholar]
- Sasse, A.D.; Sasse, E.; Carvalho, A.M.; Macedo, L.T. Androgenic suppression combined with radiotherapy for the treatment of prostate adenocarcinoma: A systematic review. BMC Cancer 2012, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; Gonzalez, D.; Warde, P.; Dubois, J.B.; Mirimanoff, R.O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; Gil, T.; et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N. Engl. J. Med. 1997, 337, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Nakamura, S.; Nishimura, T.; Yoshida, K.; Yoshioka, Y.; Koizumi, M.; Ogawa, K. Transitioning from conventional radiotherapy to intensity-modulated radiotherapy for localized prostate cancer: Changing focus from rectal bleeding to detailed quality of life analysis. J. Radiat. Res. 2014, 55, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Galalae, R.M.; Martinez, A.; Mate, T.; Mitchell, C.; Edmundson, G.; Nuernberg, N.; Eulau, S.; Gustafson, G.; Gribble, M.; Kovács, G. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Alibhai, S.M.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 2015, 67, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, P.; Ploussard, G.; Grosclaude, P.; Roumiguié, M.; Soulié, M.; Beauval, J.B.; Malavaud, B. Current impact of age and comorbidity assessment on prostate cancer treatment choice and over/undertreatment risk. World J. Urol. 2017, 35, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Masui, K.; Suzuki, G.; Nakamura, S.; Aibe, N.; Shimizu, D.; Nishikawa, T.; Okabe, H.; Yoshida, K.; Kotsuma, T.; et al. Radiothrerapy for elderly patients aged ≥75 years with clinically localized prostate cancer—Is there a role of brachytherapy? J. Clin. Med. 2018, 7, 424. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Masui, K.; Iwata, T.; Naitoh, Y.; Yamada, K.; Miki, T.; Okihara, K. Permanent prostate brachytherapy and short-term androgen deprivation for intermediate-risk prostate cancer in Japanese men: Outcome and toxicity. Brachytherapy 2015, 14, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Okihara, K.; Kobayashi, K.; Iwata, T.; Naitoh, Y.; Kamoim, K.; Kawauchim, A.; Yamadam, K.; Mikim, T. Assessment of permanent brachytherapy combined with androgen deprivation therapy in an intermediate-risk prostate cancer group without a Gleason score of 4 + 3: A single Japanese institutional experience. Int. J. Urol. 2014, 21, 271–276. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Nose, T.; Yoshida, K.; Inoue, T.; Yamazaki, H.; Tanaka, E.; Shiomi, H.; Imai, A.; Nakamura, S.; Shimamoto, S.; et al. High-dose-rate interstitial brachytherapy as a monotherapy for localized prostate cancer: Treatment description and preliminary results of a phase I/II clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 675–681. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamazaki, H.; Takenaka, T.; Kotsuma, T.; Yoshida, M.; Masui, K.; Yoshioka, Y.; Narumi, Y.; Oka, T.; Tanaka, E. High-dose-rate interstitial brachytherapy in combination with androgen deprivation therapy for prostate cancer: Are high-risk patients good candidates? Strahlenther. Onkol. 2014, 190, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Yamazaki, H.; Shimizu, D.; Suzuki, G.; Masui, K.; Nakamura, S.; Okabe, H.; Nishikawa, T.; Yoshida, K. Long-term outcomes of a dose-reduction trial to decrease late gastrointestinal toxicity in patients with prostate cancer receiving soft tissue-matched Image-guided intensity-modulated radiotherapy. Anticancer Res. 2018, 38, 385–391. [Google Scholar] [PubMed]
- Shimizu, D.; Yamazaki, H.; Nishimura, T.; Aibe, N.; Okabe, H. Long-term tumor control and late toxicity in patients with prostate cancer receiving hypofractionated (2.2 Gy) soft-tissue-matched image-guided intensity-modulated radiotherapy. Anticancer. Res. 2017, 37, 5829–5835. [Google Scholar] [PubMed]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Stevens, R.E.; Hodges, C.V. Studies on prostatic cancer. II. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg. 1941, 3, 209–223. [Google Scholar] [CrossRef]
- Akaza, H. Asian trends in primary androgen depletion therapy on prostate cancer. Cancer Biol. Med. 2013, 10, 187–191. [Google Scholar] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Kee, D.L.C.; Gal, J.; Falk, A.T.; Schiappa, R.; Chand, M.E.; Gautier, M.; Doyen, J.; Hannoun-Levi, J.M. Brachytherapy versus external beam radiotherapy boost for prostate cancer: Systematic review with meta-analysis of randomized trials. Cancer Treat Rev. 2018, 70, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; Gonzalez San Sequndo, C.; Cabeza Rodríguez, M.A.; Macias, V.; Pedro Olive, A.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localized prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet. Oncol. 2015, 16, 320–327. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2006, 24, 4448–4456. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, C.G.; Nørgaard, M.; Borre, M. Androgen-deprivation therapy in treatment of prostate cancer and risk of myocardial infarction and stroke: A nationwide Danish population-based cohort study. Eur. Urol. 2014, 65, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Lapi, F.; Azoulay, L.; Niazi, M.T.; Yin, H.; Benayoun, S.; Suissa, S. Androgen deprivation therapy and risk of acute kidney injury in patients with prostate cancer. J. Am. Med. Assoc. 2013, 310, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.; Williams, S.B.; O’Malley, A.J.; Smith, M.R.; Nguyen, P.L.; Keating, N.L. Androgen-deprivation therapy for nonmetastatic prostate cancer is associated with an increased risk of peripheral arterial disease and venous thromboembolism. Eur. Urol. 2012, 61, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Beyer, D.C.; McKeough, T.; Thomas, T. Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Abdollah, F.; Sammon, J.D.; Reznor, G.; Sood, A.; Schmid, M.; Klett, D.E.; Sun, M.; Aizer, A.A.; Choueiri, T.K.; Hu, J.C.; et al. Edical androgen deprivation therapy and increased non-cancer mortality in non-metastatic prostate cancer patients aged ≥66 years. Eur. J. Surg. Oncol. 2015, 41, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Chen, M.H.; Braccioforte, M.H.; Moran, B.J.; D’Amico, A.V. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA 2009, 302, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Morgans, A.K.; Fan, K.H.; Koyama, T.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Stanford, J.L.; Stroup, A.M.; Resnick, M.J.; et al. Influence of age on incident diabetes and cardiovascular disease in prostate cancer survivors receiving androgen deprivation therapy. J. Urol. 2015, 193, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
| Variables | Strata | ADT (+) n = 867 | ADT (−) n = 258 | p Value | ||
|---|---|---|---|---|---|---|
| ADT ≥ 2 Years n = 135 | ADT < 2 Years n = 732 | ADT (+) (%) | ||||
| No. or Median (Range) | No. or Median (Range) | No. or Median (Range) | ||||
| Age | 50 | 11 | 67 | (68%) | 36 | 0.0591 |
| 60 | 40 | 279 | (75%) | 107 | ||
| 70 | 50 | 222 | (80%) | 69 | ||
| 75 | 34 | 164 | (81%) | 46 | ||
| T category | 1 | 11 | 271 | (68%) | 133 | <0.0001 |
| 2 | 32 | 367 | (77%) | 119 | ||
| 3 | 87 | 90 | (97%) | 6 | ||
| 4 | 5 | 4 | (100%) | 0 | ||
| Pretreatment PSA | ng/mL | 23.4 (3.19–378) | 8.62 (1.4–337) | 6.5 (2.34–61) | <0.0001 | |
| Gleason score | ≤6 | 27 | 295 | (67%) | 159 | <0.0001 |
| 7 | 35 | 298 | (82%) | 75 | exc NA | |
| 8≤ | 58 | 137 | (89%) | 23 | ||
| NA | 15 | 2 | (94%) | 1 | ||
| NCCN risk classification | Low | 0 | 145 | (54%) | 122 | <0.0001 |
| Intermediate | 19 | 356 | (79%) | 100 | ||
| High | 116 | 231 | (91%) | 36 | ||
| IG-IMRT | 24 | 149 | (65%) | 93 | <0.0001 | |
| BT | 111 | 583 | (81%) | 165 | ||
| LDR-BT | 2 | 397 | (82%) | 87 | ||
| HDR-BT | 109 | 186 | (79%) | 78 | ||
| Follow-up | Months | 82.9 (14–241) | 83.0 (5–192) | 77.2 (14.5–161) | 0.0037 | |
| Variables | Strata | Died n = 81 | Died (+) % | Alive n = 1044 | p Value | |
|---|---|---|---|---|---|---|
| No. | No. | |||||
| Total | ADT (−) | 10 | (4%) | 862 | <0.0001 | |
| ADT < 2Y | 48 | (7%) | 70 | |||
| ADT ≥ 2Y | 23 | (17%) | 112 | |||
| NCCN risk classification | Low | ADT (−) | 4 | (3%) | 118 | 0.7114 |
| ADT < 2Y | 6 | (4%) | 139 | |||
| ADT ≥ 2Y | 0 | NA | 0 | |||
| Intermediate | ADT (−) | 3 | (3%) | 97 | 0.3683 | |
| ADT < 2Y | 19 | (5%) | 337 | |||
| ADT ≥ 2Y | 2 | (11%) | 17 | |||
| High | ADT (−) | 3 | (8%) | 33 | 0.0798 | |
| ADT < 2Y | 23 | (10%) | 208 | |||
| ADT ≥ 2Y | 21 | (18%) | 95 | |||
| Age | Young <75 | ADT (−) | 8 | (4%) | 204 | 0.0017 |
| ADT < 2Y | 32 | (6%) | 536 | |||
| ADT ≥ 2Y | 14 | (14%) | 87 | |||
| Elder ≥75 | ADT (−) | 2 | (4%) | 44 | 0.0050 | |
| ADT < 2Y | 16 | (10%) | 148 | |||
| ADT ≥ 2Y | 9 | (26%) | 25 |
| Variables | Strata | OCM (+) n = 71 | OCM (+) (%) | OCM (−) n = 1054 | p Value |
|---|---|---|---|---|---|
| No. or Median (Range) | No. or Median (Range) | ||||
| Age | <60 | 2 | (2%) | 112 | 0.0017 |
| 60-69 | 19 | (4%) | 407 | ||
| 70–74 | 24 | (7%) | 317 | ||
| 75≤ | 26 | (11%) | 218 | ||
| T category | 1 | 18 | (4%) | 397 | 0.0004 |
| 2 | 29 | (6%) | 489 | ||
| 3 | 24 | (13%) | 159 | ||
| 4 | 0 | (0%) | 9 | ||
| iPSA | ng/mL | 11.95 (2.68–245) | 8.40 (1.4–375) | 0.0151 | |
| Gleason score | ≤6 | 28 | (6%) | 453 | 0.9507 |
| 7 | 25 | (6%) | 383 | exc NA | |
| 8≤ | 14 | (6%) | 204 | ||
| NA | 4 | (22%) | 14 | ||
| NCCN risk classification | Low | 10 | (4%) | 257 | 0.0018 |
| Intermediate | 23 | (5%) | 452 | ||
| High | 38 | (10%) | 345 | ||
| PSA failure | Yes | 1 | (1%) | 71 | 0.1272 |
| No | 70 | (7%) | 983 | ||
| ADT | Yes | 61 | (7%) | 797 | 0.0422 |
| No | 10 | (4%) | 257 | ||
| Duration | 6 (0–173) | 6 (0–45) | 0.0486 | ||
| IG-IMRT | 13 | (5%) | 253 | <0.0001 | |
| BT | 58 | (7%) | 801 | ||
| HDR-BT | 43 | (12%) | 330 | ||
| LDR-BT | 15 | (3%) | 471 | ||
| Variables | Strata | OCM (+) n = 71 | OCM (+) % | OCM (−) n = 1054 | p Value | |
|---|---|---|---|---|---|---|
| No. | No. | |||||
| Total | ADT (−) | 10 | (4%) | 257 | 0.0007 | |
| ADT < 2Y | 43 | (6%) | 680 | |||
| ADT ≥ 2Y | 18 | (13%) | 117 | |||
| NCCN risk classification | Low | ADT (−) | 4 | (3%) | 118 | 0.7126 |
| ADT < 2Y | 6 | (4%) | 139 | |||
| ADT ≥ 2Y | 0 | NA | 0 | |||
| Intermediate | ADT (−) | 2 | (2%) | 98 | 0.1945 | |
| ADT < 2Y | 19 | (5%) | 337 | |||
| ADT ≥ 2Y | 2 | (11%) | 17 | |||
| High | ADT (−) | 3 | (8%) | 33 | 0.2477 | |
| ADT < 2Y | 19 | (8%) | 212 | |||
| ADT ≥ 2Y | 16 | (14%) | 100 | |||
| Age | Young <75 | ADT (−) | 7 | (3%) | 205 | 0.0439 |
| ADT < 2Y | 28 | (5%) | 540 | |||
| ADT ≥ 2Y | 10 | (10%) | 91 | |||
| Elder ≥75 | ADT (−) | 2 | (4%) | 44 | 0.0185 | |
| ADT < 2Y | 16 | (10%) | 148 | |||
| ADT ≥ 2Y | 8 | (24%) | 26 |
| Variables | Strata | Elder ≥75 n = 244 | Young <75 n = 881 | p Value | ||
|---|---|---|---|---|---|---|
| No. or Median (Range) | (%) | No. or Median (Range) | (%) | |||
| T category | 1 | 73 | (30%) | 342 | (39%) | 0.0219 |
| 2 | 127 | (52%) | 391 | (44%) | ||
| 3 | 44 | (18%) | 139 | (16%) | ||
| 4 | 0 | (0%) | 9 | (1%) | ||
| Pretreatment PSA | ng/mL | 9.74 (1.97–245) | 8.32 (1.4–378) | 0.0449 | ||
| Gleason score | ≤6 | 82 | (34%) | 399 | (45%) | 0.0004 |
| 7 | 93 | (38%) | 315 | (36%) | exc NA | |
| 8≤ | 66 | (27%) | 152 | (17%) | ||
| NA | 3 | (1%) | 15 | (2%) | ||
| NCCN risk classification | Low | 36 | (15%) | 231 | (26%) | 0.0005 |
| Intermediate | 108 | (44%) | 367 | (42%) | ||
| High | 100 | (41%) | 283 | (32%) | ||
| PSA failure | Yes | 14 | (6%) | 58 | (7%) | 0.6329 |
| No | 230 | (94%) | 823 | (93%) | ||
| ADT | Yes | 198 | (81%) | 669 | (76%) | 0.0866 |
| No | 46 | (19%) | 212 | (24%) | ||
| Duration | 6 (0–120) | 6 (0–257) | 0.0274 | |||
| OCM | Yes | 26 | (11%) | 45 | (5%) | 0.0016 |
| No | 218 | (89%) | 836 | (95%) | ||
| IG-IMRT | 83 | (34%) | 183 | (21%) | <0.0001 | |
| BT | 161 | (66%) | 698 | (79%) | ||
| HDR-BT | 91 | (37%) | 282 | (32%) | ||
| LDR-BT | 70 | (29%) | 416 | (47%) | ||
| Total | ADT (−) n = 258 | ADT < 2Y n = 732 | ADT ≥ 2Y n = 135 | |||
| Cardiovascular | 3 | (0.4%) | 2 | (1.5%) | ||
| Cerebrovascular | 3 | (0.4%) | 2 | (1.5%) | ||
| Other malignancies | 7 | (2.7%) | 25 | (3.4%) | 7 | (5.2%) |
| Other | 2 | (0.8%) | 5 | (0.7%) | 2 | (1.5%) |
| Unknown | 2 | (0.8%) | 6 | (0.8%) | 5 | (3.7%) |
| Elder ≥ 75 | n = 46 | n = 164 | n = 34 | |||
| Cardiovascular | 2 | (1.2%) | ||||
| Cerebrovascular | 1 | (2.9%) | ||||
| Other malignancies | 1 | (2.2%) | 11 | (6.7%) | 2 | (5.9%) |
| Other | 1 | (2.2%) | 2 | (1.2%) | 1 | (2.9%) |
| Unknown | 1 | (0.6%) | 4 | (11.8%) | ||
| Young < 75 | n = 212 | n = 568 | n = 101 | |||
| Cardiovascular | 1 | (0.2%) | 2 | (2.0%) | ||
| Cerebrovascular | 3 | (0.5%) | 1 | (1.0%) | ||
| Other malignancies | 6 | (2.8%) | 14 | (2.5%) | 5 | (5.0%) |
| Other | 1 | (0.5%) | 3 | (0.5%) | 1 | (1.0%) |
| Unknown | 2 | (0.9%) | 5 | (0.9%) | 1 | (1.0%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, H.; Masui, K.; Suzuki, G.; Nakamura, S.; Aibe, N.; Shimizu, D.; Yamada, K.; Okihara, K.; Shiraishi, T.; Kotsuma, T.; et al. Effect of Androgen Deprivation Therapy on Other-Cause of Mortality in Elderly Patients with Clinically Localized Prostate Cancer Treated with Modern Radiotherapy: Is There a Negative Impact? J. Clin. Med. 2019, 8, 338. https://doi.org/10.3390/jcm8030338
Yamazaki H, Masui K, Suzuki G, Nakamura S, Aibe N, Shimizu D, Yamada K, Okihara K, Shiraishi T, Kotsuma T, et al. Effect of Androgen Deprivation Therapy on Other-Cause of Mortality in Elderly Patients with Clinically Localized Prostate Cancer Treated with Modern Radiotherapy: Is There a Negative Impact? Journal of Clinical Medicine. 2019; 8(3):338. https://doi.org/10.3390/jcm8030338
Chicago/Turabian StyleYamazaki, Hideya, Koji Masui, Gen Suzuki, Satoaki Nakamura, Norihiro Aibe, Daisuke Shimizu, Kei Yamada, Koji Okihara, Takumi Shiraishi, Tadayuki Kotsuma, and et al. 2019. "Effect of Androgen Deprivation Therapy on Other-Cause of Mortality in Elderly Patients with Clinically Localized Prostate Cancer Treated with Modern Radiotherapy: Is There a Negative Impact?" Journal of Clinical Medicine 8, no. 3: 338. https://doi.org/10.3390/jcm8030338
APA StyleYamazaki, H., Masui, K., Suzuki, G., Nakamura, S., Aibe, N., Shimizu, D., Yamada, K., Okihara, K., Shiraishi, T., Kotsuma, T., Yoshida, K., Tanaka, E., Otani, K., Yoshioka, Y., Ogawa, K., Nishikawa, T., & Okabe, H. (2019). Effect of Androgen Deprivation Therapy on Other-Cause of Mortality in Elderly Patients with Clinically Localized Prostate Cancer Treated with Modern Radiotherapy: Is There a Negative Impact? Journal of Clinical Medicine, 8(3), 338. https://doi.org/10.3390/jcm8030338







