Neoadjuvant Chemotherapy with Taxane and Platinum Followed by Radical Hysterectomy for Stage IB2-IIB Cervical Cancer: Impact of Histology Type on Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Eligibility
2.2. Clinical Information
2.3. Study Definition
2.4. Statistical Consideration
3. Results
3.1. Study Cohort
3.2. Chemotherapy Choices per Histology Type
3.3. Patient Demographics per Histology Type
3.4. Histology-specific Surgical-Pathological Factors
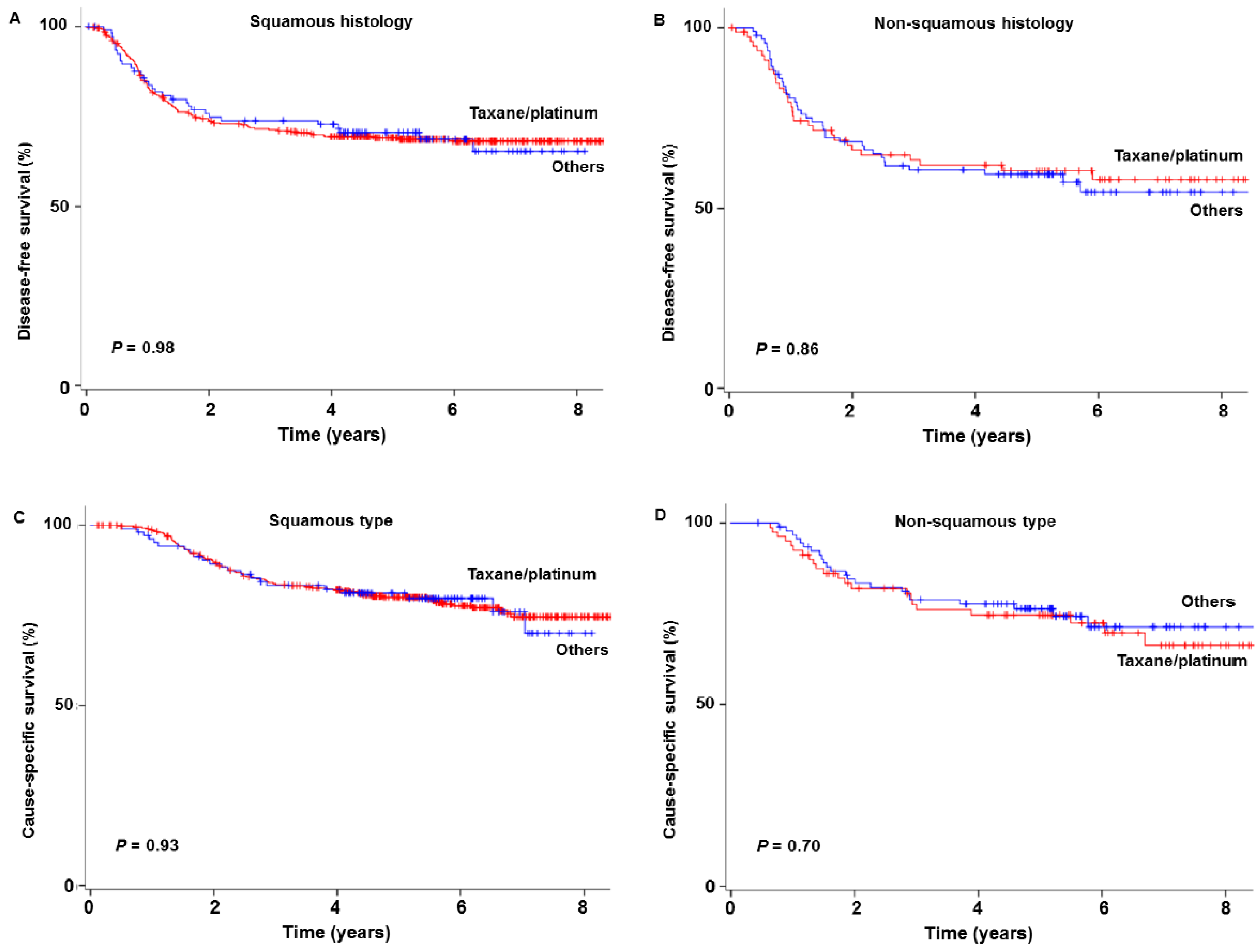
3.5. Survival Outcome
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Clinical Practice Guideline in Oncology. Cervical Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 19 December 2018).
- Saito, T.; Katabuchi, H. Annual Report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: Patient Annual Report for 2013 and Treatment Annual Report for 2008. J. Obstet. Gynaecol. Res. 2016, 42, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Mikami, M.; Aoki, Y.; Sakamoto, M.; Shimada, M.; Takeshima, N.; Fujiwara, H.; Matsumoto, T.; Kita, T.; Takizawa, K.; Disease Committee of Uterine Cervical and Vulvar Cancer, Japanese Gynecologic Oncology Group. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (Bulky Tumors) in Japan: A survey of the Japanese Gynecologic Oncology Group. Int. J. Gynecol. Cancer 2014, 24, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- Mabuchi, S.; Okazawa, M.; Isohashi, F.; Matsuo, K.; Ohta, Y.; Suzuki, O.; Yoshioka, Y.; Enomoto, T.; Kamiura, S.; Kimura, T. Radical hysterectomy with adjuvant radiotherapy versus definitive radiotherapy alone for FIGO stage IIB cervical cancer. Gynecol. Oncol. 2011, 123, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Falcetta, F.S.; Medeiros, L.R.; Edelweiss, M.I.; Pohlmann, P.R.; Stein, A.T.; Rosa, D.D. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst. Rev. 2016, 11, CD005342. [Google Scholar] [CrossRef]
- Eddy, G.L.; Bundy, B.N.; Creasman, W.T.; Spirtos, N.M.; Mannel, R.S.; Hannigan, E.; O’Connor, D. Treatment of (“bulky”) stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy: A phase III trial of the gynecologic oncology group. Gynecol. Oncol. 2007, 106, 362–369. [Google Scholar] [CrossRef]
- Rydzewska, L.; Tierney, J.; Vale, C.L.; Symonds, P.R. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst. Rev. 2013, 12, CD007406. [Google Scholar]
- Gien, L.T.; Beauchemin, M.C.; Thomas, G. Adenocarcinoma: A unique cervical cancer. Gynecol. Oncol. 2010, 116, 140–146. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Albert Einstein College of Medicine; Analytical Biological Services; Barretos Cancer Hospital; Baylor College of Medicine; Beckman Research Institute of City of Hope; Buck Institute for Research on Aging; Canada’s Michael Smith Genome Sciences Centre; Harvard Medical School; Helen, F. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar]
- Kitagawa, R.; Katsumata, N.; Shibata, T.; Kamura, T.; Kasamatsu, T.; Nakanishi, T.; Nishimura, S.; Ushijima, K.; Takano, M.; Satoh, T.; et al. Paclitaxel Plus Carboplatin Versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. J. Clin. Oncol. 2015, 33, 2129–2135. [Google Scholar] [CrossRef]
- Monk, B.J.; Sill, M.W.; McMeekin, D.S.; Cohn, D.E.; Ramondetta, L.M.; Boardman, C.H.; Benda, J.; Cella, D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J. Clin. Oncol. 2009, 27, 4649–4655. [Google Scholar] [CrossRef]
- Moore, D.H.; Blessing, J.A.; McQuellon, R.P.; Thaler, H.T.; Cella, D.; Benda, J.; Miller, D.S.; Olt, G.; King, S.; Boggess, J.F.; et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: A gynecologic oncology group study. J. Clin. Oncol. 2004, 22, 3113–3119. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Long, H.J., 3rd; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014, 370, 734–743. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Aoki, Y.; Sakamoto, M.; Takeshima, N.; Fujiwara, H.; Matsumoto, T.; Mikami, M.; Sugiyama, T. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: Systemic chemotherapy versus pelvic irradiation. Int. J. Cancer 2017, 141, 1042–1051. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Mikami, M. Ovarian conservation for young women with clinical stage IB-IIB cervical cancer in Japan. J. Gynecol. Oncol. 2017, 28, e60. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Yamaguchi, S.; Kanao, H.; Nakanishi, T.; Saito, T.; Kamiura, S.; Iwata, T.; Mikami, M.; Sugiyama, T. Identifying a candidate population for ovarian conservation in young women with clinical stage IB-IIB cervical cancer. Int. J. Cancer 2018, 142, 1022–1032. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Saito, T.; Takehara, K.; Tokunaga, H.; Watanabe, Y.; Todo, Y.; Morishige, K.I.; Mikami, M.; Sugiyama, T. Risk stratification models for para-aortic lymph node metastasis and recurrence in stage IB-IIB cervical cancer. J. Gynecol. Oncol. 2018, 29, e11. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Yokota, H.; Satoh, T.; Katabuchi, H.; Kodama, S.; Sasaki, H.; Matsumura, N.; Mikami, M.; Sugiyama, T. Effectiveness of adjuvant systemic chemotherapy for intermediate-risk stage IB cervical cancer. Oncotarget 2018, 8, 106866–106875. [Google Scholar] [CrossRef]
- FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynaecol. Obstet. 2014, 125, 97–98. [Google Scholar] [CrossRef]
- Ebina, Y.; Yaegashi, N.; Katabuchi, H.; Nagase, S.; Udagawa, Y.; Hachisuga, T.; Saito, T.; Mikami, M.; Aoki, Y.; Yoshikawa, H. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int. J. Clin. Oncol. 2015, 20, 240–248. [Google Scholar] [CrossRef]
- ONDA, T.; Satoh, T.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Takehara, K.; Miyamoto, K.; Wakabayashi, M.; Okamoto, A.; Ushijima, K.; et al. Comparison of survival between upfront primary debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomized trial: JCOG0602. In Proceedings of the 2018 Annual Meeting of American Society of Clinical Oncology, Chicago, IL, USA, 1–5 June 2018. [Google Scholar]
- Sardi, J.E.; Giaroli, A.; Sananes, C.; Ferreira, M.; Soderini, A.; Bermudez, A.; Snaidas, L.; Vighi, S.; Gomez Rueda, N.; di Paola, G. Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: The final results. Gynecol. Oncol. 1997, 67, 61–69. [Google Scholar] [CrossRef]
- Napolitano, U.; Imperato, F.; Mossa, B.; Framarino, M.L.; Marziani, R.; Marzetti, L. The role of neoadjuvant chemotherapy for squamous cell cervical cancer (Ib-IIIb): A long-term randomized trial. Eur. J. Gynaecol. Oncol. 2003, 24, 51–59. [Google Scholar]
- Gupta, S.; Maheshwari, A.; Parab, P.; Mahantshetty, U.; Hawaldar, R.; Sastri Chopra, S.; Kerkar, R.; Engineer, R.; Tongaonkar, H.; Ghosh, J.; et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients with Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 1548–1555. [Google Scholar] [CrossRef]
- Benedetti-Panici, P.; Greggi, S.; Colombo, A.; Amoroso, M.; Smaniotto, D.; Giannarelli, D.; Amunni, G.; Raspagliesi, F.; Zola, P.; Mangioni, C.; et al. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: Results from the Italian multicenter randomized study. J. Clin. Oncol. 2002, 20, 179–188. [Google Scholar] [CrossRef]
- Katsumata, N.; Yoshikawa, H.; Hirakawa, T.; Saito, T.; Kuzuya, K.; Fujii, T.; Hiura, M.; Tsunematsu, R.; Fukuda, H.; Kamura, T. Phase III randomized trial of neoadjuvant chemotherapy (NAC) followed by radical hysterectomy (RH) versus RH for bulky stage I/II cervical cancer (JCOG 0102). In Proceedings of the 2006 ASCO Annual Meeting Proceedings 5013, Alexandria, VA, USA, 1–6 June 2006. [Google Scholar]
- Buda, A.; Fossati, R.; Colombo, N.; Fei, F.; Floriani, I.; Gueli Alletti, D.; Katsaros, D.; Landoni, F.; Lissoni, A.; Malzoni, C.; et al. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: The SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study. J. Clin. Oncol. 2005, 23, 4137–4145. [Google Scholar]
- Cai, H.B.; Chen, H.Z.; Yin, H.H. Randomized study of preoperative chemotherapy versus primary surgery for stage IB cervical cancer. J. Obstet. Gynaecol. Res. 2006, 32, 315–323. [Google Scholar] [CrossRef]
- Chen, H.; Liang, C.; Zhang, L.; Huang, S.; Wu, X. Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) cervical cancer: Randomized study. Gynecol. Oncol. 2008, 110, 308–315. [Google Scholar] [CrossRef]
- Shimada, M.; Nagao, S.; Fujiwara, K.; Takeshima, N.; Takizawa, K.; Shoji, T.; Sugiyama, T.; Yamaguchi, S.; Nishimura, R.; Kigawa, J. Neoadjuvant chemotherapy with docetaxel and carboplatin followed by radical hysterectomy for stage IB2, IIA2, and IIB patients with non-squamous cell carcinoma of the uterine cervix. Int. J. Clin. Oncol. 2016, 21, 1128–1135. [Google Scholar] [CrossRef]
- Gadducci, A.; Barsotti, C.; Laliscia, C.; Cosio, S.; Fanucchi, A.; Tana, R.; Fabrini, M.G. Dose-dense Paclitaxel- and Carboplatin-based Neoadjuvant Chemotherapy Followed by Surgery or Concurrent Chemo-radiotherapy in Cervical Cancer: A Preliminary Analysis. Anticancer Res. 2017, 37, 1249–1255. [Google Scholar]
- Yang, L.; Guo, J.; Shen, Y.; Cai, J.; Xiong, Z.; Dong, W.; Min, J.; Wang, Z. Clinical efficacy and safety of paclitaxel plus carboplatin as neoadjuvant chemotherapy prior to radical hysterectomy and pelvic lymphadenectomy for Stage IB2-IIB cervical cancer. Int. J. Clin. Exp. Med. 2015, 8, 13690–13698. [Google Scholar]
- Angioli, R.; Plotti, F.; Luvero, D.; Aloisi, A.; Guzzo, F.; Capriglione, S.; Terranova, C.; De Cicco Nardone, C.; Benedetti-Panici, P. Feasibility and safety of carboplatin plus paclitaxel as neoadjuvant chemotherapy for locally advanced cervical cancer: A pilot study. Tumour. Biol. 2013, 35, 2741–2746. [Google Scholar] [CrossRef]
- Singh, R.B.; Chander, S.; Mohanti, B.K.; Pathy, S.; Kumar, S.; Bhatla, N.; Thulkar, S.; Vishnubhatla, S.; Kumar, L. Neoadjuvant chemotherapy with weekly paclitaxel and carboplatin followed by chemoradiation in locally advanced cervical carcinoma: A pilot study. Gynecol. Oncol. 2013, 129, 124–128. [Google Scholar] [CrossRef]
- Prueksaritanond, N.; Chaisarn, P.; Yanaranop, M. The efficacy of neoadjuvant paclitaxel-carboplatin chemotherapy followed by radical hysterectomy compared to radical hysterectomy alone in bulky stage IB2-IIA cervical cancer. J. Med. Assoc. Thail. 2012, 95 (Suppl. 3), S55–S61. [Google Scholar]
- Mori, T.; Hosokawa, K.; Sawada, M.; Kuroboshi, H.; Tatsumi, H.; Koshiba, H.; Okubo, T.; Kitawaki, J. Neoadjuvant weekly carboplatin and paclitaxel followed by radical hysterectomy for locally advanced cervical cancer: Long-term results. Int. J. Gynecol. Cancer 2010, 20, 611–616. [Google Scholar] [CrossRef]
- Dueñas-Gonzalez, A.; López-Graniel, C.; González-Enciso, A.; Cetina, L.; Rivera, L.; Mariscal, I.; Montalvo, G.; Gómez, E.; de la Garza, J.; Chanona, G.; et al. A phase II study of multimodality treatment for locally advanced cervical cancer: Neoadjuvant carboplatin and paclitaxel followed by radical hysterectomy and adjuvant cisplatin chemoradiation. Ann. Oncol. 2003, 14, 1278–1284. [Google Scholar] [CrossRef]
- Meden, H.; Fattahi-Meibodi, A.; Osmers, R.; Krauss, T.; Kuhn, W. Wertheim’s hysterectomy after neoadjuvant carboplatin-based chemotherapy in patients with cervical cancer stage IIB and IIIB. Anticancer Res. 1998, 18, 4575–4579. [Google Scholar]
- Curtin, J.P.; Blessing, J.A.; Webster, K.D.; Rose, P.G.; Mayer, A.R.; Fowler, W.C., Jr.; Malfetano, J.H.; Alvarez, R.D. Paclitaxel, an active agent in nonsquamous carcinomas of the uterine cervix: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2001, 19, 1275–1278. [Google Scholar] [CrossRef]
- Kastritis, E.; Bamias, A.; Efstathiou, E.; Gika, D.; Bozas, G.; Zorzou, P.; Sarris, K.; Papadimitriou, C.; Dimopoulos, M.A. The outcome of advanced or recurrent non-squamous carcinoma of the uterine cervix after platinum-based combination chemotherapy. Gynecol. Oncol. 2005, 99, 376–382. [Google Scholar] [CrossRef]
- Nagao, S.; Fujiwara, K.; Oda, T.; Ishikawa, H.; Koike, H.; Tanaka, H.; Kohno, I. Combination chemotherapy of docetaxel and carboplatin in advanced or recurrent cervix cancer. A pilot study. Gynecol. Oncol. 2005, 96, 805–809. [Google Scholar] [CrossRef]
- Kim, H.S.; Sardi, J.E.; Katsumata, N.; Ryu, H.S.; Nam, J.H.; Chung, H.H.; Park, N.H.; Song, Y.S.; Behtash, N.; Kamura, T.; et al. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: An international collaborative meta-analysis. Eur. J. Surg. Oncol. 2013, 39, 115–124. [Google Scholar] [CrossRef]
- Da Costa, A.A.; Valadares, C.V.; Baiocchi, G.; Mantoan, H.; Saito, A.; Sanches, S.; Guimarães, A.P.; Achatz, M.I. Neoadjuvant Chemotherapy Followed by Interval Debulking Surgery and the Risk of Platinum Resistance in Epithelial Ovarian Cancer. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S971–S978. [Google Scholar] [CrossRef]
- Matsuo, K.; Eno, M.L.; Im, D.D.; Rosenshein, N.B. Chemotherapy time interval and development of platinum and taxane resistance in ovarian, fallopian, and peritoneal carcinomas. Arch. Gynecol. Obstet. 2009, 281, 325–328. [Google Scholar] [CrossRef]
- Xie, Q.; Liang, J.; Rao, Q.; Xie, X.; Li, R.; Liu, Y.; Zhou, H.; Han, J.; Yao, T.; Lin, Z. Aldehyde Dehydrogenase 1 Expression Predicts Chemoresistance and Poor Clinical Outcomes in Patients with Locally Advanced Cervical Cancer Treated with Neoadjuvant Chemotherapy Prior to Radical Hysterectomy. Ann. Surg. Oncol. 2015, 23, 163–170. [Google Scholar] [CrossRef]
- Scambia, G.; Benedetti Panici, P.; Foti, E.; Amoroso, M.; Salerno, G.; Ferrandina, G.; Battaglia, F.; Greggi, S.; De Gaetano, A.; Puglia, G.; et al. Squamous cell carcinoma antigen: Prognostic significance and role in the monitoring of neoadjuvant chemotherapy response in cervical cancer. J. Clin. Oncol. 1994, 12, 2309–2316. [Google Scholar] [CrossRef]
- Matsuo, K.; Mandelbaum, R.S.; Machida, H.; Purushotham, S.; Grubbs, B.H.; Roman, L.D.; Wright, J.D. Association of tumor differentiation grade and survival of women with squamous cell carcinoma of the uterine cervix. J. Gynecol. Oncol. 2018, 29, e91. [Google Scholar] [CrossRef]
- Salihi, R.; Leunen, K.; Moerman, P.; Amant, F.; Neven, P.; Vergote, I. Neoadjuvant Weekly Paclitaxel-Carboplatin Is Effective in Stage I-II Cervical Cancer. Int. J. Gynecol. Cancer 2017, 27, 1256–1260. [Google Scholar] [CrossRef]
| Characteristic | All | Squamous | Non-Squamous | P-Value |
|---|---|---|---|---|
| Number | n = 684 | n = 511 | n = 173 | |
| Regimen | <0.001 | |||
| Irinotecan based | 223 (32.6%) | 204 (39.9%) | 19 (11.0%) | |
| Taxane/platinum | 199 (29.1%) | 106 (20.7%) | 93 (53.8%) | |
| Mitomycin based | 91 (13.3%) | 80 (15.7%) | 11 (6.4%) | |
| Fluorouracil based | 71 (10.4%) | 52 (10.2%) | 19 (11.0%) | |
| Platinum monotherapy | 45 (6.6%) | 36 (7.0%) | 9 (5.2%) | |
| Ifosfamide based | 26 (3.8%) | 21 (4.1%) | 5 (2.9%) | |
| Others | 29 (4.2%) | 12 (2.3%) | 17 (9.8%) | |
| Cycles | 0.16 | |||
| 1 | 195 (28.5%) | 144 (28.2%) | 51 (29.5%) | |
| 2 | 382 (55.8%) | 295 (57.7%) | 87 (50.3%) | |
| 3 | 84 (12.3%) | 55 (10.8%) | 29 (16.8%) | |
| ≥4 | 23 (3.3%) | 17 (3.3%) | 6 (3.5%) |
| Squamous | Non-Squamous | |||||
|---|---|---|---|---|---|---|
| Characteristic | Others | Taxane/Platinum | P-Value | Others | Taxane/Platinum | P-Value |
| Number | n = 405 (79.3%) | n = 106 (20.7%) | n = 80 (46.2%) | n = 93 (53.8%) | ||
| Age | 50.1 (SD ±11.3) | 49.7 (SD ±12.7) | 0.73 | 49.1 (SD ±11.6) | 49.8 (SD ±10.6) | 0.68 |
| <50 | 191 (78.9%) | 51 (21.1%) | 42 (50.6%) | 41 (49.4%) | ||
| ≥50 | 214 (79.6%) | 55 (20.4%) | 38 (42.2%) | 52 (57.8%) | ||
| Year | 0.005 | <0.001 | ||||
| 2004 | 74 (90.2%) | 8 (9.8%) | 13 (54.2%) | 11 (45.8%) | ||
| 2005 | 85 (80.2%) | 21 (19.8%) | 24 (68.6%) | 11 (31.4%) | ||
| 2006 | 78 (75.7%) | 25 (24.3%) | 23 (59.0%) | 16 (41.0%) | ||
| 2007 | 107 (82.3%) | 23 (17.7%) | 11 (28.9%) | 27 (71.1%) | ||
| 2008 | 61 (67.8%) | 29 (32.2%) | 9 (24.3%) | 28 (75.7%) | ||
| Clinical stage | 0.42 | 0.80 | ||||
| IB2 | 15 (68.2%) | 7 (31.8%) | 7 (38.9%) | 11 (61.1%) | ||
| IIA | 74 (79.6%) | 19 (20.4%) | 15 (46.9%) | 17 (53.1%) | ||
| IIB | 316 (79.8%) | 80 (20.2%) | 58 (47.2%) | 65 (52.8%) | ||
| Hospital volume | 0.001 | 0.032 | ||||
| High | 184 (78.0%) | 52 (22.0%) | 43 (50.0%) | 43 (50.0%) | ||
| Mid-high | 137 (86.7%) | 21 (13.3%) | 30 (53.6%) | 26 (46.4%) | ||
| Low-mid | 38 (62.3%) | 23 (37.7%) | 5 (25.0%) | 15 (75.0%) | ||
| Low | 46 (82.1%) | 10 (17.9%) | 2 (18.2%) | 9 (81.8%) | ||
| Adjuvant therapy | 0.08 | 0.048 | ||||
| None | 87 (79.8%) | 22 (20.2%) | 5 (21.7%) | 18 (78.3%) | ||
| Radiotherapy alone | 96 (82.1%) | 21 (17.9%) | 14 (50.0%) | 14 (50.0%) | ||
| CCRT | 105 (84.0%) | 20 (16.0%) | 22 (62.9%) | 13 (37.1%) | ||
| Chemotherapy alone | 82 (70.1%) | 35 (29.9%) | 35 (46.1%) | 41 (53.9%) | ||
| Both | 10 (83.3%) | 2 (16.7%) | 3 (50.0%) | 3 (50.0%) | ||
| Squamous | Non-Squamous | |||||
|---|---|---|---|---|---|---|
| Characteristic | Others | Taxane/Platinum | P-Value | Others | Taxane/Platinum | P-Value |
| Number | n = 405 | n = 106 | n = 80 | n = 93 | ||
| Parametria | 0.13 | 0.15 | ||||
| Not involved | 273 (67.4%) | 80 (75.5%) | 47 (58.8%) | 65 (69.9%) | ||
| Involved | 132 (32.6%) | 26 (24.5%) | 33 (41.3%) | 28 (30.1%) | ||
| Tumor size | 0.06 | 0.033 | ||||
| ≤4 cm | 155 (40.6%) | 32 (30.2%) | 32 (42.7%) | 24 (26.7%) | ||
| >4 cm | 227 (59.4%) | 74 (69.8%) | 43 (57.3%) | 66 (73.3%) | ||
| Stromal invasion | 0.99 | 0.50 | ||||
| Inner half | 150 (37.8%) | 40 (37.7%) | 25 (33.8%) | 26 (28.0%) | ||
| Outer half | 247 (62.2%) | 66 (62.3%) | 49 (66.2%) | 67 (72.0%) | ||
| LVSI | 0.38 | 0.63 | ||||
| No | 171 (43.2%) | 51 (48.6%) | 27 (35.5%) | 37 (40.2%) | ||
| Yes | 225 (56.8%) | 54 (51.4%) | 49 (64.5%) | 55 (59.8%) | ||
| Uterine corpus | 0.37 | 0.25 | ||||
| Not involved | 334 (83.9%) | 93 (87.7%) | 50 (63.3%) | 67 (72.0%) | ||
| Involved | 64 (16.1%) | 13 (12.3%) | 29 (36.7%) | 26 (28.0%) | ||
| Ovary | 0.50 | 0.55 | ||||
| Not involved | 394 (99.5%) | 102 (99.0%) | 76 (95.0%) | 86 (92.5%) | ||
| Involved | 2 (0.5%) | 1 (1.0%) | 4 (5.0%) | 7 (7.5%) | ||
| Peritoneal cytology | 0.99 | 0.73 | ||||
| No malignancy | 156 (96.9%) | 59 (98.3%) | 39 (90.7%) | 34 (87.2%) | ||
| Malignancy | 5 (3.1%) | 1 (1.7%) | 4 (9.3%) | 5 (12.8%) | ||
| Pelvic lymph node | 0.42 | 0.19 | ||||
| Not involved | 273 (68.8%) | 79 (74.5%) | 44 (56.4%) | 64 (68.8%) | ||
| Single metastasis | 42 (10.6%) | 11 (10.4%) | 11 (14.1%) | 7 (7.5%) | ||
| Multiple metastases | 82 (20.7%) | 16 (15.1%) | 23 (29.5%) | 22 (23.7%) | ||
| Para-aortic lymph node | 0.78 | 0.69 | ||||
| Not involved | 369 (95.8%) | 103 (97.2%) | 75 (97.4%) | 88 (95.7%) | ||
| Involved | 16 (4.2%) | 3 (2.8%) | 2 (2.6%) | 4 (4.3%) | ||
| Type | Adjustment Factors | Characteristics | Disease-Free Survival | Cause-Specific Survival | ||||
|---|---|---|---|---|---|---|---|---|
| Year | Volume | Adjuvant | HR (95%CI) | P-Value | HR (95%CI) | P-Value | ||
| Squamous | + | Others | 1 | 1 | ||||
| Taxane/platinum | 1.01 (0.68–1.50) | 0.95 | 1.00 (0.62–1.60) | 0.99 | ||||
| + | + | Others | 1 | 1 | ||||
| Taxane/platinum | 1.00 (0.67–1.49) | 0.99 | 0.97 (0.60–1.57) | 0.90 | ||||
| + | + | + | Others | 1 | 1 | |||
| Taxane/platinum | 0.88 (0.58–1.34) | 0.56 | 0.87 (0.53–1.43) | 0.58 | ||||
| Non-squamous | + | Others | 1 | 1 | ||||
| Taxane/platinum | 1.03 (0.63–1.69) | 0.89 | 1.26 (0.68–2.32) | 0.47 | ||||
| + | + | Others | 1 | 1 | ||||
| Taxane/platinum | 1.03 (0.63–1.69) | 0.91 | 1.19 (0.65–2.21) | 0.57 | ||||
| + | + | + | Others | 1 | 1 | |||
| Taxane/platinum | 0.84 (0.50–1.39) | 0.49 | 0.91 (0.49–1.70) | 0.78 | ||||
| Squamous | Non-Squamous | ||||||
| Taxane/Platinum | Irinotecan Based | Ifosfamide Based | Fluorouracil Based | Mitomycin Based | Platinum Alone | ||
| Taxane/platinum | 0.70 | 0.78 | 0.48 | 0.22 | 0.22 | ||
| Irinotecan based | 0.65 | 0.99 | 0.88 | 0.21 | 0.45 | ||
| Ifosfamide based | 0.19 | 0.11 | 0.84 | 0.48 | 0.79 | ||
| Fluorouracil based | 0.91 | 0.62 | 0.32 | 0.18 | 0.64 | ||
| Mitomycin based | 0.94 | 0.59 | 0.25 | 0.98 | 0.11 | ||
| Platinum alone | 0.44 | 0.28 | 0.62 | 0.60 | 0.57 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, K.; Shimada, M.; Yamaguchi, S.; Kigawa, J.; Tokunaga, H.; Tabata, T.; Kodama, J.; Kawana, K.; Mikami, M.; Sugiyama, T. Neoadjuvant Chemotherapy with Taxane and Platinum Followed by Radical Hysterectomy for Stage IB2-IIB Cervical Cancer: Impact of Histology Type on Survival. J. Clin. Med. 2019, 8, 156. https://doi.org/10.3390/jcm8020156
Matsuo K, Shimada M, Yamaguchi S, Kigawa J, Tokunaga H, Tabata T, Kodama J, Kawana K, Mikami M, Sugiyama T. Neoadjuvant Chemotherapy with Taxane and Platinum Followed by Radical Hysterectomy for Stage IB2-IIB Cervical Cancer: Impact of Histology Type on Survival. Journal of Clinical Medicine. 2019; 8(2):156. https://doi.org/10.3390/jcm8020156
Chicago/Turabian StyleMatsuo, Koji, Muneaki Shimada, Satoshi Yamaguchi, Junzo Kigawa, Hideki Tokunaga, Tsutomu Tabata, Junichi Kodama, Kei Kawana, Mikio Mikami, and Toru Sugiyama. 2019. "Neoadjuvant Chemotherapy with Taxane and Platinum Followed by Radical Hysterectomy for Stage IB2-IIB Cervical Cancer: Impact of Histology Type on Survival" Journal of Clinical Medicine 8, no. 2: 156. https://doi.org/10.3390/jcm8020156
APA StyleMatsuo, K., Shimada, M., Yamaguchi, S., Kigawa, J., Tokunaga, H., Tabata, T., Kodama, J., Kawana, K., Mikami, M., & Sugiyama, T. (2019). Neoadjuvant Chemotherapy with Taxane and Platinum Followed by Radical Hysterectomy for Stage IB2-IIB Cervical Cancer: Impact of Histology Type on Survival. Journal of Clinical Medicine, 8(2), 156. https://doi.org/10.3390/jcm8020156






