Predictors of Uric Acid Stones: Mean Stone Density, Stone Heterogeneity Index, and Variation Coefficient of Stone Density by Single-Energy Non-Contrast Computed Tomography and Urinary pH
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. NCCT Determinants and Stone Composition Analyses
2.3. Statistical Analyses
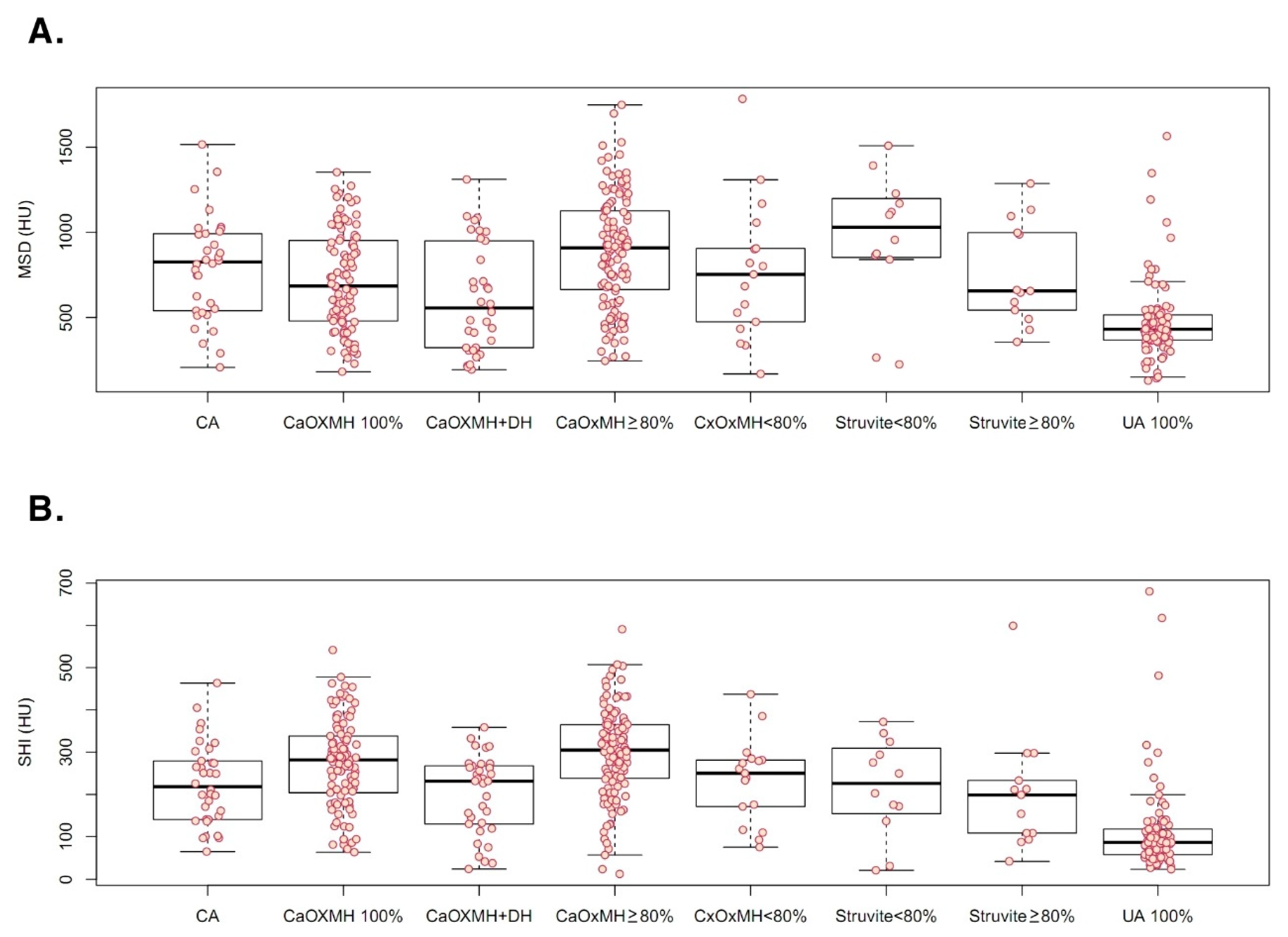
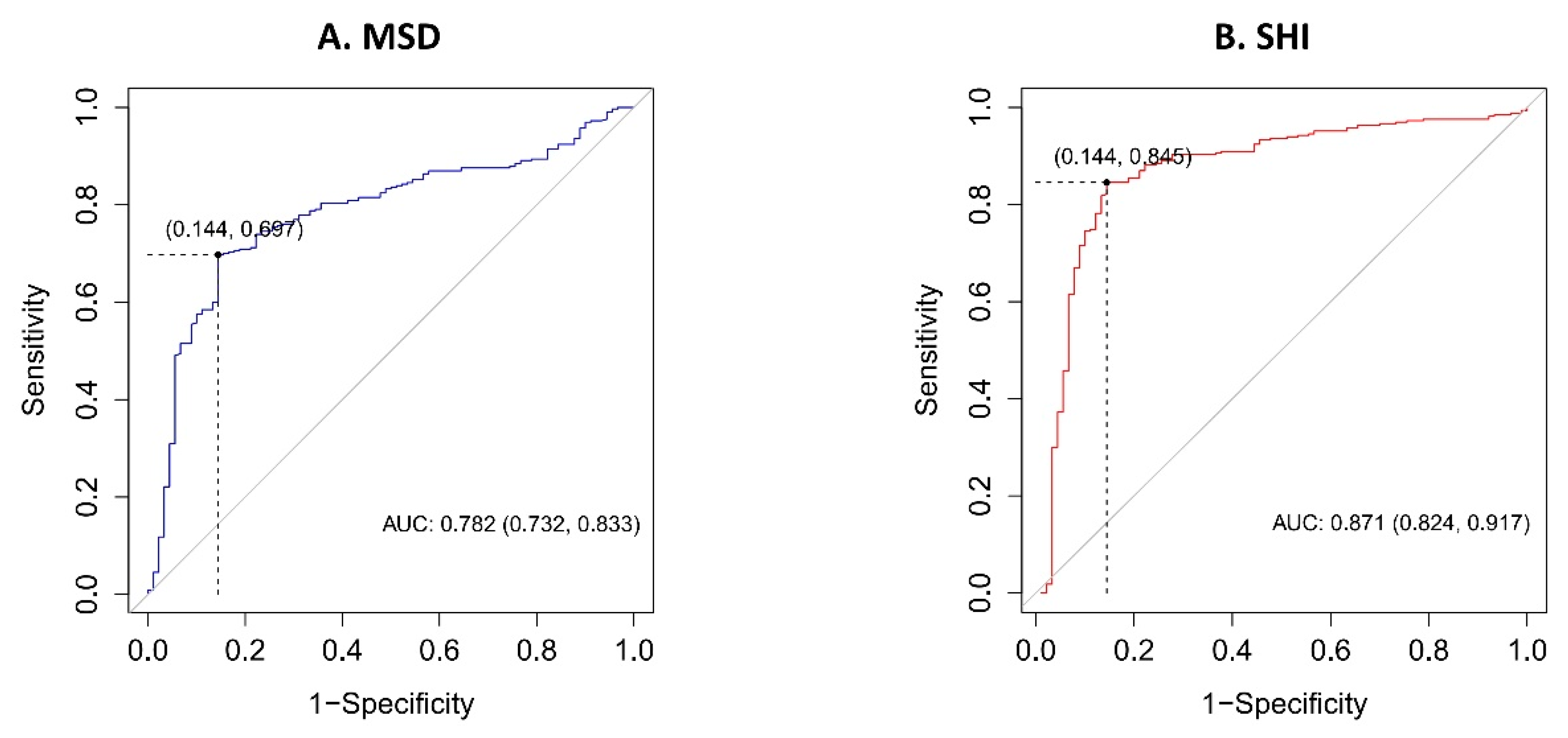
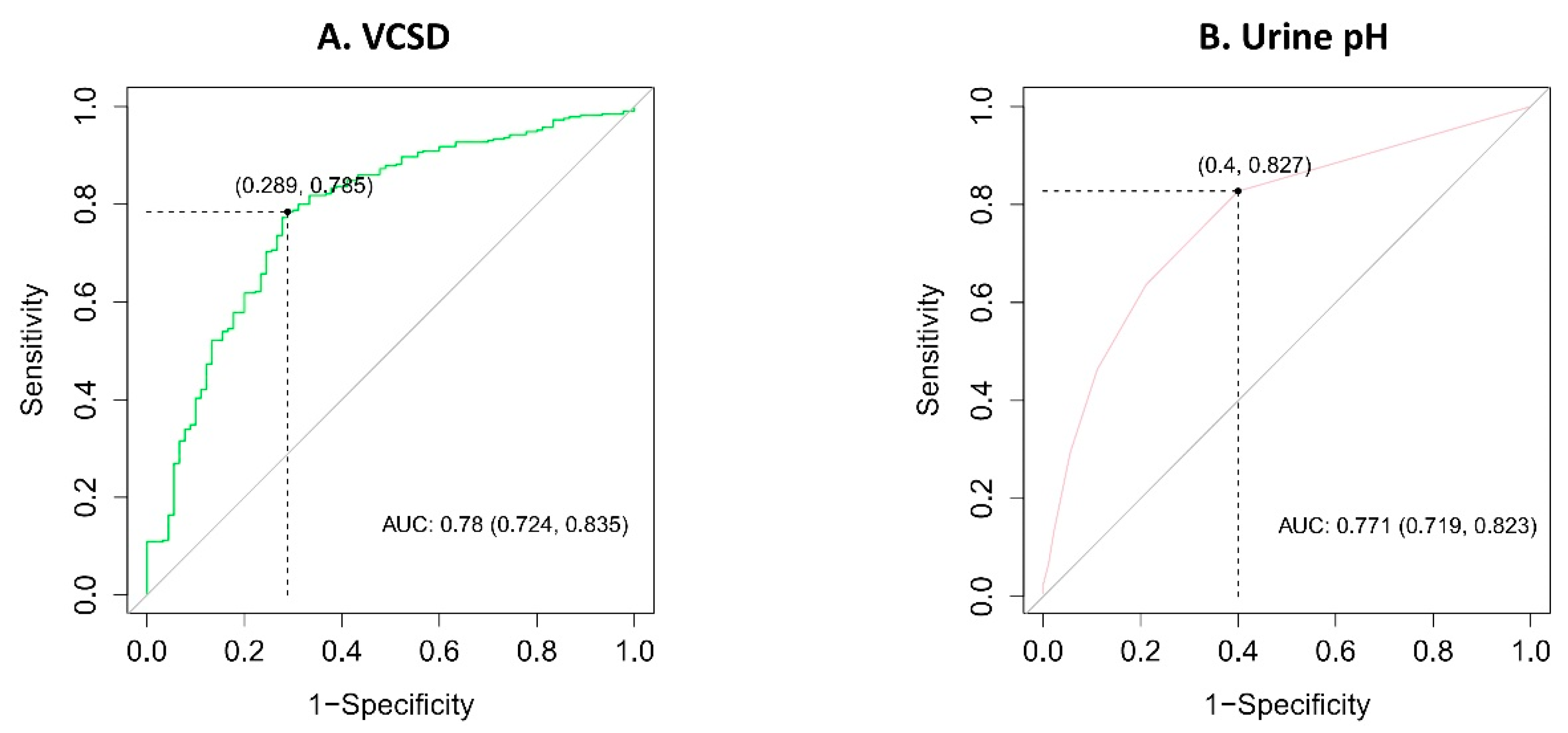
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kang, S.K.; Cho, K.S.; Kang, D.H.; Jung, H.D.; Kwon, J.K.; Lee, J.Y. Systematic review and meta-analysis to compare success rates of retrograde intrarenal surgery versus percutaneous nephrolithotomy for renal stones >2cm: An update. Medicine 2017, 96, e9119. [Google Scholar] [CrossRef]
- Turk, C.; Petrik, A.; Sarica, K.; Seitz, C.; Skolarikos, A.; Straub, M.; Knoll, T. Eau guidelines on diagnosis and conservative management of urolithiasis. Eur. Urol. 2016, 69, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Turk, C.; Petrik, A.; Sarica, K.; Seitz, C.; Skolarikos, A.; Straub, M.; Knoll, T. Eau guidelines on interventional treatment for urolithiasis. Eur. Urol. 2016, 69, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Pittomvils, G.; Vandeursen, H.; Wevers, M.; Lafaut, J.P.; De Ridder, D.; De Meester, P.; Boving, R.; Baert, L. The influence of internal stone structure upon the fracture behaviour of urinary calculi. Ultrasound Med. Boil. 1994, 20, 803–810. [Google Scholar] [CrossRef]
- Zhong, P.; Preminger, G.M. Mechanisms of differing stone fragility in extracorporeal shockwave lithotripsy. J. Endourol. 1994, 8, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Teichman, J.M.; Vassar, G.J.; Bishoff, J.T.; Bellman, G.C. Holmium: Yag lithotripsy yields smaller fragments than lithoclast, pulsed dye laser or electrohydraulic lithotripsy. J. Urol. 1998, 159, 17–23. [Google Scholar] [CrossRef]
- Chugtai, M.N.; Khan, F.A.; Kaleem, M.; Ahmed, M. Management of uric acid stone. JPMA. J. Pak. Med. Assoc. 1992, 42, 153–155. [Google Scholar]
- Moreira, D.M.; Friedlander, J.I.; Hartman, C.; Elsamra, S.E.; Smith, A.D.; Okeke, Z. Using 24-hour urinalysis to predict stone type. J. Urol. 2013, 190, 2106–2111. [Google Scholar] [CrossRef]
- Kim, J.H.; Doo, S.W.; Cho, K.S.; Yang, W.J.; Song, Y.S.; Hwang, J.; Hong, S.S.; Kwon, S.S. Which anthropometric measurements including visceral fat, subcutaneous fat, body mass index, and waist circumference could predict the urinary stone composition most? BMC Urol. 2015, 15, 17. [Google Scholar] [CrossRef]
- Chang, K.D.; Lee, J.Y.; Park, S.Y.; Kang, D.H.; Lee, H.H.; Cho, K.S. Impact of pretreatment hydronephrosis on the success rate of shock wave lithotripsy in patients with ureteral stone. Yonsei Med. J. 2017, 58, 1000–1005. [Google Scholar] [CrossRef]
- Chi, B.H.; Chang, I.H.; Lee, D.H.; Park, S.B.; Kim, K.D.; Moon, Y.T.; Hur, T. Low-dose unenhanced computed tomography with iterative reconstruction for diagnosis of ureter stones. Yonsei Med. J. 2018, 59, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.Y.; Cho, K.S.; Lee, D.H.; Han, J.H.; Kang, D.H.; Jung, H.D.; Kown, J.K.; Ham, W.S.; Choi, Y.D.; Lee, J.Y. Impact of colic pain as a significant factor for predicting the stone free rate of one-session shock wave lithotripsy for treating ureter stones: A bayesian logistic regression model analysis. PLoS ONE 2015, 10, e0123800. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, J.H.; Kang, D.H.; Chung, D.Y.; Lee, D.H.; Do Jung, H.; Kwon, J.K.; Cho, K.S. Stone heterogeneity index as the standard deviation of hounsfield units: A novel predictor for shock-wave lithotripsy outcomes in ureter calculi. Sci. Rep. 2016, 6, 23988. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Kohjimoto, Y.; Iguchi, T.; Nishizawa, S.; Iba, A.; Kikkawa, K.; Hara, I. Variation coefficient of stone density: A novel predictor of the outcome of extracorporeal shockwave lithotripsy. J. Endourol. 2017, 31, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Parks, J.H.; Worcester, E.M.; Coe, F.L.; Evan, A.P.; Lingeman, J.E. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004, 66, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Kacker, R.; Meeks, J.J.; Zhao, L.; Nadler, R.B. Decreased stone-free rates after percutaneous nephrolithotomy for high calcium phosphate composition kidney stones. J. Urol. 2008, 180, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Dretler, S.P.; Pfister, R.C. Percutaneous dissolution of renal calculi. Ann. Rev. Med. 1983, 34, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.; Sakhaee, K.; Peterson, R.D.; Poindexter, J.R.; Frawley, W.H. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001, 60, 757–761. [Google Scholar] [CrossRef]
- Spettel, S.; Shah, P.; Sekhar, K.; Herr, A.; White, M.D. Using hounsfield unit measurement and urine parameters to predict uric acid stones. Urology 2013, 82, 22–26. [Google Scholar] [CrossRef]
- Lee, J.S.; Cho, K.S.; Lee, S.H.; Yoon, Y.E.; Kang, D.H.; Jeong, W.S.; Jung, H.D.; Kwon, J.K.; Lee, J.Y. Stone heterogeneity index on single-energy noncontrast computed tomography can be a positive predictor of urinary stone composition. PLoS ONE 2018, 13, e0193945. [Google Scholar] [CrossRef]
- Wilhelm, K.; Schoenthaler, M.; Hein, S.; Adams, F.; Schlager, D.; Kuehhas, F.E.; Sevcenco, S.; Pache, G.; Langer, M.; Bulla, S.; et al. Focused dual-energy ct maintains diagnostic and compositional accuracy for urolithiasis using ultralow-dose noncontrast ct. Urology 2015, 86, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.; Strittmatter, F.; Graser, A.; Kufer, P.; Stief, C.; Staehler, M. Dual energy can accurately differentiate uric acid-containing urinary calculi from calcium stones. World J. Urol. 2016, 34, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Oh, T.H.; Seo, I.Y. Can a dual-energy computed tomography predict unsuitable stone components for extracorporeal shock wave lithotripsy? Korean J. Urol. 2015, 56, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Mandal, A.K.; Singh, S.K.; Mandal, P.; Sankhwar, S.N.; Sharma, S.K. Computerized tomography attenuation value of renal calculus: Can it predict successful fragmentation of the calculus by extracorporeal shock wave lithotripsy? A preliminary study. J. Urol. 2002, 167, 1968–1971. [Google Scholar] [CrossRef]
- Gupta, N.P.; Ansari, M.S.; Kesarvani, P.; Kapoor, A.; Mukhopadhyay, S. Role of computed tomography with no contrast medium enhancement in predicting the outcome of extracorporeal shock wave lithotripsy for urinary calculi. BJU Int. 2005, 95, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- El-Nahas, A.R.; El-Assmy, A.M.; Mansour, O.; Sheir, K.Z. A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: The value of high-resolution noncontrast computed tomography. Eur. Urol. 2007, 51, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Kohjimoto, Y.; Iwahashi, Y.; Iguchi, T.; Nishizawa, S.; Kikkawa, K.; Hara, I. Noncontrast computed tomography parameters for predicting shock wave lithotripsy outcome in upper urinary tract stone cases. BioMed Res. Int. 2018, 2018, 9253952. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Kim, J.C.; Kang, D.H.; Lee, J.Y. Digital videoscopic retrograde intrarenal surgeries for renal stones: Time-to-maximal stone length ratio analysis. Yonsei Med. J. 2018, 59, 303–309. [Google Scholar] [CrossRef]
- Jung, H.D.; Kim, J.C.; Ahn, H.K.; Kwon, J.H.; Han, K.; Han, W.K.; Kim, M.D.; Lee, J.Y. Real-time simultaneous endoscopic combined intrarenal surgery with intermediate-supine position: Washout mechanism and transport technique. Investig. Clin. Urol. 2018, 59, 348–354. [Google Scholar] [CrossRef]

| Total | CaOx Compound | Infectious | Uric Acid | p-Value | |
|---|---|---|---|---|---|
| No. of patients | 420 | 271 | 59 | 90 | |
| Age | 55.55 ± 15.46 | 54.45 ± 15.75 | 52.51 ± 14.43 | 60.88 ± 14.07 | 0.001 a |
| Sex | 0.002 b | ||||
| Male | 256 (60.95%) | 164 (60.52%) | 26 (44.07%) | 66 (73.33%) | |
| Female | 164 (39.05%) | 107 (39.48%) | 33 (55.93%) | 24 (26.67%) | |
| Procedures | |||||
| SWL | 11 (2.62%) | 9 (3.32%) | 1 (1.69%) | 1 (1.11%) | |
| laparoscopic pyelolithotomy | 3 (0.71%) | 2 (0.74%) | 0 (0.00%) | 1 (1.11%) | |
| laparoscopic ureterolithotomy | 30 (7.14%) | 23 (8.49%) | 5 (8.47%) | 2 (2.22%) | |
| PCNL | 63 (15.00%) | 27 (9.96%) | 19 (32.20%) | 17 (18.89%) | |
| RIRS | 106 (25.24%) | 63 (23.25%) | 10 (16.95%) | 33 (36.67%) | |
| URSL | 169 (40.24%) | 133 (49.08%) | 15 (25.42%) | 21 (23.33%) | |
| vesicolitholapaxy | 25 (5.95%) | 5 (1.85%) | 9 (15.25%) | 11 (12.22%) | |
| Spontaneous passage | 13 (3.10%) | 9 (3.32%) | 0 (0.00%) | 4 (4.44%) | |
| Location | |||||
| Kidney | 149 (35.48%) | 77 (28.41%) | 31 (52.54%) | 41 (45.56%) | |
| Upper ureter | 128 (30.48%) | 93 (34.32%) | 12 (20.34%) | 23 (25.56%) | |
| Mid-ureter | 20 (4.76%) | 19 (7.01%) | 0 (0.00%) | 1 (1.11%) | |
| Lower ureter | 100 (23.81%) | 78 (28.78%) | 8 (13.56%) | 14 (15.56%) | |
| Bladder | 23 (5.48%) | 4 (1.48%) | 8 (13.56%) | 11 (12.22%) | |
| MSL | 14.03 ± 10.90 | 11.05 ± 6.94 | 21.90 ± 16.96 | 17.84 ± 11.94 | <0.001 a |
| MSD | 722.49 ± 337.79 | 782.60 ± 333.52 | 818.11 ± 324.46 | 478.84 ± 232.41 | <0.001 a |
| SHI | 230.71 ± 125.22 | 272.10 ± 107.97 | 219.00 ± 110.24 | 113.79 ± 106.79 | <0.001 a |
| VCSD | 32.75 ± 13.72 | 37.13 ± 12.99 | 27.63 ± 11.46 | 22.93 ± 10.77 | <0.001 a |
| Urine pH | 6.05 ± 0.94 | 6.14 ± 0.87 | 6.61 ± 1.10 | 5.41 ± 0.64 | <0.001 a |
| Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Univariate | |||
| Age | 1.032 | 1.015–1.050 | <0.001 |
| Sex (Male) | 2.026 | 1.224–3.448 | 0.007 |
| MSL | 1.036 | 1.016–1.057 | <0.001 |
| MSD | 0.996 | 0.995–0.997 | <0.001 |
| SHI | 0.985 | 0.982–0.988 | <0.001 |
| VCSD | 0.914 | 0.891–0.936 | <0.001 |
| Urinary pH | 0.244 | 0.158–0.360 | <0.001 |
| Multivariate (with MSD & SHI) | |||
| Age | 1.033 | 1.010–1.057 | 0.005 |
| Sex (Male) | 1.516 | 0.766–3.048 | 0.236 |
| MSL | 1.052 | 1.021–1.087 | 0.001 |
| MSD | 0.998 | 0.996–1.000 | 0.038 |
| SHI | 0.989 | 0.984–0.994 | <0.001 |
| Urinary pH | 0.359 | 0.226–0.543 | <0.001 |
| Multivariate (with VCSD) | |||
| Age | 1.021 | 1.001–1.042 | 0.048 |
| Sex (Male) | 1.859 | 1.012–3.495 | 0.049 |
| MSL | 0.997 | 0.971–1.022 | 0.791 |
| VCSD | 0.909 | 0.881–0.936 | <0.001 |
| Urinary pH | 0.275 | 0.173–0.416 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.C.; Cho, K.S.; Kim, D.K.; Chung, D.Y.; Jung, H.D.; Lee, J.Y. Predictors of Uric Acid Stones: Mean Stone Density, Stone Heterogeneity Index, and Variation Coefficient of Stone Density by Single-Energy Non-Contrast Computed Tomography and Urinary pH. J. Clin. Med. 2019, 8, 243. https://doi.org/10.3390/jcm8020243
Kim JC, Cho KS, Kim DK, Chung DY, Jung HD, Lee JY. Predictors of Uric Acid Stones: Mean Stone Density, Stone Heterogeneity Index, and Variation Coefficient of Stone Density by Single-Energy Non-Contrast Computed Tomography and Urinary pH. Journal of Clinical Medicine. 2019; 8(2):243. https://doi.org/10.3390/jcm8020243
Chicago/Turabian StyleKim, Jong Chan, Kang Su Cho, Do Kyung Kim, Doo Yong Chung, Hae Do Jung, and Joo Yong Lee. 2019. "Predictors of Uric Acid Stones: Mean Stone Density, Stone Heterogeneity Index, and Variation Coefficient of Stone Density by Single-Energy Non-Contrast Computed Tomography and Urinary pH" Journal of Clinical Medicine 8, no. 2: 243. https://doi.org/10.3390/jcm8020243
APA StyleKim, J. C., Cho, K. S., Kim, D. K., Chung, D. Y., Jung, H. D., & Lee, J. Y. (2019). Predictors of Uric Acid Stones: Mean Stone Density, Stone Heterogeneity Index, and Variation Coefficient of Stone Density by Single-Energy Non-Contrast Computed Tomography and Urinary pH. Journal of Clinical Medicine, 8(2), 243. https://doi.org/10.3390/jcm8020243






