Complications of Resection Arthroplasty in Two-Stage Revision for the Treatment of Periprosthetic Hip Joint Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Surgical and Antimicrobial Treatment
2.5. Demographics and Infection Characteristics
2.6. Statistical Analysis
3. Results
3.1. Local Complications and Their Temporal Appearance During the Two-Stage Revision
3.2. Systemic Complications
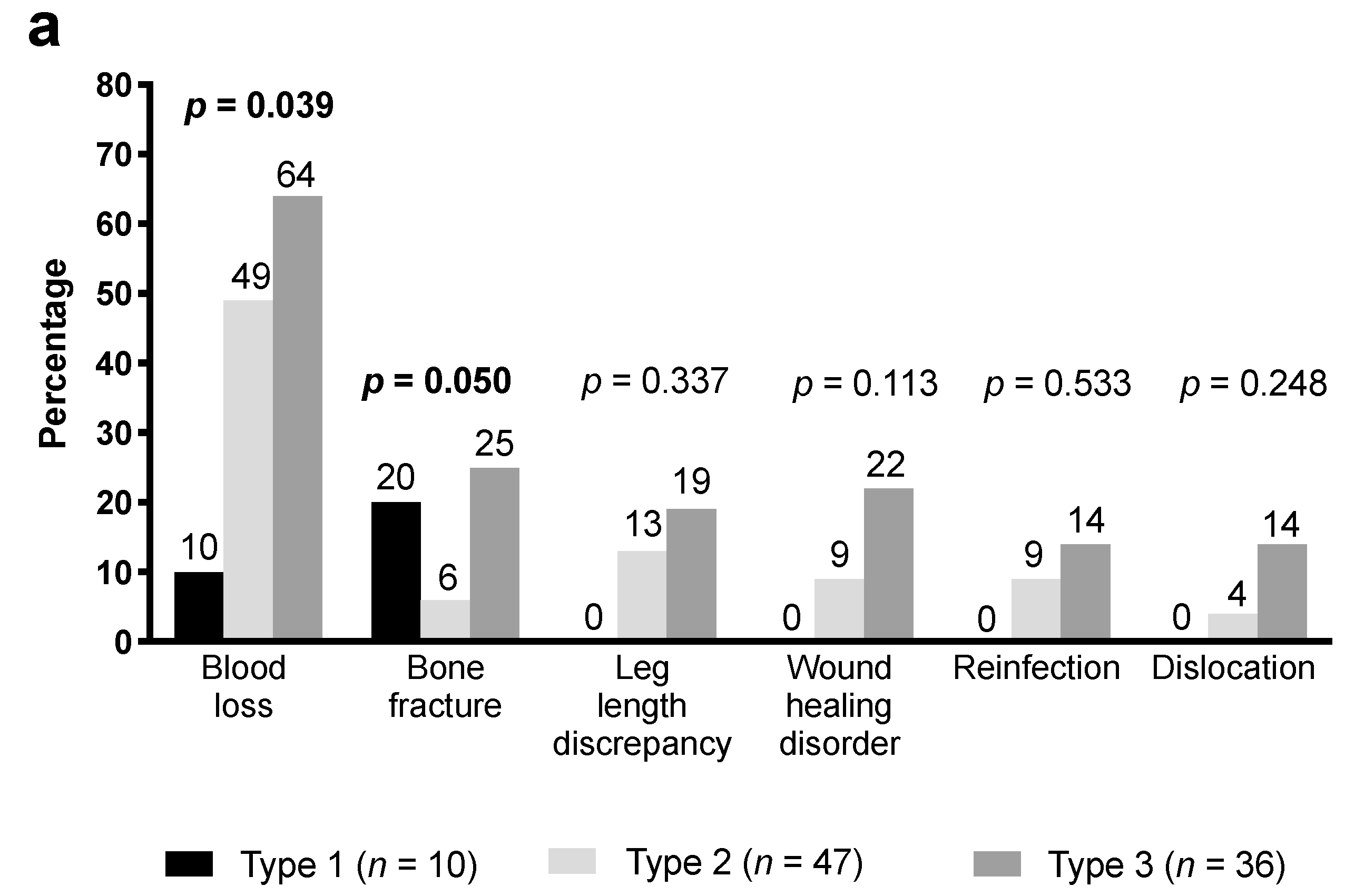
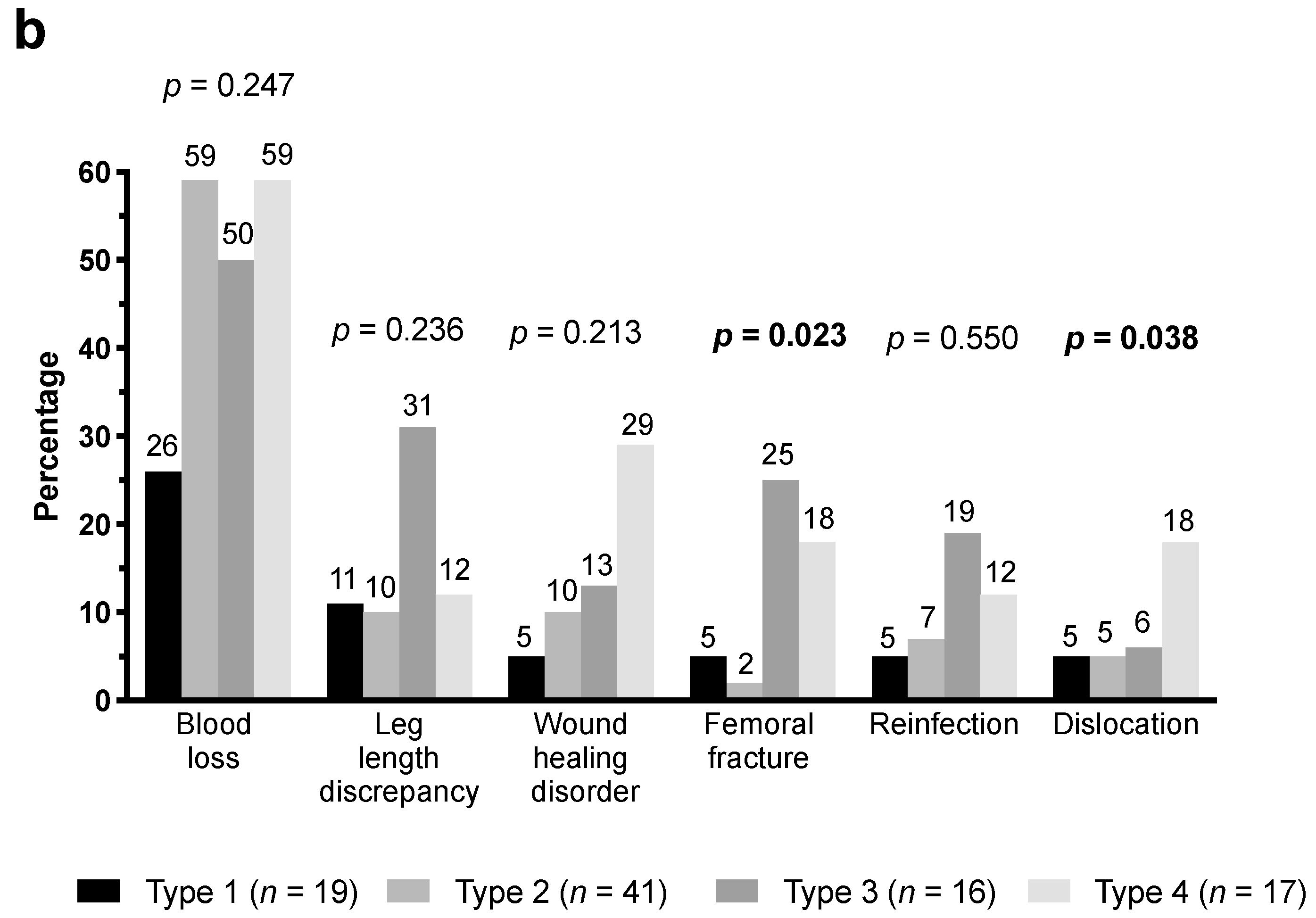
3.3. Bone Defects and Consecutive Complications
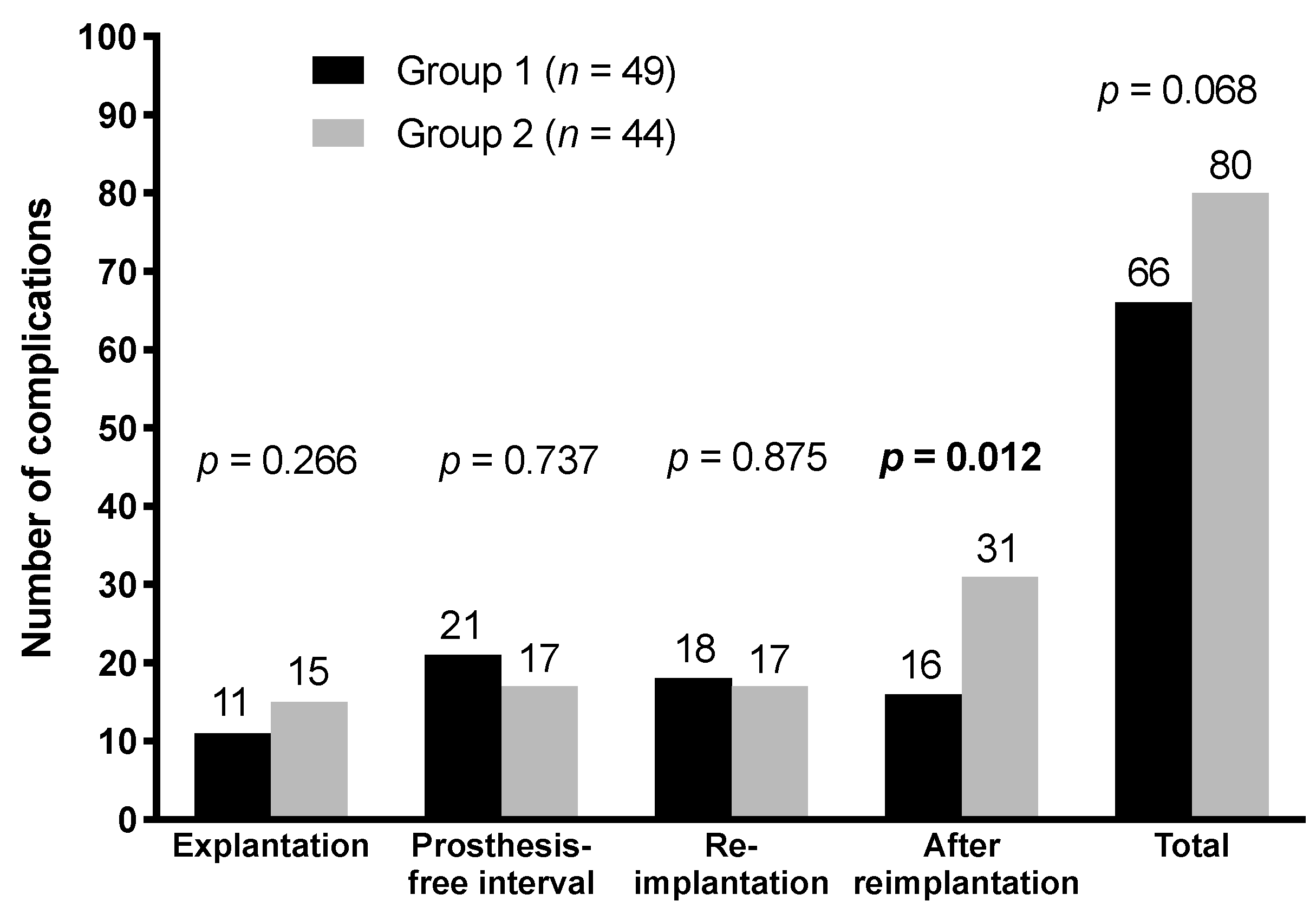
3.4. Comparison of Groups
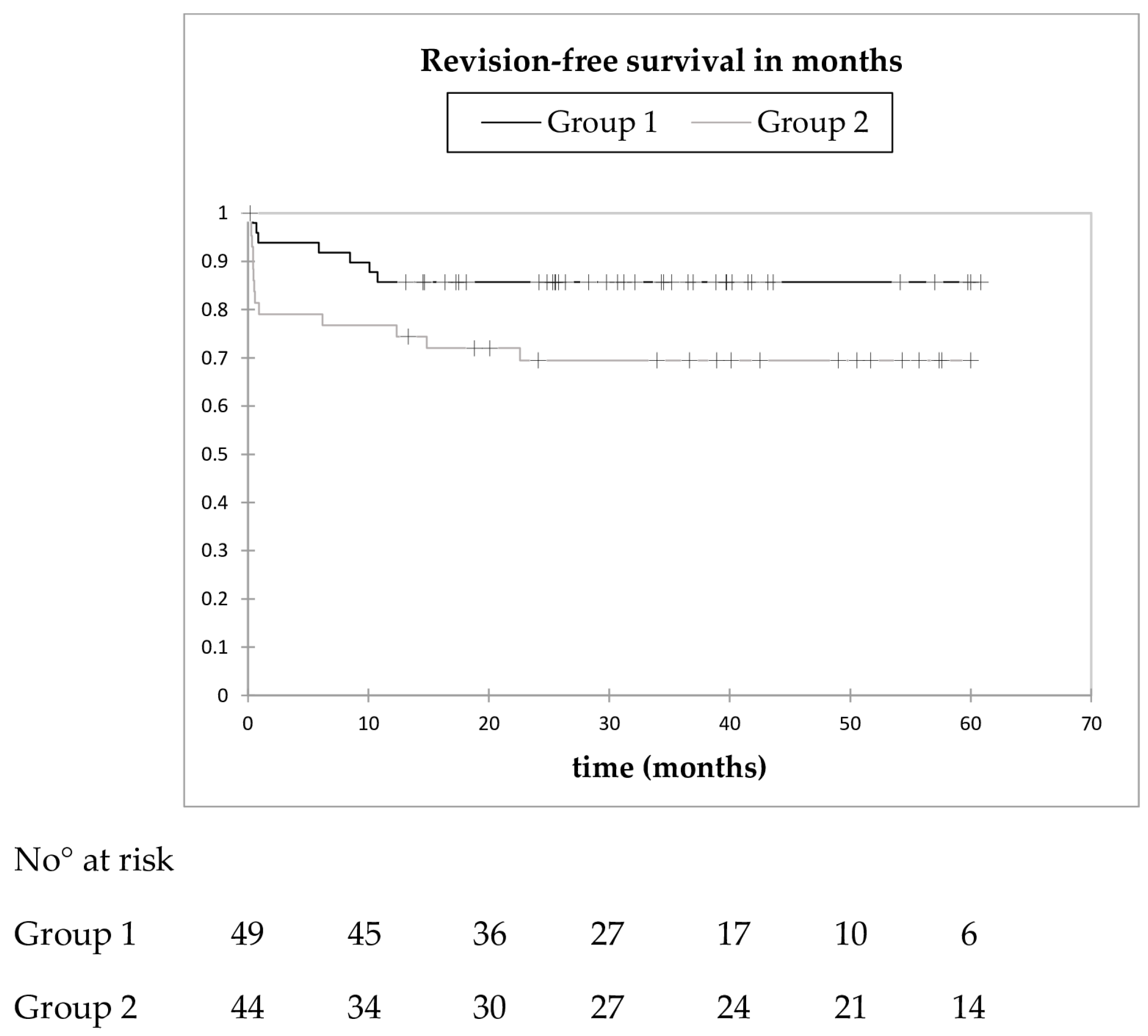
3.5. Revision-Free Survival
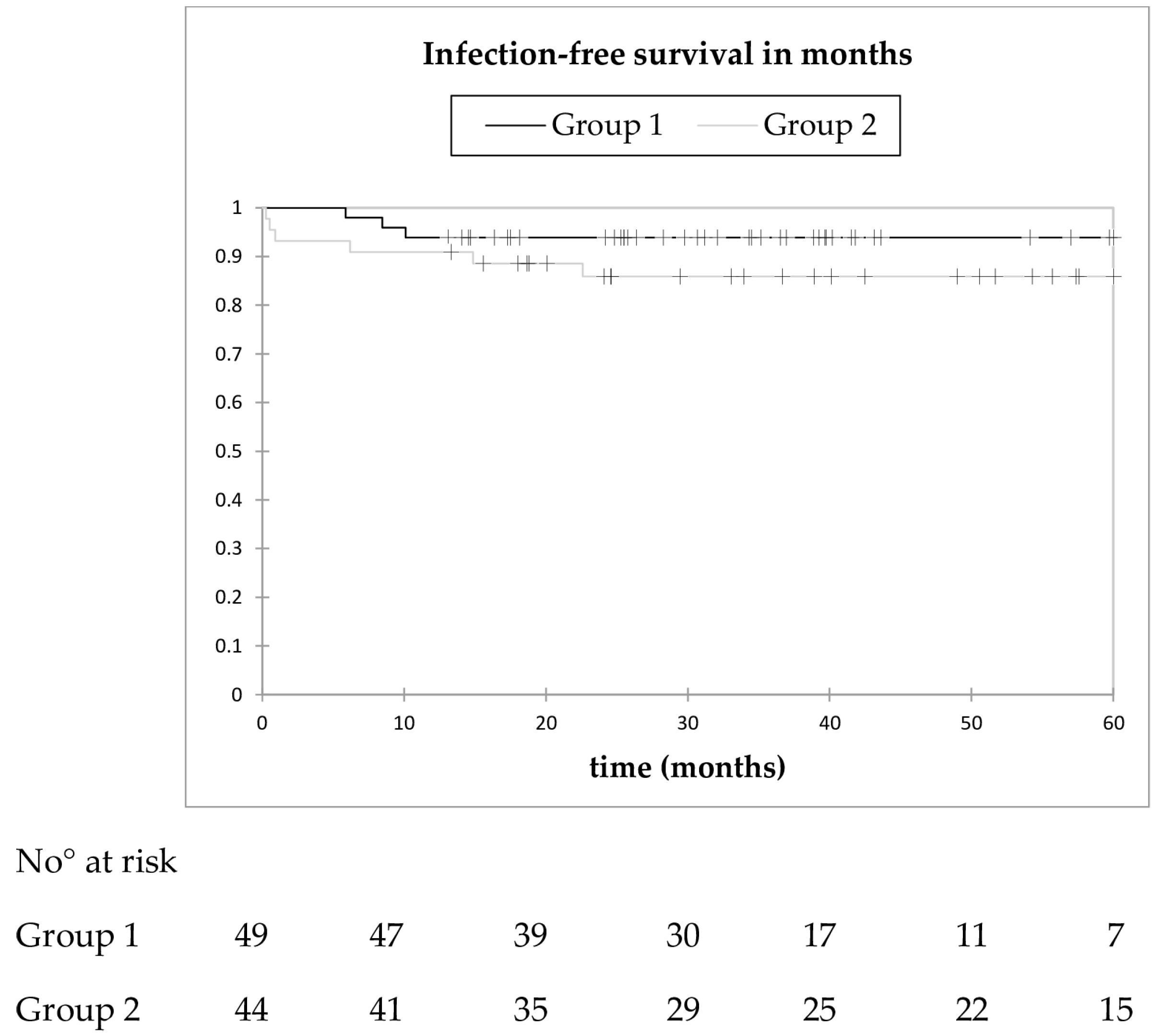
3.6. Infection-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janssen, D.M.; Geurts, J.A.; Jutten, L.M.; Walenkamp, G.H. 2-stage revision of 120 deep infected hip and knee prostheses using gentamicin-PMMA beads. Acta Orthop. 2016, 87, 324–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pattyn, C.; De Geest, T.; Ackerman, P.; Audenaert, E. Preformed gentamicin spacers in two-stage revision hip arthroplasty: Functional results and complications. Int. Orthop. 2011, 35, 1471–1476. [Google Scholar] [CrossRef]
- Engesaeter, L.B.; Dale, H.; Schrama, J.C.; Hallan, G.; Lie, S.A. Surgical procedures in the treatment of 784 infected THAs reported to the Norwegian Arthroplasty Register. Acta Orthop. 2011, 82, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.A.; Farris, A. Outcome of infected total joint replacement. Orthopedics 2010, 33. [Google Scholar] [CrossRef]
- Younger, A.S.; Duncan, C.P.; Masri, B.A.; McGraw, R.W. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. J. Arthroplast. 1997, 12, 615–623. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Shih, C.H.; Chang, Y.H.; Lee, M.S.; Shih, H.N.; Yang, W.E. Two-stage revision hip arthroplasty for infection: Comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J. Bone Jt. Surg. 2004, 86, 1989–1997. [Google Scholar] [CrossRef]
- Duncan, C.P.; Masri, B.A. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. Instr. Course Lect. 1995, 44, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hope, P.G.; Kristinsson, K.G.; Norman, P.; Elson, R.A. Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. J. Bone Jt. Surg. 1989, 71, 851–855. [Google Scholar] [CrossRef]
- Lautenschlager, E.P.; Jacobs, J.J.; Marshall, G.W.; Meyer, P.R., Jr. Mechanical properties of bone cements containing large doses of antibiotic powders. J. Biomed. Mater. Res. 1976, 10, 929–938. [Google Scholar] [CrossRef]
- Van Diemen, M.P.; Colen, S.; Dalemans, A.A.; Stuyck, J.; Mulier, M. Two-stage revision of an infected total hip arthroplasty: A follow-up of 136 patients. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 2013, 23, 445–450. [Google Scholar] [CrossRef]
- Girdlestone, G.R. Arthrodesis and Other Operations for Tuberculosis of the Hip; The Robert Jones Birthday Volume; Milford, H., Ed.; Oxford University Press: London, UK, 1928; pp. 347–374. [Google Scholar]
- Girdlestone, G.R. Acute pyogenic arthritis of the hip: An operation giving free access and effective drainage. Lancet 1943, 241, 419–421. [Google Scholar] [CrossRef]
- Trampuz, A.; Perka, C.; Borens, O. Prosthetic joint infection: New developments in diagnosis and treatmen]. Dtsch. Med. Wochenschr. 2013, 138, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Paprosky, W.G.; Greidanus, N.V.; Antoniou, J. Minimum 10-year-results of extensively porous-coated stems in revision hip arthroplasty. Clin. Orthop. Relat. Res. 1999, 369, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Paprosky, W.G.; Perona, P.G.; Lawrence, J.M. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J. Arthroplast. 1994, 9, 33–44. [Google Scholar] [CrossRef]
- Sigmund, I.K.; Yermak, K.; Perka, C.; Trampuz, A.; Renz, N. Is the Enzyme-linked Immunosorbent Assay More Accurate Than the Lateral Flow Alpha Defensin Test for Diagnosing Periprosthetic Joint Infection? Clin. Orthop. Relat. Res. 2018, 476, 1645–1654. [Google Scholar] [CrossRef]
- Diaz-Ledezma, C.; Higuera, C.A.; Parvizi, J. Success after treatment of periprosthetic joint infection: A Delphi-based international multidisciplinary consensus. Clin. Orthop. Relat. Res. 2013, 471, 2374–2382. [Google Scholar] [CrossRef]
- Li, H.K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kumin, M.; et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef]
- Faschingbauer, M.; Reichel, H.; Bieger, R.; Kappe, T. Mechanical complications with one hundred and thirty eight (antibiotic-laden) cement spacers in the treatment of periprosthetic infection after total hip arthroplasty. Int. Orthop. 2015, 39, 989–994. [Google Scholar] [CrossRef]
- Yang, F.S.; Lu, Y.D.; Wu, C.T.; Blevins, K.; Lee, M.S.; Kuo, F.C. Mechanical failure of articulating polymethylmethacrylate (PMMA) spacers in two-stage revision hip arthroplasty: The risk factors and the impact on interim function. BMC Musculoskelet. Disord. 2019, 20, 372. [Google Scholar] [CrossRef]
- Jung, J.; Schmid, N.V.; Kelm, J.; Schmitt, E.; Anagnostakos, K. Complications after spacer implantation in the treatment of hip joint infections. Int. J. Med. Sci. 2009, 6, 265–273. [Google Scholar] [CrossRef]
- Garcia-Rey, E.; Cruz-Pardos, A.; Madero, R. Clinical outcome following conversion of Girdlestone’s resection arthroplasty to total hip replacement: A retrospective matched case-control study. Bone Jt. J. 2014, 96, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Charlton, W.P.; Hozack, W.J.; Teloken, M.A.; Rao, R.; Bissett, G.A. Complications associated with reimplantation after girdlestone arthroplasty. Clin. Orthop. Relat. Res. 2003, 407, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Marczak, D.; Synder, M.; Sibinski, M.; Polguj, M.; Dudka, J.; Kowalczewski, J. Two stage revision hip arthroplasty in periprosthetic joint infection. Comparison study: With or without the use of a spacer. Int. Orthop. 2017, 41, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.J.; Fitzgerald, R.H., Jr.; Ilstrup, D.M. Two-stage reconstruction of a total hip arthroplasty because of infection. J. Bone Jt. Surg. 1989, 71, 828–834. [Google Scholar] [CrossRef]
- Gomez, M.M.; Tan, T.L.; Manrique, J.; Deirmengian, G.K.; Parvizi, J. The Fate of Spacers in the Treatment of Periprosthetic Joint Infection. J. Bone Jt. Surg. 2015, 97, 1495–1502. [Google Scholar] [CrossRef]
- Winkler, T.; Stuhlert, M.G.W.; Lieb, E.; Muller, M.; von Roth, P.; Preininger, B.; Trampuz, A.; Perka, C.F. Outcome of short versus long interval in two-stage exchange for periprosthetic joint infection: A prospective cohort study. Arch. Orthop. Trauma. Surg. 2019, 139, 295–303. [Google Scholar] [CrossRef]
- Noordin, S.; Masri, B.A.; Duncan, C.P.; Garbuz, D.S. Acetabular bone loss in revision total hip arthroplasty: Principles and techniques. Instr. Course Lect. 2010, 59, 27–36. [Google Scholar]
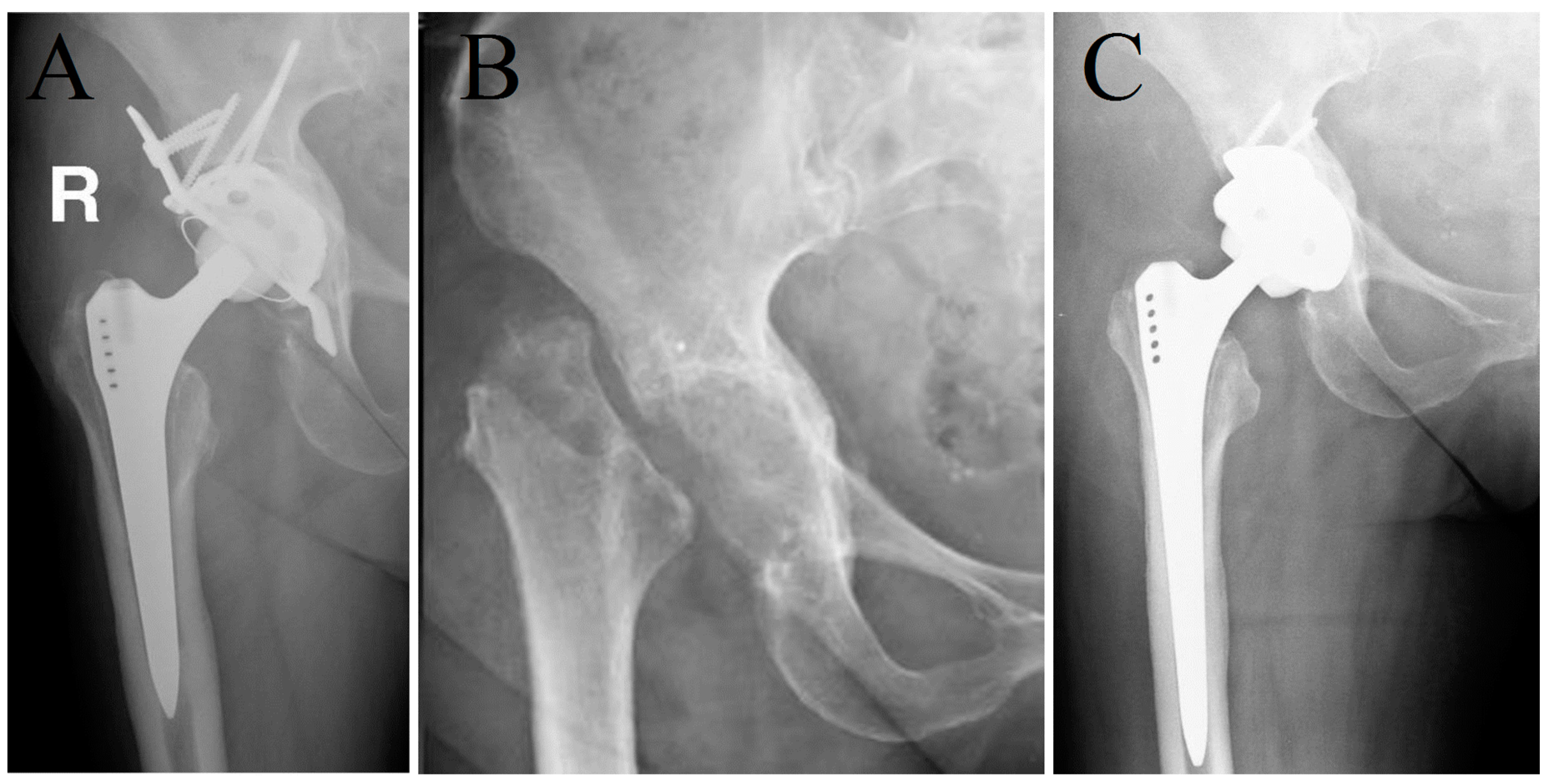
| Variable | Group 1 (n = 49) | Group 2 (n = 44) | p-Value |
|---|---|---|---|
| Patient age, median (range)—years | 75 (51–90) | 77 (50–88) | 0.790 |
| Female sex | 28 (57) | 20 (45) | 0.302 |
| Body mass index (kg/m2) | 29.2 (16.2–46.4) | 25.7 (18.8–37) | 0.014 |
| ASA score | 2.0 (2–3) | 2.0 (1–3) | 0.874 |
| No. of previous surgeries | 2 (0–8) | 2 (0–10) | 0.544 |
| Acetabular bone defect (Paprosky [15]) | |||
| Type 1 | 9 (18) | 1 (2) | 0.017 |
| Type 2 | 24 (49) | 23 (52) | 0.836 |
| Type 3 | 16 (33) | 20 (45) | 0.286 |
| Femoral bone defect (Paprosky [14]) | |||
| Type 1 | 14 (29) | 5 (9) | 0.070 |
| Type 2 | 20 (41) | 21 (48) | 0.536 |
| Type 3 | 8 (16) | 8 (18) | 1.000 |
| Type 4 | 7 (14) | 10 (23) | 0.421 |
| Prosthesis-free interval, median (range)—weeks | 8.6 (1.0–10.0) | 12.0 (10.1–115) | <0.0001 |
| Type of prosthesis fixation | 0.052 | ||
| Cemented | 13 (27%) | 21 (48%) | |
| Uncemented | 36 (73%) | 23 (52%) | |
| Interval from reimplantation and first walk, median (range)—days | 2.0 (1–10) | 2.0 (1–6) | 0.506 |
| Microorganism | Cases with Positive Microbiology at Explantation (n = 72) | Cases with Positive Microbiology at Reimplantation (n = 17) |
|---|---|---|
| Coagulase-negative staphylococci | 53 | 17 1 |
| Staphylococcus aureus | 11 | 2 |
| Cutibacterium acnes | 8 | 1 |
| Streptococcus spp. | 5 | 0 |
| Enterococcus spp. | 5 | 0 |
| Gram-negative bacteria 2 | 5 | 0 |
| Others 3 | 4 | 1 |
| Complication | All Cases (n = 93) | Group 1 (n = 49) | Group 2 (n = 44) | p-Value |
|---|---|---|---|---|
| At explantation | ||||
| Blood loss 1 | 22 (24) | 9 (18) | 13 (30) | 0.230 |
| Bone fracture 2 | 3 (3) | 2 (4) | 1 (2) | 1.000 |
| Nerve palsy 3 | 1 (1) | 0 (0) | 1 (2) | 0.473 |
| Total | 26 (28) | 11 (22) | 15 (34) | 0.266 |
| During resection arthroplasty | ||||
| Persistent infection | 16 (17) | 8 (16) | 8 (18) | 1.000 |
| Wound healing disorder | 6 (6) | 2 (4) | 4 (9) | 0.417 |
| Bone fracture 4 | 3 (3) | 2 (4) | 1 (2) | 1.000 |
| Others 5 | 13 (14) | 9 (18) | 4 (9) | 0.268 |
| Total | 38 (41) | 21 (43) | 17 (39) | 0.737 |
| At reimplantation | ||||
| Blood loss 6 | 25 (27) | 13 (27) | 12 (27) | 1.000 |
| Bone fracture 7 | 5 (5) | 3 (6) | 2 (5) | 1.000 |
| New infection | 3 (3) | 1 (2) | 2 (5) | 0.601 |
| Nerve palsy 8 (reversible) | 2 (2) | 1 (2) | 1 (2) | 1.000 |
| Total | 35 (38) | 18 (37) | 17 (39) | 0.875 |
| After Reimplantation | ||||
| Leg length discrepancy 9 | 13 (14) | 4 (8) | 9 (20) | 0.134 |
| Reinfection | 9 (10) | 3 (6) | 6 (14) | 0.299 |
| Dislocation | 7 (8) | 3 (6) | 4 (9) | 0.704 |
| Wound healing disturbance 10 | 6 (7) | 0 (0) | 6 (14) | 0.009 |
| Bone fracture 11 | 3 (3) | 2 (4) | 1 (2) | 1.000 |
| Aseptic loosening 12 | 2 (2) | 0 (0) | 2 (5) | 0.226 |
| Others 13 | 7 (8) | 4 (8) | 3 (7) | 0.688 |
| Total | 47 (51) | 16 (33) | 31 (70) | 0.012 |
| Total | 146 | 66 | 80 | 0.068 |
| Complications | All Cases (n = 93) | Group 1 (n = 49) | Group 2 (n = 44) | p-Value |
|---|---|---|---|---|
| Allergic reaction to antibiotics | 6 | 5 | 1 | 0.120 |
| Cardiovascular events 1 | 6 | 5 | 1 | 0.208 |
| Thromboembolic events 2 | 4 | 0 | 4 | 0.047 |
| Hepatic insufficiency * | 2 | 0 | 2 | 0.131 |
| Sepsis/Systemic inflammatory response syndrome * | 2 | 0 | 2 | 0.131 |
| Others 3 | 6 | 2 | 4 | 0.417 |
| Total | 26 | 12 | 14 | 0.501 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigmund, I.K.; Winkler, T.; Önder, N.; Perka, C.; Renz, N.; Trampuz, A. Complications of Resection Arthroplasty in Two-Stage Revision for the Treatment of Periprosthetic Hip Joint Infection. J. Clin. Med. 2019, 8, 2224. https://doi.org/10.3390/jcm8122224
Sigmund IK, Winkler T, Önder N, Perka C, Renz N, Trampuz A. Complications of Resection Arthroplasty in Two-Stage Revision for the Treatment of Periprosthetic Hip Joint Infection. Journal of Clinical Medicine. 2019; 8(12):2224. https://doi.org/10.3390/jcm8122224
Chicago/Turabian StyleSigmund, Irene K., Tobias Winkler, Nuri Önder, Carsten Perka, Nora Renz, and Andrej Trampuz. 2019. "Complications of Resection Arthroplasty in Two-Stage Revision for the Treatment of Periprosthetic Hip Joint Infection" Journal of Clinical Medicine 8, no. 12: 2224. https://doi.org/10.3390/jcm8122224
APA StyleSigmund, I. K., Winkler, T., Önder, N., Perka, C., Renz, N., & Trampuz, A. (2019). Complications of Resection Arthroplasty in Two-Stage Revision for the Treatment of Periprosthetic Hip Joint Infection. Journal of Clinical Medicine, 8(12), 2224. https://doi.org/10.3390/jcm8122224







