Delayed Occurrence of Hypertrophic Olivary Degeneration after Therapy of Posterior Fossa Tumors: A Single Institution Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Magnetic Resonance Imaging
3. Results
3.1. Patient Characteristics
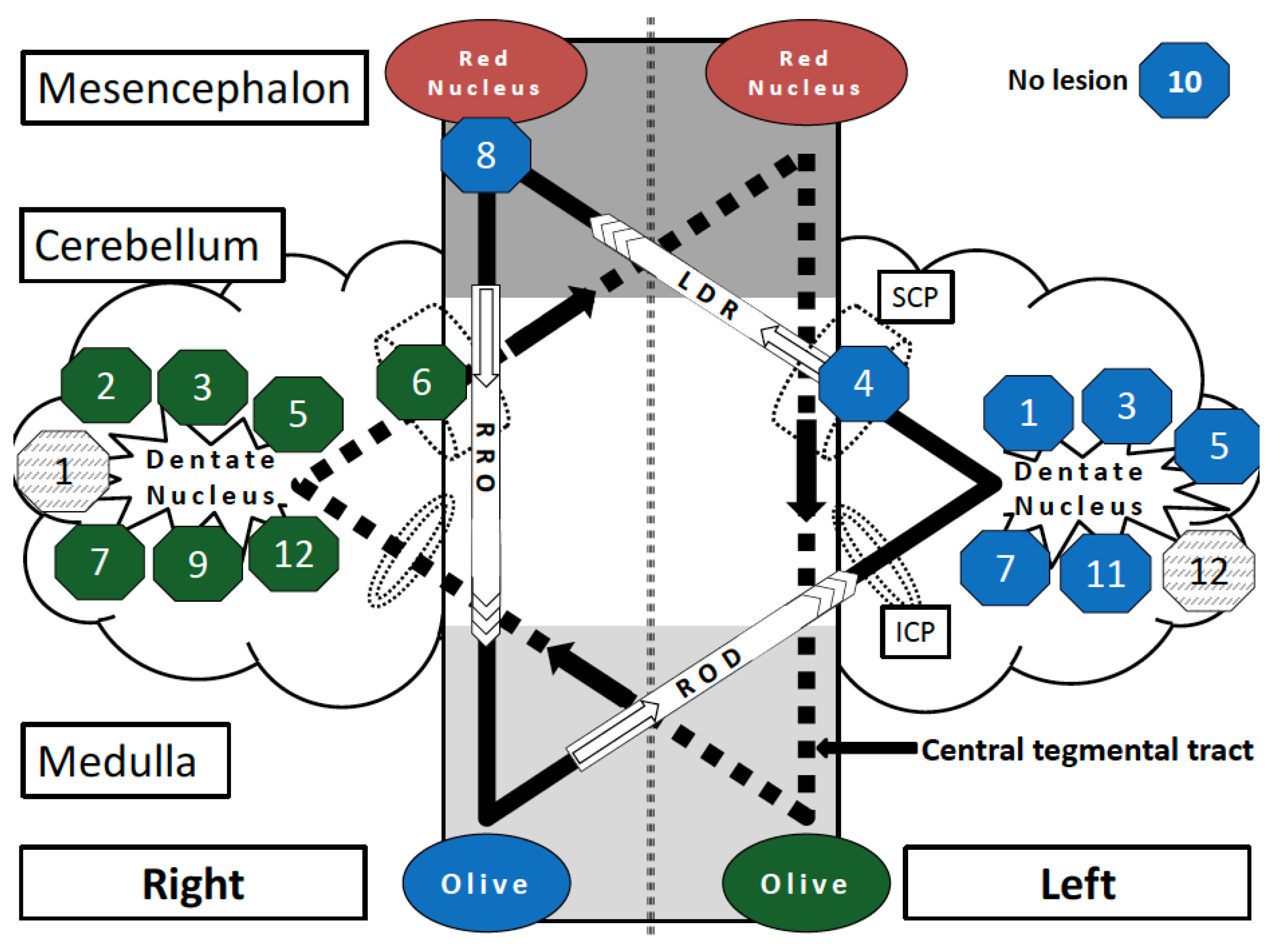
3.2. Patterns of Lesion
3.3. Clinical Presentation
3.4. Delay in Diagnosing HOD
3.5. Model of HOD Prediction
3.6. Image Series before and after HOD Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goto, N.; Kaneko, M. Olivary enlargement: Chronological and morphometric analyses. Acta Neuropathol. 1981, 54, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Lapresle, J. Rhythmic palatal myoclonus and the dentato-olivary pathway. J. Neurol. 1979, 220, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.F.C. Sur la dégénération pseudo-hypertrophique de l’olive bulbaire. Rev. Neurol. 1913, 26, 48–52. [Google Scholar]
- Wang, H.; Wang, Y.; Wang, R.; Li, Y.; Wang, P.; Li, J.; Du, J. Hypertrophic olivary degeneration: A comprehensive review focusing on etiology. Brain Res. 2019, 1718, 53–63. [Google Scholar] [CrossRef]
- Onen, M.R.; Moore, K.; Cikla, U.; Ucer, M.; Schmidt, B.; Field, A.S.; Baskaya, M.K. Hypertrophic Olivary Degeneration: Neurosurgical Perspective and Literature Review. World Neurosurg. 2018, 112, e763–e771. [Google Scholar] [CrossRef]
- Kitajima, M.; Korogi, Y.; Shimomura, O.; Sakamoto, Y.; Hirai, T.; Miyayama, H.; Takahashi, M. Hypertrophic olivary degeneration: MR imaging and pathologic findings. Radiology 1994, 192, 539–543. [Google Scholar] [CrossRef]
- Goyal, M.; Versnick, E.; Tuite, P.; Cyr, J.S.; Kucharczyk, W.; Montanera, W.; Willinsky, R.; Mikulis, D. Hypertrophic olivary degeneration: Metaanalysis of the temporal evolution of MR findings. AJNR Am. J. Neuroradiol. 2000, 21, 1073–1077. [Google Scholar] [PubMed]
- Gautier, J.C.; Blackwood, W. Enlargement of the inferior olivary nucleus in association with lesions of the central tegmental tract or dentate nucleus. Brain 1961, 84, 341–361. [Google Scholar] [CrossRef]
- Murdoch, S.; Shah, P.; Jampana, R. The Guillain-Mollaret triangle in action. Pract. Neurol. 2016, 16, 243–246. [Google Scholar] [CrossRef]
- Tilikete, C.; Desestret, V. Hypertrophic Olivary Degeneration and Palatal or Oculopalatal Tremor. Front. Neurol. 2017, 8, 302. [Google Scholar] [CrossRef]
- Carr, C.M.; Hunt, C.H.; Kaufmann, T.J.; Kotsenas, A.L.; Krecke, K.N.; Wood, C.P. Frequency of bilateral hypertrophic olivary degeneration in a large retrospective cohort. J. Neuroimaging 2015, 25, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Carota, A.; Düron, N.; Cereda, C.; Bassetti, C.L. Vertical pendular nystagmus and hypertrophic inferior olivary nuclei degeneration: An “odd couple”. J. Neurol. 2012, 259, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Graf, H.; Gorges, M.; Kassubek, J. Hypertrophe Degeneration des Nucleus olivaris. Nervenheilkunde 2013, 32, 532–537. [Google Scholar] [CrossRef]
- Foerch, C.; Schaller, M.A.; Lapa, S.; Filipski, K.; Steinmetz, H.; Kang, J.-S.; Zöllner, J.P.; Wagner, M. Hypertrophe Degeneration der Olive: Ursache neuerlicher neurologischer Symptome nach Schlaganfall. Nervenarzt 2019, 90, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Hatzoglou, V.; Karimi, S.; Young, R.J. Hypertrophic olivary degeneration resulting from posterior fossa masses and their treatments. Clin. Imaging 2015, 39, 787–790. [Google Scholar] [CrossRef]
- Yokota, T.; Hirashima, F.; Furukawa, T.; Tsukagoshi, H.; Yoshikawa, H. MRI findings of inferior olives in palatal myoclonus. J. Neurol. 1989, 236, 115–116. [Google Scholar] [CrossRef]
- Sen, D.; Gulati, Y.S.; Malik, V.; Mohimen, A.; Sibi, E.; Reddy, D.C. MRI and MR tractography in bilateral hypertrophic olivary degeneration. Indian. J. Radiol. Imaging 2014, 24, 401–405. [Google Scholar] [CrossRef]
- Sarawagi, R.; Murugesan, A. Hypertrophic olivary degeneration—A report of two cases. J. Clin. Imaging Sci. 2015, 5, 8. [Google Scholar] [CrossRef]
- Guillain, G. Deux cas de myoclonies synchrones et rythmées vélo-pharyngo-laryngo-oculo-diaphragmatiques: Le problème anatomique et physiologique. Rev. Neurol. 1931, 2, 545–566. [Google Scholar]
- Brinar, V.V.; Barun, B.; Zadro, I.; Ozretic, D.; Habek, M. Progressive ataxia and palatal tremor. Arch. Neurol. 2008, 65, 1248–1249. [Google Scholar] [CrossRef]
- Samuel, M.; Torun, N.; Tuite, P.J.; Sharpe, J.A.; Lang, A.E. Progressive ataxia and palatal tremor (PAPT): Clinical and MRI assessment with review of palatal tremors. Brain 2004, 127, 1252–1268. [Google Scholar] [CrossRef]
- Yun, J.H.; Ahn, J.S.; Park, J.C.; Kwon, D.H.; Kwun, B.D.; Kim, C.J. Hypertrophic olivary degeneration following surgical resection or gamma knife radiosurgery of brainstem cavernous malformations: An 11-case series and a review of literature. Acta Neurochir. 2013, 155, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Kinoshita, T.; Kinoshita, F.; Ogawa, T. Hypertrophic olivary degeneration after gamma-knife radiosurgery for pontine metastasis. Magn. Reson. Med. Sci. 2012, 11, 299–302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vélez, M.; Cosentino, C.; Torres, L. Levodopa-responsive rubral (Holmes’) tremor. Mov. Disord. 2002, 17, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, B.; Rosenberg, M.; Scherokman, B.; Gunderson, C.H.; McBurney, J.W.; McClintock, W. Effectiveness of trihexyphenidyl against pendular nystagmus and palatal myoclonus: Evidence of cholinergic dysfunction. Mov. Disord. 1987, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Raina, G.B.; Cersosimo, M.G.; Folgar, S.S.; Giugni, J.C.; Calandra, C.; Paviolo, J.P.; Tkachuk, V.A.; Zuñiga Ramirez, C.; Tschopp, A.L.; Calvo, D.S.; et al. Holmes tremor: Clinical description, lesion localization, and treatment in a series of 29 cases. Neurology 2016, 86, 931–938. [Google Scholar] [CrossRef]
- Deuschl, G.; Raethjen, J.; Hellriegel, H.; Elble, R. Treatment of patients with essential tremor. Lancet Neurol. 2011, 10, 148–161. [Google Scholar] [CrossRef]
- Wang, D.; Sanchez, J.; Foote, K.D.; Sudhyadhom, A.; Bhatti, M.T.; Lewis, S.; Okun, M.S. Failed DBS for palliation of visual problems in a case of oculopalatal tremor. Parkinsonism Relat. Disord. 2009, 15, 71–73. [Google Scholar] [CrossRef]


| Case | Age, Sex | Etiology | Tumor Localization | Surgery | Surgical Approach (side) | Non-Surgical Therapy |
|---|---|---|---|---|---|---|
| 1 | 15, male | Medulloblastoma | Fourth ventricle, Lepto-meningeal and spinal | Partial resection | Transvermal (midline) | RCH (craniospinal RT (41 Gy), posterior fossa + tuber cinereum boost (14.4 Gy), MET-HIT 2000-AB4-M2-4) |
| 2 | 60, male | Brain metastasis (adenocarcinoma) | Cerebellum, right | Resection | Paramedian (right) | - |
| 3 | 26, male | Medulloblastoma | Fourth ventricle | Resection | No data | RCH (craniospinal RT (40 Gy), posterior fossa boost (60 Gy), residual tumor boost (68–72 Gy), HIT-SKK) |
| 4 | 56, male | Glioblastoma | Mesencephalon | Biopsy | Frontal biopsy (right) | RCH (RT (46 Gy), boost (8 Gy), temozolomide) |
| 5 | 19, female | Medulloblastoma | Fourth ventricle | Resection | Telovelar (midline) | RCH (craniospinal RT (23.4 Gy), posterior fossa boost (19.8 Gy), vincristine) |
| 6 | 71, female | Glioblastoma | Temporopolar, right | Resection | Temporal (right) | RCH (60 Gy, temozolomide), CH (dose-dense temozolomide), Re-RT (20 Gy) |
| 7 | 35, male | Medulloblastoma | Fourth ventricle | Resection | Telovelar, transvermal (midline) | RCH (craniospinal RT (35.2 Gy), posterior fossa boost (19.8 Gy), vincristine, lomustine, cisplastin) |
| 8 | 56, female | PCNSL | Pons, thalamus, and centrum semiovale, right | Biopsy | Parietal biopsy (right) | CH (rituximab, methotrexate, Ara-C) |
| 9 | 28, male | Papillary tumor of the pineal region (WHO II) | Fourth ventricle | Resection | Telovelar (midline) | Brachytherapy, radiosurgery (15 Gy) |
| 10 | 63, male | Cerebellar metastasis (SCLC) | Cerebellar vermis | - | No surgery | RCH (WBRT (30 Gy), mediastinal RT (50 Gy), cisplatin, etoposide) |
| 11 | 41, male | Medulloblastoma | Fourth ventricle, left dentate nucleus | Partial resection | Telovelar (midline) | RCH (craniospinal RT (35.2 Gy), posterior fossa boost (19.8 Gy), vincristine) |
| 12 | 12, female | Ependymoma (WHO II) | 4th ventricle | Biopsy, resection | Transvermal (midline) | CH (methotrexate, etoposide, cyclophosphamide, vincristine, carboplatin, Ara-C), RT (craniospinal RT (36 Gy), tumor boost (20 Gy)) |
| Case | Age, Sex | Diagnosis | HOD on Preoperative MRI (latency) 1 | HOD on First Postoperative MRI (latency) 2 | Latency Surgery to HOD (months) | Lesion Localization within Dentato-Rubro-Olivary Pathway | HOD Side | HOD Symptoms (PT, HT, PN) | HOD Dx |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 15, male | Medulloblastoma | no (−1d) | no (+8d) | 4 | bilateral dentate nucleus | right | no | n. a. |
| 2 | 60, male | Brain metastasis (adenocarcinoma) | no (−8d) | yes (+4w) | 1 | right dentate nucleus | left | PT | yes |
| 3 | 26, male | Medulloblastoma | n. a.* | no (+6w) | 9 | bilateral dentate nucleus | bilateral | n. a. | no |
| 4 | 56, male | Glioblastoma | no (−1d) | no (+4m) | 7 | left sup. cerebellar peduncle | right | PT | yes |
| 5 | 19, female | Medulloblastoma | no (−6d) | no (+1d) | 8 | bilateral dentate nucleus | bilateral | PT | no |
| 6 | 71, female | Glioblastoma | no (−4d) | no (+3d) | 30 | right sup. cerebellar peduncle | left | no | no |
| 7 | 35, male | Medulloblastoma | no (−3d) | no (+2d) | 10 | bilateral dentate nucleus | bilateral | no | no |
| 8 | 56, female | PCNSL | no (−2d) | no (+1d) | 1 | right red nucleus and central tegmental tract | right | no | no |
| 9 | 28, male | Papillary tumor of the pineal region (WHO II) | no (−13d) | no (+1d) | 2 | right dentate nucleus | left | no | no |
| 10 | 63, male | Cerebellar metastasis (SCLC) | no surgery | no surgery | no surgery | no visible lesion | right | no | yes |
| 11 | 41, male | Medulloblastoma | no (−31d) | no (+2d) | 3 | left dentate nucleus | right | HT | yes |
| 12 | 12, female | Ependymoma (WHO II) | n. a.* | no (+1d) | 4 | bilateral dentate nucleus | left | n. a. | no |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaller-Paule, M.A.; Foerch, C.; Kluge, S.; Baumgarten, P.; Konczalla, J.; Steinbach, J.P.; Wagner, M.; Luger, A.-L. Delayed Occurrence of Hypertrophic Olivary Degeneration after Therapy of Posterior Fossa Tumors: A Single Institution Retrospective Analysis. J. Clin. Med. 2019, 8, 2222. https://doi.org/10.3390/jcm8122222
Schaller-Paule MA, Foerch C, Kluge S, Baumgarten P, Konczalla J, Steinbach JP, Wagner M, Luger A-L. Delayed Occurrence of Hypertrophic Olivary Degeneration after Therapy of Posterior Fossa Tumors: A Single Institution Retrospective Analysis. Journal of Clinical Medicine. 2019; 8(12):2222. https://doi.org/10.3390/jcm8122222
Chicago/Turabian StyleSchaller-Paule, Martin A., Christian Foerch, Sara Kluge, Peter Baumgarten, Jürgen Konczalla, Joachim P. Steinbach, Marlies Wagner, and Anna-Luisa Luger. 2019. "Delayed Occurrence of Hypertrophic Olivary Degeneration after Therapy of Posterior Fossa Tumors: A Single Institution Retrospective Analysis" Journal of Clinical Medicine 8, no. 12: 2222. https://doi.org/10.3390/jcm8122222
APA StyleSchaller-Paule, M. A., Foerch, C., Kluge, S., Baumgarten, P., Konczalla, J., Steinbach, J. P., Wagner, M., & Luger, A.-L. (2019). Delayed Occurrence of Hypertrophic Olivary Degeneration after Therapy of Posterior Fossa Tumors: A Single Institution Retrospective Analysis. Journal of Clinical Medicine, 8(12), 2222. https://doi.org/10.3390/jcm8122222





