Identification of Diagnostic and Prognostic microRNAs for Recurrent Vitreous Hemorrhage in Patients with Proliferative Diabetic Retinopathy
Abstract
1. Introduction
2. Methods
2.1. Ethics Approval and Consent to Participate
2.2. Patient Selection
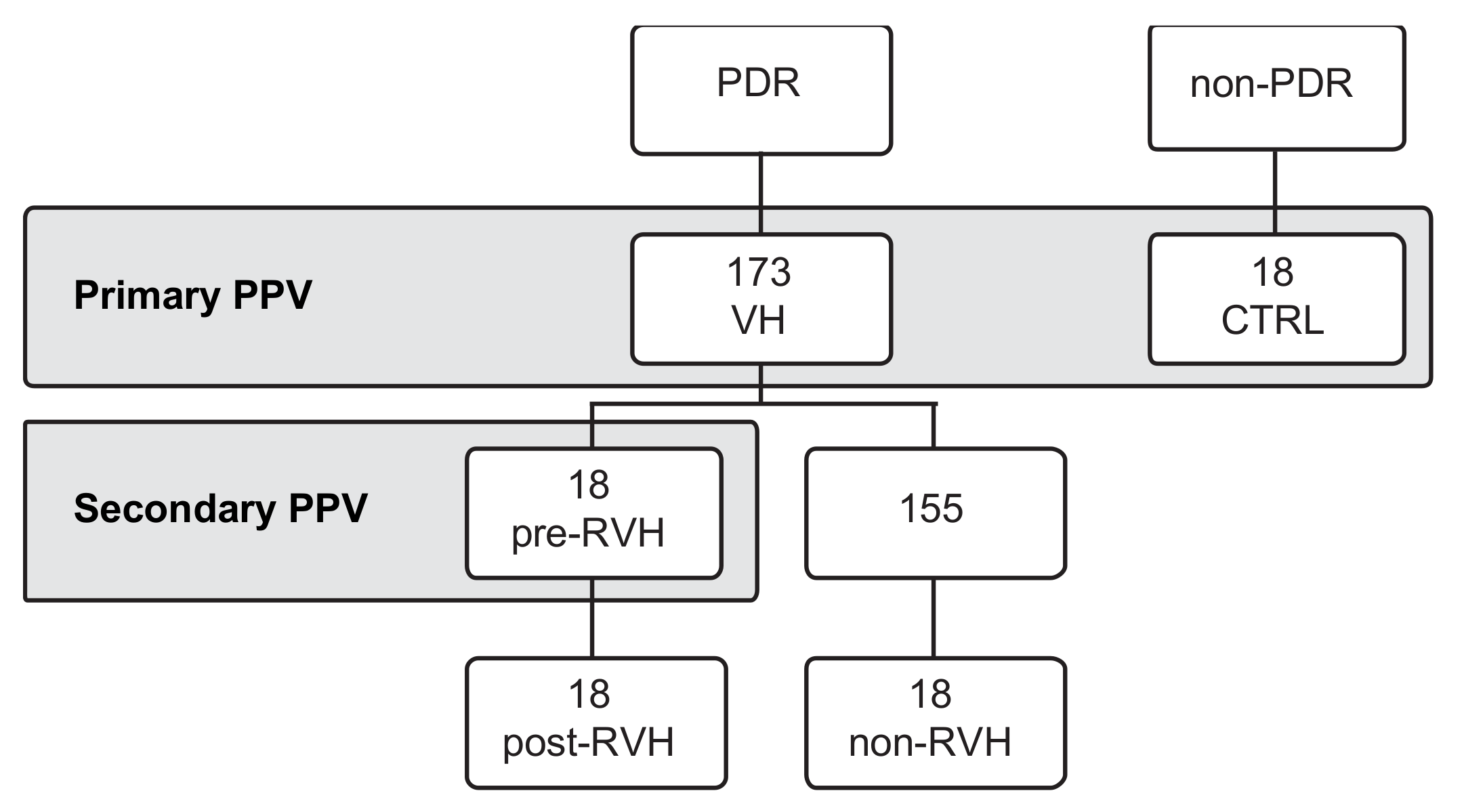
2.3. Collection of Vitreous Samples and Grouping
2.4. Detection of miRNA Presence
2.5. Reverse Transcriptase and Preamplification
2.6. Pooled Analysis
2.7. Single-Patient Analysis
2.8. Statistical Analysis
3. Results
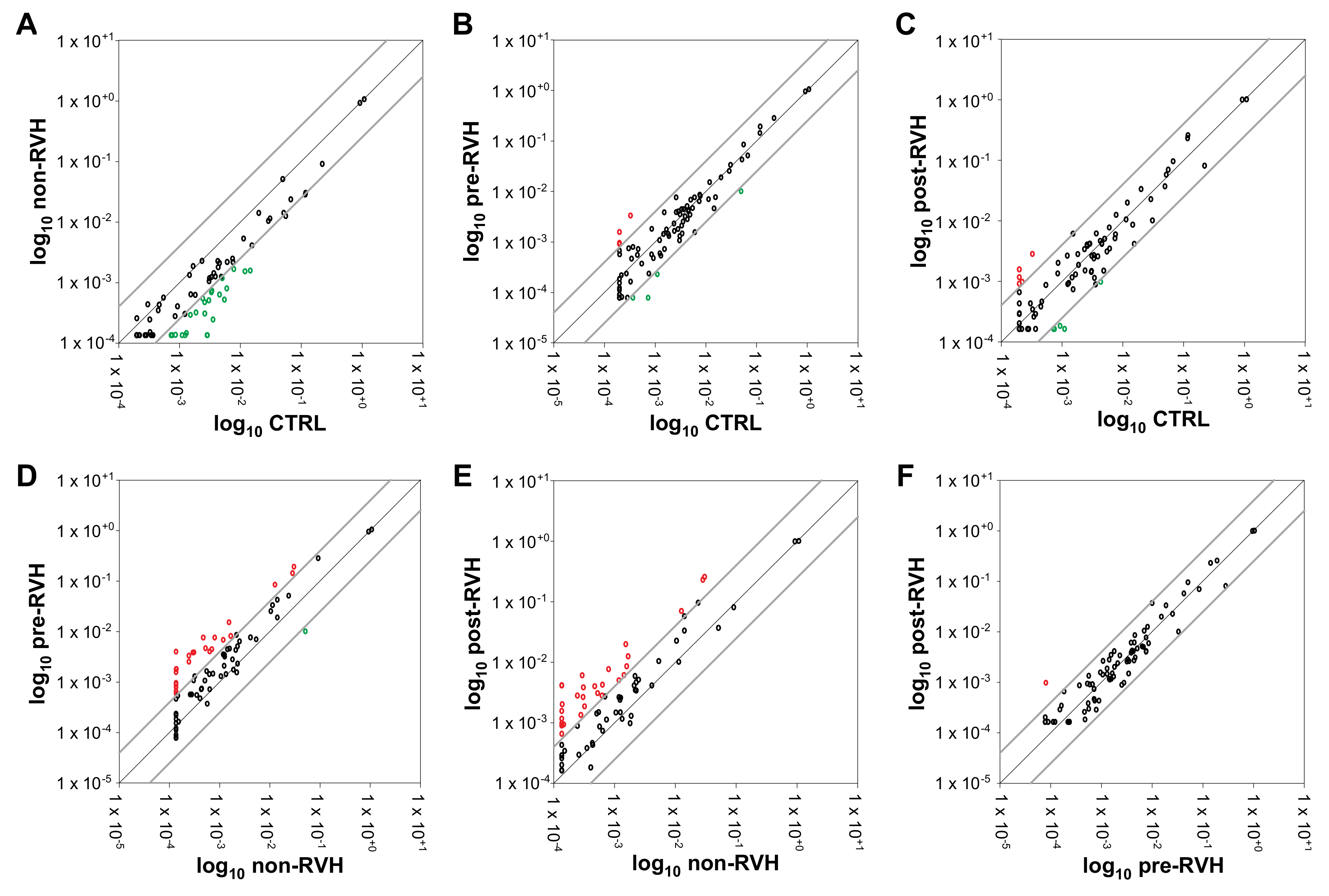
3.1. Angiogenic miRNAs Profiling Illustrated Divergences within Pooled Proliferative Diabetic Retinopathy (PDR) Vitreous
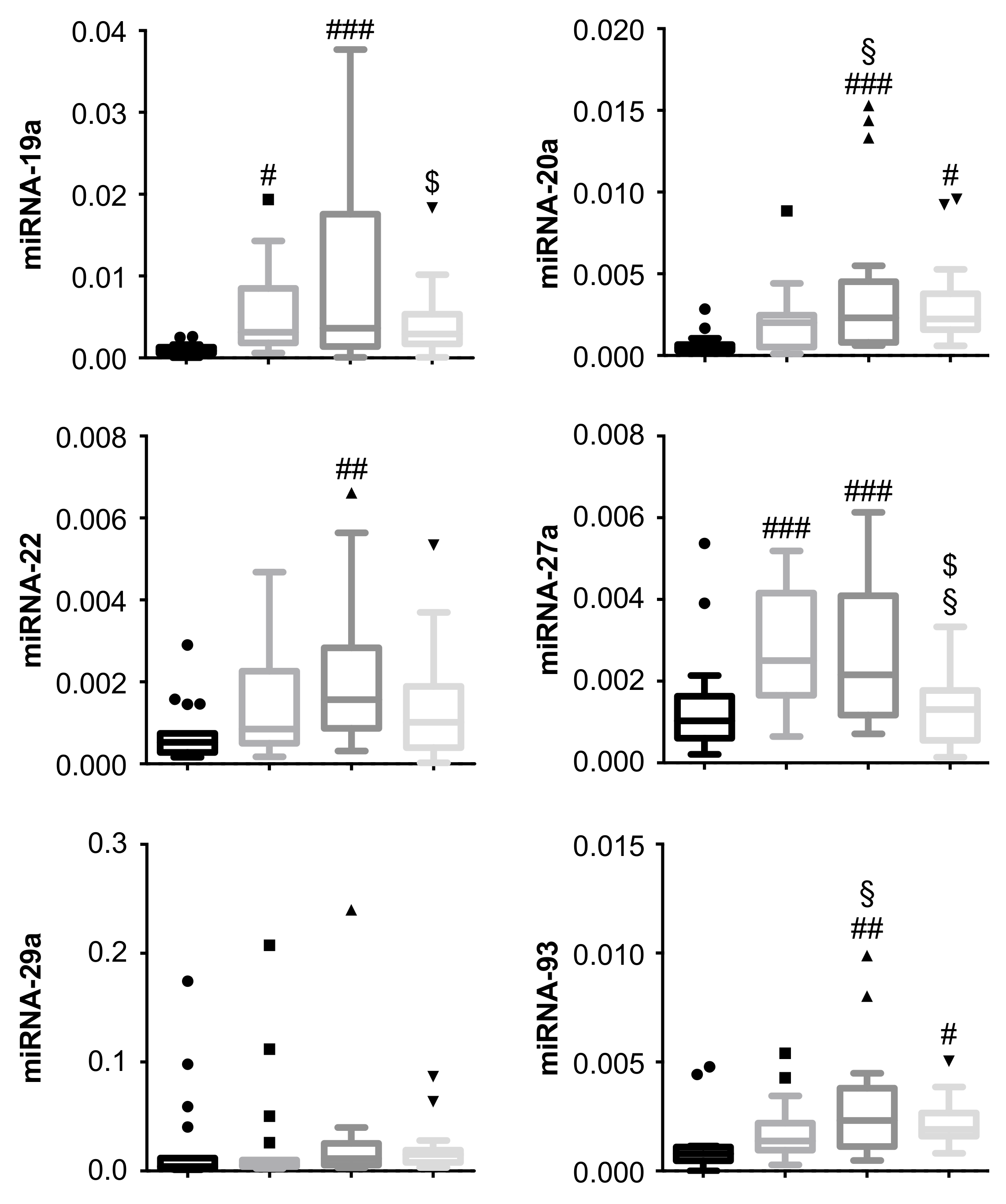
3.2. miRNA-19a and miRNA-27a Are Increased in PDR Patients, and Are Decreased in PDR Patients Undergoing Secondary pars plana vitrectomy (PPV)
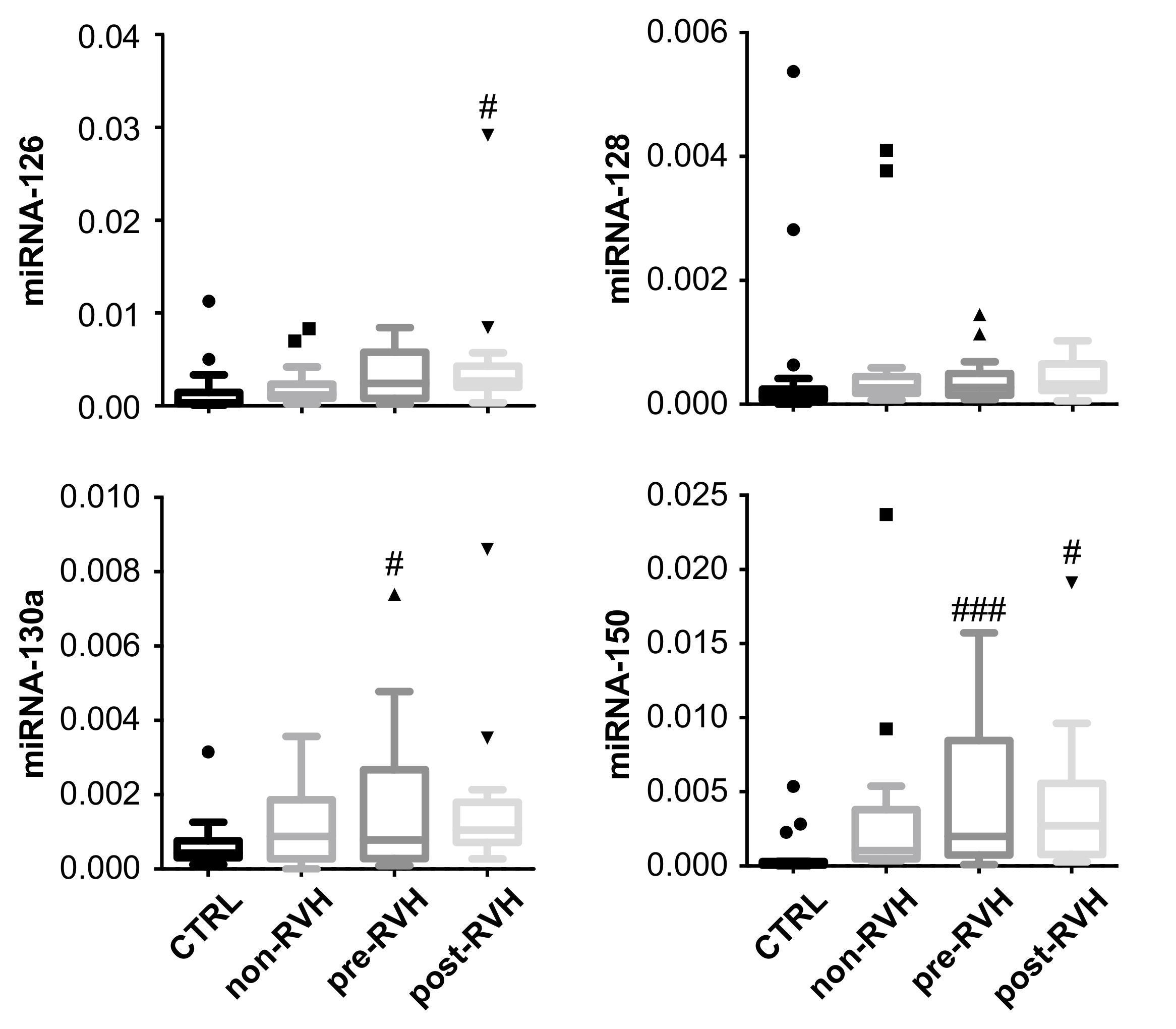
3.3. miRNA-20a and miRNA-93 Are Upregulated in PDR Patients with Recurrent Vitreous Hemorrhage (RVH) in Single-Patient Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beck, L.; D’Amore, P.A. Vascular development: cellular and molecular regulation. FASEB J. 1997, 11, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Isner, J.M.; Asahara, T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J. Clin. Invest. 1999, 103, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Fukushima, Y.; Ogura, S.; Inoue, N.; Uemura, A. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab. J. 2018, 42, 364. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. 2013, 91, 311–321. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Kvanta, A. Ocular angiogenesis: The role of growth factors. Acta Ophthalmol. Scand. 2006, 84, 282–288. [Google Scholar] [CrossRef]
- Yang, C.M.; Yeh, P.T.; Yang, C.H. Intravitreal Long-Acting Gas in the Prevention of Early Postoperative Vitreous Hemorrhage in Diabetic Vitrectomy. Ophthalmology 2007, 114, 710–715. [Google Scholar] [CrossRef]
- Brown, G.C.; Tasman, W.S.; Benson, W.E.; Mcnamara, J.A.; Eagle, R.C. Reoperation Following Diabetic Vitrectomy. Arch. Ophthalmol. 1992, 110, 506–510. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Usui, Y.; Okunuki, Y.; Ueda, S.; Kimura, K.; Muramatsu, D.; Kezuka, T.; Goto, H. Intraocular VEGF level as a risk factor for postoperative complications after vitrectomy for proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6403–6410. [Google Scholar] [CrossRef]
- Watanabe, D.; Suzuma, K.; Suzuma, I.; Ohashi, H.; Ojima, T.; Kurimoto, M.; Murakami, T.; Kimura, T.; Takagi, H. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. 2005, 139, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bellini, S.; Mastrocola, R.; Bruno, G.; Gruden, G. MicroRNA and microvascular complications of diabetes. Int. J. Endocrinol. 2018, 2018, 6890501. [Google Scholar] [CrossRef] [PubMed]
- Soifer, H.S.; Rossi, J.J.; Sætrom, P. MicroRNAs in disease and potential therapeutic applications. Mol. Ther. 2007, 15, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Kuehbacher, A.; Urbich, C.; Dimmeler, S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol. Sci. 2008, 29, 12–15. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef]
- Szemraj, M.; Bielecka-Kowalska, A.; Oszajca, K.; Krajewska, M.; Goś, R.; Jurowski, P.; Kowalski, M.; Szemraj, J. Serum MicroRNAs as Potential Biomarkers of AMD. Med. Sci. Monit. 2015, 21, 2734–2742. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, J.; Gan, L.; Zhang, Y.; Guo, R.; Cao, X.; Lau, W.B.; Ma, X.; Wang, Y. CTRP3 is a novel biomarker for diabetic retinopathy and inhibits HGHL-induced VCAM-1 expression in an AMPK-dependent manner. PLoS ONE 2017, 12, e0178253. [Google Scholar] [CrossRef]
- Kim, V.N. MicroRNA biogenesis: Coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005, 6, 376–385. [Google Scholar] [CrossRef]
- Huang, P.; Sun, J.; Wang, F.; Luo, X.; Feng, J.; Gu, Q.; Liu, T.; Sun, X. MicroRNA expression patterns involved in amyloid beta-induced retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Rutar, M.V.; Provis, J.M.; Natoli, R.C. Identification of miRNAs in a model of retinal degenerations. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, G.; Oh, D.J.; Kang, M.H.; Rhee, D.J. Coordinated regulation of extracellular matrix synthesis by the microRNA-29 family in the trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, M.V.; Januszewski, A.S.; Jenkins, A.J.; Hardikar, A.A. Circulating microRNA Biomarkers of Diabetic Retinopathy. Diabetes 2016, 65, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Su, G. Roles of miRNAs and long noncoding RNAs in the progression of diabetic retinopathy. Biosci. Rep. 2017, 37, BSR20171157. [Google Scholar] [CrossRef]
- Ménard, C.; Rezende, F.A.; Miloudi, K.; Wilson, A.; Tétreault, N.; Hardy, P.; SanGiovanni, J.P.; De Guire, V.; Sapieha, P. MicroRNA signatures in vitreous humour and plasma of patients with exudative AMD. Oncotarget 2016, 7, 19171–19184. [Google Scholar] [CrossRef][Green Version]
- Tuo, J.; Shen, D.; Yang, H.H.; Chan, C.-C. Distinct microRNA-155 expression in the vitreous of patients with primary vitreoretinal lymphoma and uveitis. Am. J. Ophthalmol. 2014, 157, 728–734. [Google Scholar] [CrossRef]
- Ragusa, M.; Caltabiano, R.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Toro, M.D.; Di Pietro, C.; Purrello, M.; Reibaldi, M. MicroRNAs in vitreous humor from patients with ocular diseases. Mol. Vis. 2013, 19, 430–440. [Google Scholar]
- Zou, H.-L.; Wang, Y.; Gang, Q.; Zhang, Y.; Sun, Y. Plasma level of miR-93 is associated with higher risk to develop type 2 diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1159–1166. [Google Scholar] [CrossRef]
- Rezk, N.A.; Sabbah, N.A.; Saad, M.S.S. Role of MicroRNA 126 in screening, diagnosis, and prognosis of diabetic patients in Egypt. IUBMB Life 2016, 68, 452–458. [Google Scholar] [CrossRef]
- Mazzeo, A.; Beltramo, E.; Lopatina, T.; Gai, C.; Trento, M.; Porta, M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp. Eye Res. 2018, 176, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Liu, Y.; Wang, C.; Duan, D.; Lu, N.; Wang, K.; Zhang, L.; Gu, K.; Chen, S.; et al. Comparisons of serum miRNA expression profiles in patients with diabetic retinopathy and type 2 diabetes mellitus. Clinics (Sao Paulo) 2017, 72, 111–115. [Google Scholar] [CrossRef]
- Zampetaki, A.; Willeit, P.; Burr, S.; Yin, X.; Langley, S.R.; Kiechl, S.; Klein, R.; Rossing, P.; Chaturvedi, N.; Mayr, M. Angiogenic microRNAs Linked to Incidence and Progression of Diabetic Retinopathy in Type 1 Diabetes. Diabetes 2016, 65, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Qing, S.; Yuan, S.; Yun, C.; Hui, H.; Mao, P.; Wen, F.; Ding, Y.; Liu, Q. Serum miRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell. Physiol. Biochem. 2014, 34, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Keino, H.; Inoue, M.; Ishida, H.; Hirakata, A. Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Huang, Y.-F. Postvitrectomy diabetic vitreous hemorrhage in proliferative diabetic retinopathy. J. Res. Med. Sci. 2012, 17, 865–871. [Google Scholar]
- Kovacs, B.; Lumayag, S.; Cowan, C.; Xu, S. microRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4402–4409. [Google Scholar] [CrossRef]
- Agrawal, S. MicroRNA signature and function in retinal neovascularization. World J. Biol. Chem. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Lv, Y.N.; Ou-yang, A.J.; Fu, L.S. MicroRNA-27a Negatively Modulates the Inflammatory Response in Lipopolysaccharide-Stimulated Microglia by Targeting TLR4 and IRAK4. Cell. Mol. Neurobiol. 2017, 37, 195–210. [Google Scholar] [CrossRef]
- Tang, X.; Dai, Y.; Wang, X.; Zeng, J.; Li, G. MicroRNA-27a protects retinal pigment epithelial cells under high glucose conditions by targeting TLR4. Exp. Ther. Med. 2018, 16, 452–458. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, M.; Liu, Y.; Zhao, X.; Lian, P.; Chen, Y.; Liu, B.; Lu, L. Landscape of microRNA in the aqueous humour of proliferative diabetic retinopathy as assessed by next-generation sequencing. Clin. Exp. Ophthalmol. 2019, 47, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Usui-Ouchi, A.; Ouchi, Y.; Kiyokawa, M.; Sakuma, T.; Ito, R.; Ebihara, N. Upregulation of mir-21 levels in the vitreous humor is associated with development of proliferative vitreoretinal disease. PLoS ONE 2016, 11, e0158043. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.R.; Elsayed, E.T.; Moftah, R.F. MicroRNA-200b Expression in the Vitreous Humor of Patients with Proliferative Diabetic Retinopathy. Ophthalmic Res. 2017, 58, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.N.; Dias-Neto, E.; Cardó-Vila, M.; Edwards, J.K.; Dobroff, A.S.; Giordano, R.J.; Mandelin, J.; Brentani, H.P.; Hasselgren, C.; Yao, V.J.; et al. Synchronous down-modulation of miR-17 family members is an early causative event in the retinal angiogenic switch. Proc. Natl. Acad. Sci. 2015, 112, 3770–3775. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Birnbaum, Y.; Nanhwan, M.K.; Thomas, B.; Bajaj, M.; Ye, Y. MicroRNA-dependent cross-talk between VEGF and HIF1α in the diabetic retina. Cell. Signal. 2013, 25, 2840–2847. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, Z.; Li, G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem. Biophys. Res. Commun. 2009, 386, 549–553. [Google Scholar] [CrossRef]
- Hua, Z.; Lv, Q.; Ye, W.; Wong, C.K.A.; Cai, G.; Gu, D.; Ji, Y.; Zhao, C.; Wang, J.; Yang, B.B.; et al. Mirna-directed regulation of VEGF and other angiogenic under hypoxia. PLoS ONE 2006, 1, e116. [Google Scholar] [CrossRef]
- Liang, Z.; Gao, K.P.; Wang, Y.X.; Liu, Z.C.; Tian, L.; Yang, X.Z.; Ding, J.Y.; Wu, W.T.; Yang, W.H.; Li, Y.L.; et al. RNA sequencing identified specific circulating miRNA biomarkers for early detection of diabetes retinopathy. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E374–E385. [Google Scholar] [CrossRef]
- Li, F.; Liang, X.; Chen, Y.; Li, S.; Liu, J. Role of microRNA-93 in regulation of angiogenesis. Tumor Biol. 2014, 35, 10609–10613. [Google Scholar] [CrossRef]
- Ching, A.-S.; Ahmad-Annuar, A. A Perspective on the Role of microRNA-128 Regulation in Mental and Behavioral Disorders. Front. Cell. Neurosci. 2015, 9, 465. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Chen, J.; Lin, S.; Cai, D.; Chen, C.; Chen, Z. Downregulation of MicroRNA 29a/b exacerbated diabetic retinopathy by impairing the function of Müller cells via Forkhead box protein O4. Diabetes Vasc. Dis. Res. 2018, 15, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Bai, X.; Wang, Z.; Zhang, X.; Ruan, C.; Miao, J. MicroRNA-126 inhibits ischemia-induced retinal neovascularization via regulating angiogenic growth factors. Exp. Mol. Pathol. 2011, 91, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-Z.; Yang, J.; Xue, L.-P.; Xiao, L.-B.; Li, Y. MiR-126 overexpression inhibits high glucose-induced migration and tube formation of rhesus macaque choroid-retinal endothelial cells by obstructing VEGFA and PIK3R2. J. Diabetes Complications 2017, 31, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Kim, A.J.; Chang, R.C.A.; Chang, J.Y.A.; Ying, W.; Ko, M.L.; Zhou, B.; Ko, G.Y.P. Deletion of miR-150 exacerbates retinal vascular overgrowth in high-fat-diet induced diabetic mice. PLoS ONE 2016, 11, e0157543. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhan, H.; Zhou, X.-Y.; Yao, L.; Yan, M.; Chen, A.; Liu, J.; Ren, X.; Zhang, X.; Liu, J.-X.; et al. MicroRNA-22 regulates inflammation and angiogenesis via targeting VE-cadherin. FEBS Lett. 2017, 591, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lv, X.; Chen, L.; Gu, X.; Qian, H.; Fransisca, S.; Zhang, Z.; Liu, Q.; Xie, P. Protective effects of microRNA-22-3p against retinal pigment epithelial inflammatory damage by targeting NLRP3 inflammasome. J. Cell. Physiol. 2019, 234, 18849–18857. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Zhang, X.; Fan, Y.; Fang, L.; Wang, C.; Lv, Z.; Fu, D.; Li, Y. Downregulation of MicroRNA-130a Contributes to Endothelial Progenitor Cell Dysfunction in Diabetic Patients via Its Target Runx3. PLoS ONE 2013, 8, e68611. [Google Scholar] [CrossRef]

| Patient Variables | Control | Non-Reoperated a | Reoperated b |
|---|---|---|---|
| N | 18 | 18 | 18 |
| Age (mean, range) c | 69 (29–80) | 58 (39–83) | 53 (24–81) |
| Sex (M/F) d | 12/6 | 12/6 | 8/10 |
| Surgery (MH/ERM) e | 4/14 | ||
| DM type (T1D/T2D) f | 7/11 | 8/10 | |
| DM duration (mean, range) g | 24 (5–41) | 21 (1–44) | |
| Rubeosis iridis (N) h | 2 | 3 | |
| Neovascular glaucoma (N) i | 1 | 0 | |
| Preoperative laser (N) j | 16 | 16 | |
| Peroperative laser (N) k | 17 | 15 | |
| PVD (N) l | 6 | 3 | |
| Tamponade (n/a/s/c) m | 1/10/4/3 | 3/10/1/4 | |
| 2nd PPV (mean, range) n | 13 (2–48) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammadzada, P.; Bayle, J.; Gudmundsson, J.; Kvanta, A.; André, H. Identification of Diagnostic and Prognostic microRNAs for Recurrent Vitreous Hemorrhage in Patients with Proliferative Diabetic Retinopathy. J. Clin. Med. 2019, 8, 2217. https://doi.org/10.3390/jcm8122217
Mammadzada P, Bayle J, Gudmundsson J, Kvanta A, André H. Identification of Diagnostic and Prognostic microRNAs for Recurrent Vitreous Hemorrhage in Patients with Proliferative Diabetic Retinopathy. Journal of Clinical Medicine. 2019; 8(12):2217. https://doi.org/10.3390/jcm8122217
Chicago/Turabian StyleMammadzada, Parviz, Juliette Bayle, Johann Gudmundsson, Anders Kvanta, and Helder André. 2019. "Identification of Diagnostic and Prognostic microRNAs for Recurrent Vitreous Hemorrhage in Patients with Proliferative Diabetic Retinopathy" Journal of Clinical Medicine 8, no. 12: 2217. https://doi.org/10.3390/jcm8122217
APA StyleMammadzada, P., Bayle, J., Gudmundsson, J., Kvanta, A., & André, H. (2019). Identification of Diagnostic and Prognostic microRNAs for Recurrent Vitreous Hemorrhage in Patients with Proliferative Diabetic Retinopathy. Journal of Clinical Medicine, 8(12), 2217. https://doi.org/10.3390/jcm8122217





