Bioresorbable Vascular Scaffolds—Dead End or Still a Rough Diamond?
Abstract
1. Introduction
2. Potential Advantages of BVS over Current Generation DES
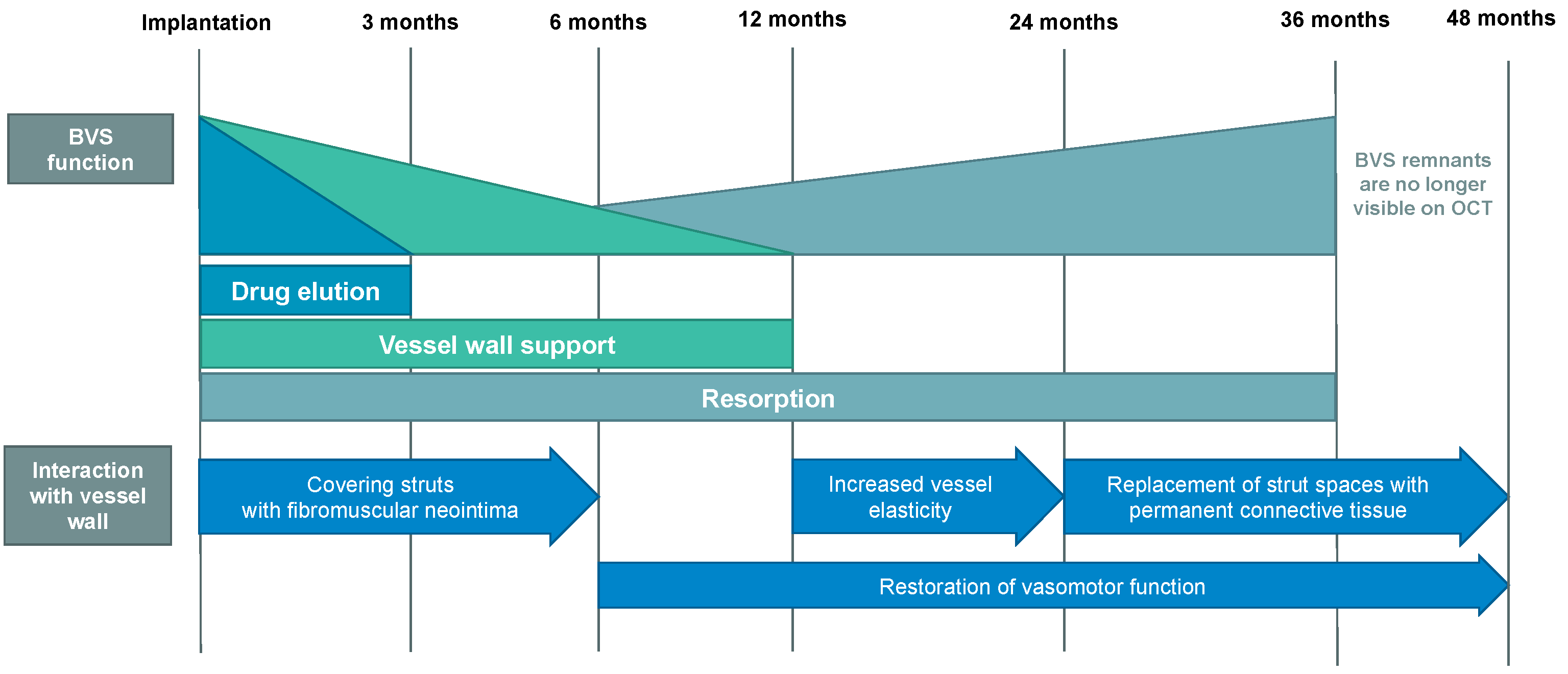
3. Overview of First and Second Generation BVS
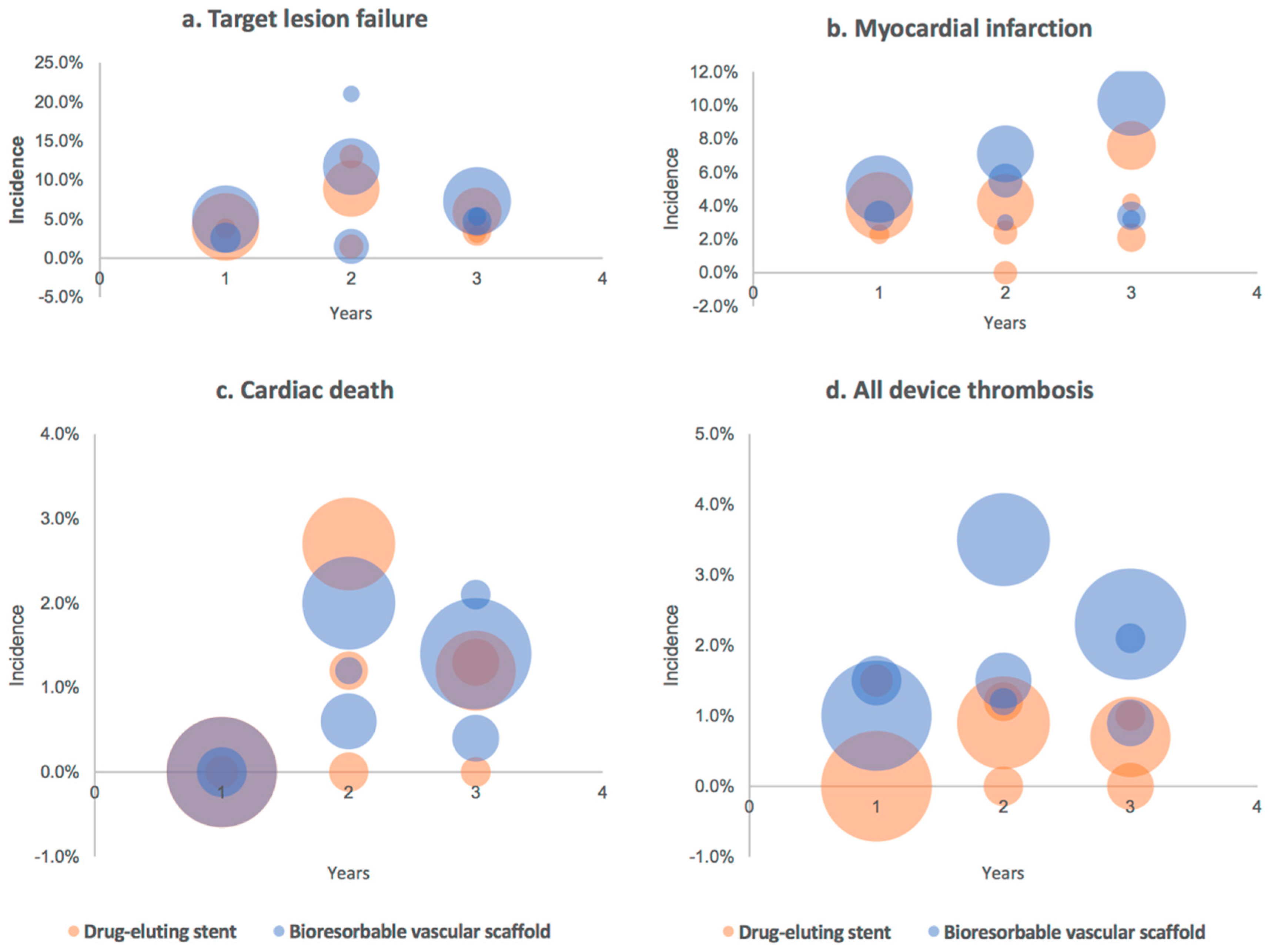
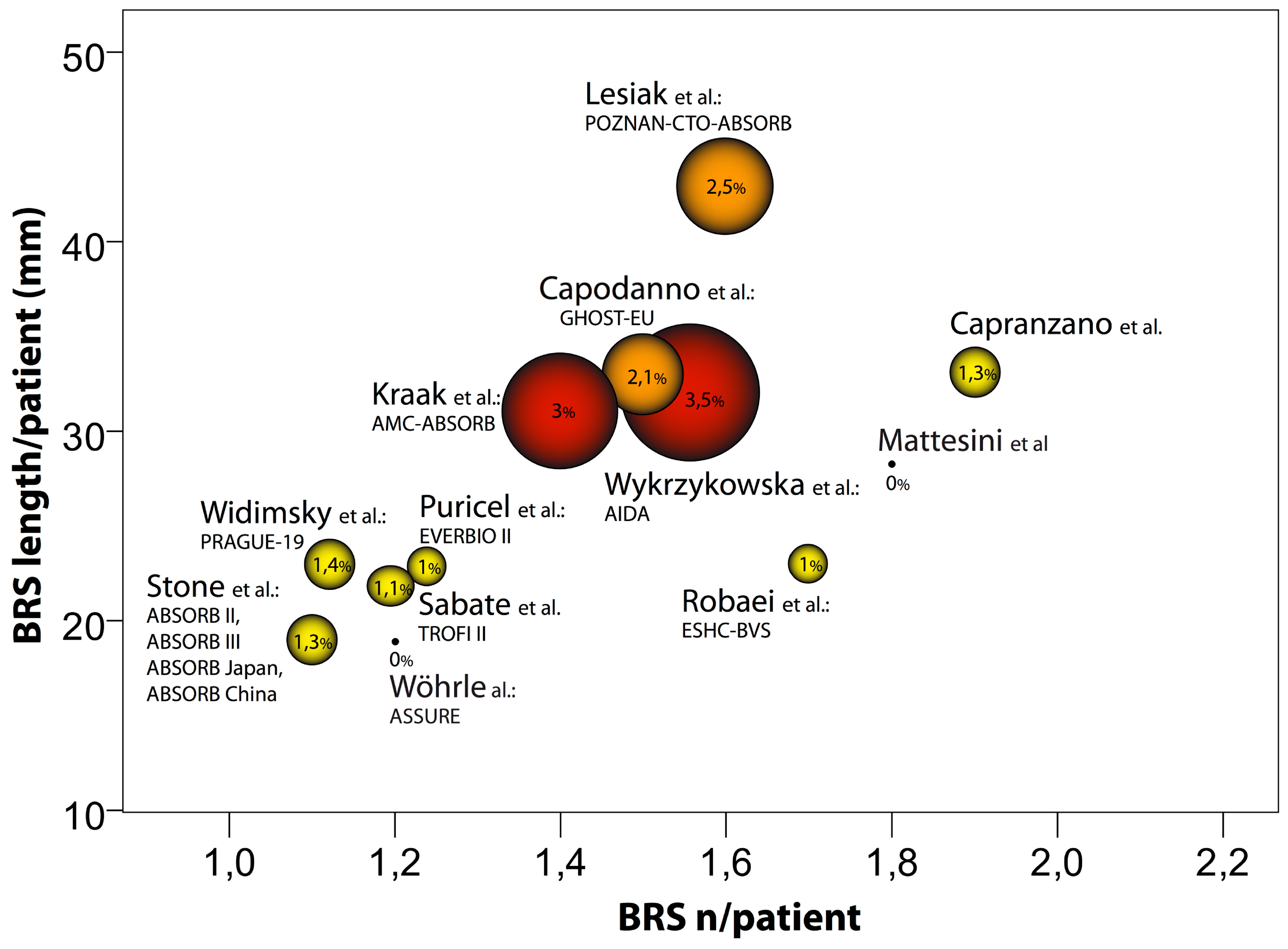
4. Real-World BVS Performance—Outcomes and Evaluation
5. Anatomy of Failure: Explanation for Unfavorable Outcomes
6. Niche for BVS and Optimization of BVS Action
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Townsend, N.; Nichols, M.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe—Epidemiological update 2015. Eur. Heart J. 2015, 36, 2696–2705. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Schmidt, T.; Abbott, J.D. Coronary Stents: History, Design, and Construction. J. Clin. Med. 2018, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Vahl, T.P.; Gasior, P.; Gongora, C.A.; Ramzipoor, K.; Lee, C.; Cheng, Y. Four-year polymer biocompatibility and vascular healing profile of a novel ultrahigh molecular weight amorphous PLLA bioresorbable vascular scaffold: An OCT study in healthy porcine coronary arteries. EuroIntervention 2016, 12, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, N.; Otaegui, I.; Rumoroso, J.R.; Gutiérrez, H.; Alfonso, F.; Marti, G. Device specificity of vascular healing following implantation of bioresorbable vascular scaffolds and bioabsorbable polymer metallic drug-eluting stents in human coronary arteries: The ESTROFA OCT BVS vs. BP-DES study. EuroIntervention 2018, 14, e1295–e1303. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Onuma, Y.; Serruys, P.W.; Stone, G.W. Bioresorbable Vascular Scaffolds for Coronary Revascularization. Circulation 2016, 134, 168–182. [Google Scholar] [CrossRef]
- Serruys, P.W.; Ormiston, J.A.; Onuma, Y.; Regar, E.; Gonzalo, N.; Garcia-Garcia, H.M. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 2009, 373, 897–910. [Google Scholar] [CrossRef]
- Tesfamariam, B. Bioresorbable vascular scaffolds: Biodegradation, drug delivery and vascular remodeling. Pharm. Res. 2016, 107, 163–171. [Google Scholar] [CrossRef]
- Montone, R.A.; Niccoli, G.; De Marco, F.; Minelli, S.; D’Ascenzo, F.; Testa, L. Temporal Trends in Adverse Events After Everolimus-Eluting Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Metallic Stent Implantation: A Meta-Analysis of Randomized Controlled Trials. Circulation 2017, 135, 2145–2154. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhu, Q.Q.; Kang, L.N.; Li, X.L.; Xu, B. Mid- and Long-Term Outcome Comparisons of Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2017, 167, 642–654. [Google Scholar] [CrossRef]
- Gheorghe, L.; Millán, X.; Jimenez-Kockar, M.; Gomez-Lara, J.; Arzamendi, D.; Danduch, L.; Agudelo, V.; Serra, A. Bioresorbable vascular scaffolds in coronary chronic total occlusions: Clinical, vasomotor and optical coherence tomography findings at three-year follow-up (ABSORB-CTO study). EuroIntervention 2019, 15, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Virmani, R.; Finn, A.V. Bioresorbable vascular scaffolds: Implication of very late scaffold thrombosis. Coron. Artery Dis. 2017, 28, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Cuesta, J. Bioresorbable Vascular Scaffolds Restenosis: Pathophysiology and Predictors. JACC Cardiovasc. Interv. 2017, 10, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Indolfi, C. Letter by De Rosa and Indolfi regarding article, “Clinical presentation and outcomes of coronary in-stent restenosis across 3-stent generations”. Circ. Cardiovasc. Interv. 2015, 8. [Google Scholar] [CrossRef]
- Indolfi, C.; Mongiardo, A.; Spaccarotella, C.; Caiazzo, G.; Torella, D.; De Rosa, S. Neointimal proliferation is associated with clinical restenosis 2 years after fully bioresorbable vascular scaffold implantation. Circ. Cardiovasc. Imaging 2014, 7, 755–757. [Google Scholar] [CrossRef][Green Version]
- Serruys, P.W.; Onuma, Y.; Garcia-Garcia, H.M.; Muramatsu, T.; Dudek, D.; Thuesen, L. Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: A multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention 2014, 9, 1271–1284. [Google Scholar] [CrossRef]
- Serruys, P.W.; Chevalier, B.; Sotomi, Y.; Cequier, A.; Carrié, D.; Piek, J.J.; Van Boven, J.; Marcello, D.; Dariusz, D.; McClean, D.; et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): A 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016, 388, 2479–2491. [Google Scholar] [CrossRef]
- Chevalier, B.; Cequier, A.; Dudek, D.; Haude, M.; Carrie, D.; Sabaté, M.; Windecker, S.; Reith, S.; de Sousa Almeida, M.; Campo, G.; et al. Four-year follow-up of the randomised comparison between an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II Trial). EuroIntervention 2018, 13, 1561–1564. [Google Scholar] [CrossRef]
- Wykrzykowska, J.J.; Kraak, R.P.; Hofma, S.H.; van der Schaaf, R.J.; Arkenbout, E.K.; IJsselmuiden, A.J.; Joëlle, E.; Ivo, M.; Ruben, Y.G.; Karel, T.K.; et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N. Engl. J. Med. 2017, 376, 2319–2328. [Google Scholar] [CrossRef]
- Rapetto, C.; Leoncini, M. Magmaris: A new generation metallic sirolimus-eluting fully bioresorbable scaffold: Present status and future perspectives. J. Thorac. Dis. 2017, 9 (Suppl. S9), 903–913. [Google Scholar] [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Böse, D. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet 2013, 381, 836–844. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Evald, H.C.; William, W.; Franz-Josef, N.; Christoph, K.; et al. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur. Heart J. 2016, 37, 2701–2709. [Google Scholar] [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Vermeersch, P. Safety and performance of the DRug-Eluting Absorbable Metal Scaffold (DREAMS) in patients with de novo coronary lesions: 3-year results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. EuroIntervention 2016, 12, e160–e166. [Google Scholar] [CrossRef] [PubMed]
- Haude, M.; Ince, H.; Kische, S.; Abizaid, A.; Tölg, R.; Alves, L.P. Sustained safety and clinical performance of a drug-eluting absorbable metal scaffold up to 24 months: Pooled outcomes of BIOSOLVE-II and BIOSOLVE-III. EuroIntervention 2017, 13, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Verheye, S.; Wlodarczak, A.; Montorsi, P.; Bennett, J.; Torzewski, J.; Haude, M. Safety and performance of a resorbable magnesium scaffold under real-world conditions: 12-month outcomes of the first 400 patients enrolled in the BIOSOLVE-IV registry. EuroIntervention 2019. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; di Palma, G.; Cerrato, E.; Latini, R.A.; Elwany, M.; Orrego, P.S.; Seregni, R.G. Clinical and angiographic outcome of a single center, real world population treated with a dedicated technique of implantation for bioresorbable vascular scaffolds. The FAtebenefratelli Bioresorbable Vascular Scaffold (FABS) registry. J. Interv. Cardiol. 2017, 30, 427–432. [Google Scholar] [CrossRef]
- Li, H.; Rha, S.W.; Choi, C.U.; Oh, D.J. Optical Coherence Tomography and Stent Boost Imaging Guided Bioresorbable Vascular Scaffold Overlapping for Coronary Chronic Total Occlusion Lesion. Yonsei Med. J. 2017, 58, 1071–1074. [Google Scholar] [CrossRef]
- Caiazzo, G.; Longo, G.; Giavarini, A.; Kilic, I.D.; Fabris, E.; Serdoz, R.; Alessio, M.; Nicolas, F.; Gioel, G.; Seccob, S.; et al. Optical coherence tomography guidance for percutaneous coronary intervention with bioresorbable scaffolds. Int. J. Cardiol. 2016, 221, 352–358. [Google Scholar] [CrossRef]
- Caiazzo, G.; Kilic, I.D.; Fabris, E.; Serdoz, R.; Mattesini, A.; Foin, N. Absorb bioresorbable vascular scaffold: What have we learned after 5 years of clinical experience? Int. J. Cardiol. 2015, 201, 129–136. [Google Scholar] [CrossRef]
- Onuma, Y.; Dudek, D.; Thuesen, L.; Webster, M.; Nieman, K.; Garcia-Garcia, H.M. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: The ABSORB cohort A trial. JACC Cardiovasc. Interv. 2013, 6, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Khamis, H.; Sanad, O.; Elrabbat, K.; Attia, A.; Adel, M.; Alkady, H.; Masoud, A. Bioresorbable vascular scaffold (BVS) for the treatment of native coronary artery stenosis: One year outcome. Egypt. Heart J. 2016, 68, 253–259. [Google Scholar] [CrossRef][Green Version]
- Otsuka, F.; Vorpahl, M.; Nakano, M.; Foerst, J.; Newell, J.B.; Sakakura, K.; Robert, K.; Elena, L.; Aloke, V.F.; Frank, D.K.; et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 2014, 129, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Kimura, T.; Morimoto, T.; Nakagawa, Y.; Inoue, K.; Soga, Y. Very long-term (15 to 20 years) clinical and angiographic outcome after coronary bare metal stent implantation. Circ. Cardiovasc. Interv. 2010, 3, 468–475. [Google Scholar] [CrossRef][Green Version]
- Pavasini, R.; Serenelli, M.; Gallo, F.; Bugani, G.; Geraci, S.; Vicinelli, P.; Campo, G. Effectiveness and safety of the ABSORB bioresorbable vascular scaffold for the treatment of coronary artery disease: Systematic review and meta-analysis of randomized clinical trials. J. Thorac. Dis. 2017, 9 (Suppl. S9), 887–897. [Google Scholar] [CrossRef]
- Onuma, Y.; Serruys, P.W. Bioresorbable scaffold: The advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011, 123, 779–797. [Google Scholar] [CrossRef]
- Campos, C.M.; Muramatsu, T.; Iqbal, J.; Zhang, Y.J.; Onuma, Y.; Garcia-Garcia, H.M. Bioresorbable drug-eluting magnesium-alloy scaffold for treatment of coronary artery disease. Int. J. Mol. Sci. 2013, 14, 24492–24500. [Google Scholar] [CrossRef]
- Regazzoli, D.; Leone, P.P.; Colombo, A.; Latib, A. New generation bioresorbable scaffold technologies: An update on novel devices and clinical results. J. Thorac. Dis. 2017, 9 (Suppl. S9), 979–985. [Google Scholar] [CrossRef]
- Waksman, R.; Zumstein, P.; Pritsch, M.; Wittchow, E.; Haude, M.; Lapointe-Corriveau, C. Second-generation magnesium scaffold Magmaris: Device design and preclinical evaluation in a porcine coronary artery model. EuroIntervention 2017, 13, 440–449. [Google Scholar] [CrossRef]
- Giacchi, G.; Ortega-Paz, L.; Brugaletta, S.; Ishida, K.; Sabaté, M. Bioresorbable vascular scaffolds technology: Current use and future developments. Med. Devices 2016, 9, 185–198. [Google Scholar] [CrossRef]
- Foin, N.; Lee, R.D.; Torii, R.; Guitierrez-Chico, J.L.; Mattesini, A.; Nijjer, S. Impact of stent strut design in metallic stents and biodegradable scaffolds. Int. J. Cardiol. 2014, 177, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Bil, J.; Gil, R.J. Bioresorbable vascular scaffolds—What does the future bring? J. Thorac. Dis. 2016, 8, E741–E745. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katagiri, Y.; Stone, G.W.; Onuma, Y.; Serruys, P.W. State of the art: The inception, advent and future of fully bioresorbable scaffolds. EuroIntervention 2017, 13, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Joner, M.; Ruppelt, P.; Zumstein, P.; Lapointe-Corriveau, C.; Leclerc, G.; Bulin, A. Preclinical evaluation of degradation kinetics and elemental mapping of first- and second-generation bioresorbable magnesium scaffolds. EuroIntervention 2018, 14, e1040–e1048. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Kumar, S.; Fusaro, M.; Amoroso, N.; Attubato, M.J.; Feit, F. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: A mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 2012, 125, 2873–2891. [Google Scholar] [CrossRef] [PubMed]
- Cassese, S.; Katagiri, Y.; Byrne, R.A.; Brugaletta, S.; Alfonso, F.; Räber, L. Angiographic and clinical outcomes of STEMI patients treated with bioresorbable or metallic everolimus-eluting stents. A pooled analysis of individual patient data from 2 randomized trials. EuroIntervention 2019. [Google Scholar] [CrossRef]
- La Manna, A.; Chisari, A.; Giacchi, G.; Capodanno, D.; Longo, G.; Di Silvestro, M. Everolimus-eluting bioresorbable vascular scaffolds for treatment of complex chronic total occlusions. EuroIntervention 2017, 13, 355–363. [Google Scholar] [CrossRef]
- La Manna, A.; Chisari, A.; Giacchi, G.; Capodanno, D.; Longo, G.; Di Silvestro, M. Systemic Pharmacokinetics of Everolimus Eluted from the Absorb Bioresorbable Vascular Scaffold: An ABSORB III Substudy. J. Am. Coll. Cardiol. 2015, 66, 2467–2469. [Google Scholar] [CrossRef][Green Version]
- Tanabe, K.; Popma, J.J.; Kozuma, K.; Saito, S.; Muramatsu, T.; Nakamura, S. Multislice computed tomography assessment of everolimus-eluting Absorb bioresorbable scaffolds in comparison with metallic drug-eluting stents from the ABSORB Japan randomised trial. EuroIntervention 2018, 14, e1020–e1028. [Google Scholar] [CrossRef]
- Ang, H.Y.; Bulluck, H.; Wong, P.; Venkatraman, S.S.; Huang, Y.; Foin, N. Bioresorbable stents: Current and upcoming bioresorbable technologies. Int. J. Cardiol. 2017, 228, 931–939. [Google Scholar] [CrossRef]
- Ali, Z.A.; Serruys, P.W.; Kimura, T.; Gao, R.; Ellis, S.G.; Kereiakes, D.J. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: A systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet 2017, 390, 760–772. [Google Scholar] [CrossRef]
- Varcoe, R.L.; Thomas, S.D.; Rapoza, R.J.; Kum, S. Lessons Learned Regarding Handling and Deployment of the Absorb Bioresorbable Vascular Scaffold in Infrapopliteal Arteries. J. Endovasc. Ther. 2017, 24, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, S.; De Rosa, S.; Ambrosio, G.; Mongiardo, A.; Spaccarotella, C.; Polimeni, A.; Jolanda, S.; Daniele, T.; Gianluca, C.; Ciro, I. The duration of balloon inflation affects the luminal diameter of coronary segments after bioresorbable vascular scaffolds deployment. BMC Cardiovasc. Disord. 2015, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Mattesini, A.; Bartolini, S.; Dini, C.S.; Valente, S.; Parodi, G.; Stolcova, M.; Di Mario, C. The DESolve novolimus bioresorbable Scaffold: From bench to bedside. J. Thorac. Dis. 2017, 9 (Suppl. S9), 950–958. [Google Scholar] [CrossRef] [PubMed]
- Nef, H.M.; Wiebe, J.; Foin, N.; Blachutzik, F.; Dörr, O.; Toyloy, S.; Hamm, C.W. A new novolimus-eluting bioresorbable coronary scaffold: Present status and future clinical perspectives. Int. J. Cardiol. 2017, 227, 127–133. [Google Scholar] [CrossRef]
- Abizaid, A.; Costa, R.A.; Schofer, J.; Ormiston, J.; Maeng, M.; Witzenbichler, B. Serial Multimodality Imaging and 2-Year Clinical Outcomes of the Novel DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold System for the Treatment of Single De Novo Coronary Lesions. JACC Cardiovasc. Interv. 2016, 9, 565–574. [Google Scholar] [CrossRef]
- Ormiston, J.A.; Webber, B.; Ubod, B.; Darremont, O.; Webster, M.W. An independent bench comparison of two bioresorbable drug-eluting coronary scaffolds (Absorb and DESolve) with a durable metallic drug-eluting stent (ML8/Xpedition). EuroIntervention 2015, 11, 60–67. [Google Scholar] [CrossRef]
- Schmidt, W.; Behrens, P.; Brandt-Wunderlich, C.; Siewert, S.; Grabow, N.; Schmitz, K.P. In vitro performance investigation of bioresorbable scaffolds - Standard tests for vascular stents and beyond. Cardiovasc. Revasc. Med. 2016, 17, 375–383. [Google Scholar] [CrossRef]
- Verheye, S.; Ormiston, J.A.; Stewart, J.; Webster, M.; Sanidas, E.; Costa, R.; Ribamar, C., Jr.; Daniel, C.; Andrea, S.A.; Ibraim, P.; et al. A next-generation bioresorbable coronary scaffold system: From bench to first clinical evaluation: 6- and 12-month clinical and multimodality imaging results. JACC Cardiovasc. Interv. 2014, 7, 89–99. [Google Scholar] [CrossRef]
- de Pommereau, A.; de Hemptinne, Q.; Varenne, O.; Picard, F. Bioresorbable vascular scaffolds: Time to absorb past lessons or fade away? Arch. Cardiovasc. Dis. 2018. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet 2016, 387, 31–39. [Google Scholar] [CrossRef]
- Ghimire, G.; Spiro, J.; Kharbanda, R.; Roughton, M.; Barlis, P.; Mason, M. Initial evidence for the return of coronary vasoreactivity following the absorption of bioabsorbable magnesium alloy coronary stents. EuroIntervention 2009, 4, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Varenhorst, C.; Lindholm, M.; Sarno, G.; Olivecrona, G.; Jensen, U.; Nilsson, J.; Jörg, C.; Stefan, J.; Bo, L. Stent thrombosis rates the first year and beyond with new- and old-generation drug-eluting stents compared to bare metal stents. Clin. Res. Cardiol. 2018, 107, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, K.; Gratz, M.; Koeck, K.; Mostertz, J.; Begunk, R.; Loebler, M.; Beatrice, S.; Anne, S.; Petra, H.; Georg, H.; et al. Magnesium used in bioabsorbable stents controls smooth muscle cell proliferation and stimulates endothelial cells in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Berthon, N.; Laurant, P.; Fellmann, D.; Berthelot, A. Effect of magnesium on mRNA expression and production of endothelin-1 in DOCA-salt hypertensive rats. J. Cardiovasc. Pharmacol. 2003, 42, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Ellis, S.G. 3-Year Clinical Outcomes with Everolimus-Eluting Bioresorbable Coronary Scaffolds: The ABSORB III Trial. J. Am. Coll. Cardiol. 2017. [Google Scholar] [CrossRef]
- Ellis, S.G.; Kereiakes, D.J. Everolimus-Eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results; American College of Cardiology Annual Scientific Session: Washington, DC, USA, 2017. [Google Scholar]
- Stone, G.W.; Ellis, S.G.; Gori, T.; Metzger, D.C.; Stein, B.; Erickson, M. Blinded outcomes and angina assessment of coronary bioresorbable scaffolds: 30-day and 1-year results from the ABSORB IV randomised trial. Lancet 2018, 392, 1530–1540. [Google Scholar] [CrossRef]
- Arroyo, D.; Gendre, G.; Schukraft, S.; Kallinikou, Z.; Müller, O.; Baeriswyl, G.; Stauffera, J.C.; Goya, J.J.; Tognia, M.; Cooka, S.; et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds: Two-year clinical outcomes of the EVERBIO II trial. Int. J. Cardiol. 2017, 243, 121–125. [Google Scholar] [CrossRef]
- Katagiri, Y.; Onuma, Y. Three-year follow-up of the randomised comparison between an everolimus-eluting bioresorbable scaffold and a durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction (TROFI II trial). EuroIntervention 2018, 14, e1224–e1226. [Google Scholar] [CrossRef]
- Kimura, T.; Kozuma, K.; Tanabe, K.; Nakamura, S.; Yamane, M.; Muramatsu, T. A randomized trial evaluating everolimus-eluting Absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur. Heart J. 2015, 36, 3332–3342. [Google Scholar] [CrossRef]
- Serruys, P.W.; Ormiston, J.; van Geuns, R.J.; de Bruyne, B.; Dudek, D.; Christiansen, E.; Bernard, C.; Pieter, S.; Dougal, M.; Jacques, K.; et al. A Polylactide Bioresorbable Scaffold Eluting Everolimus for Treatment of Coronary Stenosis: 5-Year Follow-Up. J. Am. Coll. Cardiol. 2016, 67, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Toušek, P.; Kočka, V.; Malý, M.; Kozel, M.; Petr, R.; Hajsl, M. Long-term follow-up after bioresorbable vascular scaffold implantation in STEMI patients: PRAGUE-19 study update. EuroIntervention 2016, 12, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yang, Y.; Han, Y.; Huo, Y.; Wang, L.; Qi, X. Comparison of everolimus-eluting bioresorbable vascular scaffolds and metallic stents: Three-year clinical outcomes from the ABSORB China randomised trial. EuroIntervention 2018, 14, e554–e561. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Alfonso, F.; Schneider, S.; Maeng, M.; Wiebe, J.; Kretov, E. Prospective, randomized trial of bioresorbable scaffolds vs. everolimus-eluting stents in patients undergoing coronary stenting for myocardial infarction: The Intracoronary Scaffold Assessment a Randomized evaluation of Absorb in Myocardial Infarction (ISAR-Absorb MI) trial. Eur. Heart J. 2019, 40, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A.; Vrolix, M.; Costa, J.R.; Chamie, D.; Abizaid, A.; Castro, J. TCT-330 Multi-Center Evaluation of a Novel 120 μm Novolimus-Eluting, Fully Bioresorbable Coronary Scaffold: First Report of 6-month Imaging and 12-Month Clinical Results. J. Am. Coll. Cardiol. 2017, 70, 135–136. [Google Scholar] [CrossRef]
- Nef, H.; Wiebe, J.; Boeder, N.; Dörr, O.; Bauer, T.; Hauptmann, K.E. A multicenter post-marketing evaluation of the Elixir DESolve((R)) Novolimus-eluting bioresorbable coronary scaffold system: First results from the DESolve PMCF study. Catheter. Cardiovasc. Interv. 2018, 92, 1021–1027. [Google Scholar] [CrossRef]
- de Ribamar Costa, J.; Abizaid, A.; Chamie, D.; Lansky, A.; Kochman, J.; Koltowski, L. Initial results of the fantom 1 trial: A first-in-man evaluation of a novel, radiopaque sirolimus-eluting bioresorbable vascular scaffold. J. Am. Coll. Cardiol. 2016, 67, 232. [Google Scholar] [CrossRef]
- Abizaid, A.; Carrié, D.; Frey, N.; Lutz, M.; Weber-Albers, J.; Dudek, D. 6-Month Clinical and Angiographic Outcomes of a Novel Radiopaque Sirolimus-Eluting Bioresorbable Vascular Scaffold: The FANTOM II Study. JACC Cardiovasc. Interv. 2017, 10, 1832–1838. [Google Scholar] [CrossRef]
- Kraak, R.P.; Grundeken, M.J.; Hassell, M.E.; Elias, J.; Koch, K.T.; Henriques, J.P. Two-year clinical outcomes of Absorb bioresorbable vascular scaffold implantation in complex coronary artery disease patients stratified by SYNTAX score and ABSORB II study enrolment criteria. EuroIntervention 2016, 12, e557–e565. [Google Scholar] [CrossRef]
- Puricel, S.; Arroyo, D.; Corpataux, N.; Baeriswyl, G.; Lehmann, S.; Kallinikou, Z. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J. Am. Coll. Cardiol. 2015, 65, 791–801. [Google Scholar] [CrossRef]
- Ellis, S.G. Fantom Bioresorbable Scaffold: Verse, But Not Yet Chorus (An Incomplete Composition). JACC Cardiovasc. Interv. 2017, 10, 1839–1840. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.G.; Gori, T.; Serruys, P.W.; Nef, H.; Steffenino, G.; Brugaletta, S. Clinical, Angiographic, and Procedural Correlates of Very Late Absorb Scaffold Thrombosis: Multistudy Registry Results. JACC Cardiovasc. Interv. 2018, 11, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, E.; Reeves, R.R. Bioresorbable Vascular Scaffolds: Back to the Drawing Board. JACC Cardiovasc. Interv. 2018, 11, 645–647. [Google Scholar] [CrossRef]
- Suwannasom, P.; Sotomi, Y.; Ishibashi, Y.; Cavalcante, R.; Albuquerque, F.N.; Macaya, C. The Impact of Post-Procedural Asymmetry, Expansion, and Eccentricity of Bioresorbable Everolimus-Eluting Scaffold and Metallic Everolimus-Eluting Stent on Clinical Outcomes in the ABSORB II Trial. JACC Cardiovasc. Interv. 2016, 9, 1231–1242. [Google Scholar] [CrossRef]
- Costa, J.R.; Abizaid, A. Bioresorbable Coronary Scaffolds: Deployment Tips and Tricks and the Future of the Technology. Methodist DeBakey Cardiovasc. J. 2018, 14, 42–49. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Bourantas, C.V.; Muramatsu, T.; Iqbal, J.; Farooq, V.; Diletti, R. Comparison of acute gain and late lumen loss after PCI with bioresorbable vascular scaffolds versus everolimus-eluting stents: An exploratory observational study prior to a randomised trial. EuroIntervention 2014, 10, 672–680. [Google Scholar] [CrossRef]
- Serruys, P.W.; Katagiri, Y.; Sotomi, Y.; Zeng, Y.; Chevalier, B.; van der Schaaf, R.J. Arterial Remodeling After Bioresorbable Scaffolds and Metallic Stents. J. Am. Coll. Cardiol. 2017, 70, 60–74. [Google Scholar] [CrossRef]
- Danzi, G.B.; Sesana, M.; Arieti, M.; Villa, G.; Rutigliano, S.; Aprile, A. Does optimal lesion preparation reduce the amount of acute recoil of the Absorbe BVS? Insights from a real-world population. Catheter. Cardiovasc. Interv. 2015, 86, 984–991. [Google Scholar] [CrossRef]
- Onuma, Y.; Sotomi, Y.; Shiomi, H.; Ozaki, Y.; Namiki, A.; Yasuda, S. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: Insights from the randomised ABSORB Japan trial. EuroIntervention 2016, 12, 1090–1101. [Google Scholar] [CrossRef]
- Kochman, J.; Tomaniak, M.; Jąkała, J.; Proniewska, K.; Legutko, J.; Roleder, T. First serial optical coherence tomography assessment at baseline, 12 and 24 months in STEMI patients treated with the second generation ABSORB bioresorbable vascular scaffold. EuroIntervention 2017. [Google Scholar] [CrossRef]
- Oberhauser, J.P.; Hossainy, S.; Rapoza, R.J. Design principles and performance of bioresorbable polymeric vascular scaffolds. EuroIntervention 2009, 5, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L.; Brugaletta, S.; Sabaté, M. Impact of PSP Technique on Clinical Outcomes Following Bioresorbable Scaffolds Implantation. J. Clin. Med. 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Imori, Y.; D’Ascenzo, F.; Gori, T.; Münzel, T.; Ugo, F.; Campo, G. Impact of postdilatation on performance of bioresorbable vascular scaffolds in patients with acute coronary syndrome compared with everolimus-eluting stents: A propensity score-matched analysis from a multicenter “real-world” registry. Cardiol. J. 2016, 23, 374–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamaji, K.; Brugaletta, S.; Sabaté, M.; Iñiguez, A.; Jensen, L.O.; Cequier, A. Effect of Post-Dilatation Following Primary PCI With Everolimus-Eluting Bioresorbable Scaffold Versus Everolimus-Eluting Metallic Stent Implantation: An Angiographic and Optical Coherence Tomography TROFI II Substudy. JACC Cardiovasc. Interv. 2017, 10, 1867–1877. [Google Scholar] [CrossRef]
- Stone, G.W.; Abizaid, A.; Onuma, Y.; Seth, A.; Gao, R.; Ormiston, J. Effect of Technique on Outcomes Following Bioresorbable Vascular Scaffold Implantation: Analysis from the ABSORB Trials. J. Am. Coll. Cardiol. 2017, 70, 2863–2874. [Google Scholar] [CrossRef]
- Polimeni, A.; Anadol, R.; Münzel, T.; Geyer, M.; De Rosa, S.; Indolfi, C.; Gori, T. Bioresorbable vascular scaffolds for percutaneous treatment of chronic total coronary occlusions: A meta-analysis. BMC Cardiovasc. Disord. 2019, 19, 59. [Google Scholar] [CrossRef]
- Abizaid, A.; Ribamar Costa, J., Jr. Bioresorbable Scaffolds for Coronary Stenosis: When and How Based Upon Current Studies. Curr. Cardiol. Rep. 2017, 19, 27. [Google Scholar] [CrossRef]
- Geraci, S.; Kawamoto, H.; Caramanno, G.; Ruparelia, N.; Capodanno, D.; Brugaletta, S. Bioresorbable Everolimus-Eluting Vascular Scaffold for Long Coronary Lesions: A Subanalysis of the International, Multicenter GHOST-EU Registry. JACC Cardiovasc. Interv. 2017, 10, 560–568. [Google Scholar] [CrossRef]
- Gori, T.; Wiebe, J.; Capodanno, D.; Latib, A.; Lesiak, M.; Pyxaras, S.A. Early and midterm outcomes of bioresorbable vascular scaffolds for ostial coronary lesions: Insights from the GHOST-EU registry. EuroIntervention 2016, 12, e550–e556. [Google Scholar] [CrossRef]
- Cassese, S.; Byrne, R.A.; Ndrepepa, G.; Kufner, S.; Wiebe, J. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: A meta-analysis of randomised controlled trials. Lancet 2016, 387, 537–544. [Google Scholar] [CrossRef]
- Stone, G.W.; Gao, R.; Kimura, T.; Kereiakes, D.J.; Ellis, S.G.; Onuma, Y. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: A patient-level, pooled meta-analysis. Lancet 2016, 387, 1277–1289. [Google Scholar] [CrossRef]
- MiYaZaKi, Y.; Zeng, Y.; TUMMala, K.; STaneTiC, B.; TiJSSen, J. Early, late and very late incidence of bioresorbable scaffold thrombosis: A systematic review and meta-analysis of randomized clinical trials and observational studies. Minerva Cardioangiol. 2017, 65, 32–51. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Escarcega, R.O.; Baker, N.C.; Benn, H.A.; Gaglia, M.A.; Torguson, R.; Waksman, R. Scaffold Thrombosis After Percutaneous Coronary Intervention with ABSORB Bioresorbable Vascular Scaffold: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Interv. 2016, 9, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Gogas, B.D.; Jeon, K.H.; Park, J.S.; Lee, W.; Yoon, C.H. Long-term safety of bioresorbable scaffolds: Insights from a network meta-analysis including 91 trials. EuroIntervention 2018, 13, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Capranzano, P.; Longo, G.; Tamburino, C.I.; Gargiulo, G.; Ohno, Y. One-year outcomes after Absorb bioresorbable vascular scaffold implantation in routine clinical practice. EuroIntervention 2016, 12, e152–e159. [Google Scholar] [CrossRef]
- Capodanno, D.; Gori, T.; Nef, H.; Latib, A.; Mehilli, J.; Lesiak, M. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: Early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015, 10, 1144–1153. [Google Scholar] [CrossRef]
- Hoppmann, P.; Kufner, S.; Cassese, S.; Wiebe, J.; Schneider, S.; Pinieck, S.; Laugwitz, K.L. Angiographic and clinical outcomes of patients treated with everolimus-eluting bioresorbable stents in routine clinical practice: Results of the ISAR-ABSORB registry. Catheter. Cardiovasc. Interv. 2016, 87, 822–829. [Google Scholar] [CrossRef]
- Tröbs, M.; Achenbach, S.; Röther, J.; Klinghammer, L.; Schlundt, C. Bioresorbable vascular scaffold thrombosis in a consecutive cohort of 550 patients. Catheter. Cardiovasc. Interv. 2016, 88, 872–880. [Google Scholar] [CrossRef]
- Gori, T.; Schulz, E.; Hink, U.; Kress, M.; Weiers, N.; Weissner, M.; Münzel, T. Clinical, Angiographic, Functional, and Imaging Outcomes 12 Months After Implantation of Drug-Eluting Bioresorbable Vascular Scaffolds in Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2015, 8, 770–777. [Google Scholar] [CrossRef][Green Version]
- Kraak, R.P.; Hassell, M.E.; Grundeken, M.J.; Koch, K.T.; Henriques, J.P.; Piek, J.J. Initial experience and clinical evaluation of the Absorb bioresorbable vascular scaffold (BVS) in real-world practice: The AMC Single Centre Real World PCI Registry. EuroIntervention 2015, 10, 1160–1168. [Google Scholar] [CrossRef][Green Version]
- Widimsky, P.; Petr, R.; Tousek, P.; Maly, M.; Linkova, H.; Vrana, J.; Kocka, V. One-Year Clinical and Computed Tomography Angiographic Outcomes After Bioresorbable Vascular Scaffold Implantation During Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction: The PRAGUE-19 Study. Circ. Cardiovasc. Interv. 2015, 8, e002933. [Google Scholar] [CrossRef] [PubMed]
- Hoye, A.; van Domburg, R.T.; Sonnenschein, K.; Serruys, P.W. Percutaneous coronary intervention for chronic total occlusion of the coronary artery with the implantation of bioresorbable everolimus-eluting scaffolds. Poznan CTO-Absorb Pilot Registry. EuroIntervention 2016, 12, e144–e151. [Google Scholar] [CrossRef]
- Robaei, D.; Back, L.; Ooi, S.Y.; Pitney, M.; Jepson, N. Twelve-Month Outcomes with a Bioresorbable Everolimus-Eluting Scaffold: Results of the ESHC-BVS Registry at Two Australian Centers. J. Invasive Cardiol. 2016, 28, 316–322. [Google Scholar] [PubMed]
- Tamburino, C.; Latib, A.; Sabate, M.; Mehilli, J.; Gori, T.; Achenbach, S. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: A European perspective. EuroIntervention 2015, 11, 45–52. [Google Scholar] [CrossRef]
- Tenekecioglu, E.; Poon, E.K.; Collet, C.; Thondapu, V.; Torii, R.; Bourantas, C.V. The Nidus for Possible Thrombus Formation: Insight from the Microenvironment of Bioresorbable Vascular Scaffold. JACC Cardiovasc. Interv. 2016, 9, 2167–2168. [Google Scholar] [CrossRef]
- Mattesini, A.; Secco, G.G.; Dall’Ara, G.; Ghione, M.; Rama-Merchan, J.C.; Lupi, A. ABSORB biodegradable stents versus second-generation metal stents: A comparison study of 100 complex lesions treated under OCT guidance. JACC Cardiovasc. Interv. 2014, 7, 741–750. [Google Scholar] [CrossRef]
- Brugaletta, S.; Gori, T.; Low, A.F.; Tousek, P.; Pinar, E.; Gomez-Lara, J. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: The BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc. Interv. 2015, 8, 189–197. [Google Scholar] [CrossRef]
- Wöhrle, J.; Naber, C.; Schmitz, T.; Schwencke, C.; Frey, N.; Butter, C.; Mathey, D.G. Beyond the early stages: Insights from the ASSURE registry on bioresorbable vascular scaffolds. EuroIntervention 2015, 11, 149–156. [Google Scholar] [CrossRef]
- Sabaté, M.; Windecker, S.; Iñiguez, A.; Okkels-Jensen, L.; Cequier, A.; Brugaletta, S.; Pilgrim, T. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: Results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur. Heart J. 2016, 37, 229–240. [Google Scholar] [CrossRef]
- Christ, G.; Hafner, T.; Siller-Matula, J.M.; Francesconi, M.; Grohs, K.; Wilhelm, E.; Podczeck-Schweighofer, A. Platelet inhibition by abciximab bolus-only administration and oral ADP receptor antagonist loading in acute coronary syndrome patients: The blocking and bridging strategy. Thromb. Res. 2013, 132, e36–e41. [Google Scholar] [CrossRef]
- Vaquerizo, B.; Barros, A.; Pujadas, S.; Bajo, E.; Jiménez, M.; Gomez-Lara, J.; Serra, A. One-Year Results of Bioresorbable Vascular Scaffolds for Coronary Chronic Total Occlusions. Am. J. Cardiol. 2016, 117, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, S.; Pan, M.; Romero, M.; de Lezo, J.S.; Mazuelos, F.; Segura, J.; Medina, A. Outcomes and computed tomography scan follow-up of bioresorbable vascular scaffold for the percutaneous treatment of chronic total coronary artery occlusion. Am. J. Cardiol. 2015, 115, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Capranzano, P.; Capodanno, D.; Brugaletta, S.; Latib, A.; Mehilli, J.; Nef, H.; Mattesini, A. Clinical outcomes of patients with diabetes mellitus treated with Absorb bioresorbable vascular scaffolds: A subanalysis of the European Multicentre GHOST-EU Registry. Catheter. Cardiovasc. Interv. 2018, 91, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, T.; Kirtane, A.J.; Serruys, P.W.; Smits, P.C.; Kedhi, E.; Kereiakes, D.; De Waha, A. Stent thrombosis with everolimus-eluting stents: Meta-analysis of comparative randomized controlled trials. Circ. Cardiovasc. Interv. 2012, 5, 357–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Authors/Task Force members; Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Jüni, P. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Serruys, P.W.; Silber, S.; Kelbaek, H.; Richardt, G.; Morel, M.A.; Windecker, S. Comparison of zotarolimus- and everolimus-eluting coronary stents: Final 5-year report of the RESOLUTE all-comers trial. Circ. Cardiovasc. Interv. 2015, 8, e002230. [Google Scholar] [CrossRef]
- Fallesen, C.O.; Antonsen, L.; Thayssen, P.; Jensen, L.O.; Lee, P.H.; Lee, S.W.; Brugaletta, S. How should I treat a bioresorbable vascular scaffold edge restenosis and intra-scaffold dissection? EuroIntervention 2018, 13, 1730–1734. [Google Scholar] [CrossRef]
- Colombo, A.; Azzalini, L. Bioresorbable scaffolds: Reflections after a setback—Losing a battle does not mean losing the war! EuroIntervention 2017, 13, 785–786. [Google Scholar] [CrossRef]
- Stettler, C.; Wandel, S.; Allemann, S.; Kastrati, A.; Morice, M.C.; Schömig, A.; Goy, J.J. Outcomes associated with drug-eluting and bare-metal stents: A collaborative network meta-analysis. Lancet 2007, 370, 937–948. [Google Scholar] [CrossRef]
- FinnAV, J.; NakazawaG, K.; NewellJ, J. Pathological correlates of late drug-eluting stent thrombosis: Strut coverage as a marker of endothelialization. Circulation 2007, 115, 2435–2441. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakazawa, G.; Joner, M.; Kolodgie, F.D.; Mont, E.K.; Gold, H.K.; Virmani, R. Vascular responses to drug eluting stents: Importance of delayed healing. Arter. Thromb. Vasc. Biol. 2007, 27, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Virmani, R. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 2006, 48, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e011245. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; White, C.J. Paclitaxel-Coated Balloons and Eluting Stents: Is There a Mortality Risk in Patients with Peripheral Artery Disease? Circulation 2019. [Google Scholar] [CrossRef]
- Ali, Z.A.; Karimi Galougahi, K.; Shlofmitz, R.; Maehara, A.; Mintz, G.S.; Abizaid, A.; Stone, G.W. Imaging-guided pre-dilatation, stenting, post-dilatation: A protocolized approach highlighting the importance of intravascular imaging for implantation of bioresorbable scaffolds. Expert Rev. Cardiovasc. Ther. 2018, 16, 431–440. [Google Scholar] [CrossRef]
- Ortega-Paz, L.; Capodanno, D.; Gori, T.; Nef, H.; Latib, A.; Caramanno, G.; Wiebe, J. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events: Development and internal validation of the PSP score. EuroIntervention 2017, 12, 2110–2117. [Google Scholar] [CrossRef]
- Markovic, S.; Kugler, C.; Rottbauer, W.; Wöhrle, J. Long-term clinical results of bioresorbable absorb scaffolds using the PSP-technique in patients with and without diabetes. J. Interv. Cardiol. 2017, 30, 325–330. [Google Scholar] [CrossRef]
- Heeger, C.H.; Schedifka, A.S.; Meincke, F.; Spangenberg, T.; Wienemann, H.; Kreidel, F. Optical coherence tomography-guided versus angiography-guided implantation of everolimus-eluting bioresorbable vascular scaffolds: Comparison of coverage, apposition and clinical outcome. The ALSTER-OCT ABSORB registry. Cardiol. J. 2018. [Google Scholar] [CrossRef]
- von Zur Mühlen, C.; Reiss, S.; Krafft, A.J.; Besch, L.; Menza, M.; Zehender, M.; Reinöhl, J. Coronary magnetic resonance imaging after routine implantation of bioresorbable vascular scaffolds allows non-invasive evaluation of vascular patency. PLoS ONE 2018, 13, e0191413. [Google Scholar] [CrossRef]
- Capodanno, D. Bioresorbable Scaffolds in Coronary Intervention: Unmet Needs and Evolution. Korean Circ. J. 2018, 48, 24–35. [Google Scholar] [CrossRef]
- Ferdous, J.; Kolachalama, V.B.; Kolandaivelu, K.; Shazly, T. Degree of bioresorbable vascular scaffold expansion modulates loss of essential function. Acta Biomater. 2015, 26, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Mattesini, A.; Boeder, N.; Valente, S.; Löblich, K.; Dörr, O.; Secco, G.G. Absorb vs. DESolve: An optical coherence tomography comparison of acute mechanical performances. EuroIntervention 2016, 12, e566–e573. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Neumann, F.J. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet 2013, 382, 614–623. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Trenk, D.; Schrör, K.; Gawaz, M.; Kristensen, S.D.; Storey, R.F.; Huber, K. Response variability to P2Y12 receptor inhibitors: Expectations and reality. JACC Cardiovasc. Interv. 2013, 6, 1111–1128. [Google Scholar] [CrossRef]
- Mangiacapra, F.; Patti, G.; Barbato, E.; Peace, A.J.; Ricottini, E.; Vizzi, V. A therapeutic window for platelet reactivity for patients undergoing elective percutaneous coronary intervention: Results of the ARMYDA-PROVE (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity for Outcome Validation Effort) study. JACC Cardiovasc. Interv. 2012, 5, 281–289. [Google Scholar] [CrossRef]
- Byrne, R.A.; Stefanini, G.G.; Capodanno, D.; Onuma, Y.; Baumbach, A.; Escaned, J. Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: Executive summary. Eur. Heart J. 2018, 39, 1591–1601. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bonello, L.; Aradi, D.; Price, M.J.; Jeong, Y.H.; Angiolillo, D.J. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J. Am. Coll. Cardiol. 2013, 62, 2261–2273. [Google Scholar] [CrossRef]
- Christ, G.; Siller-Matula, J.M.; Francesconi, M.; Dechant, C.; Grohs, K.; Podczeck-Schweighofer, A. Individualising dual antiplatelet therapy after percutaneous coronary intervention: The IDEAL-PCI registry. BMJ Open 2014, 4, e005781. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Gruber, C.; Francesconi, M.; Dechant, C.; Jilma, B.; Delle-Karth, G. The net clinical benefit of personalized antiplatelet therapy in patients undergoing percutaneous coronary intervention. Clin. Sci. 2015, 128, 121–130. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Francesconi, M.; Dechant, C.; Jilma, B.; Maurer, G. Personalized antiplatelet treatment after percutaneous coronary intervention: The MADONNA study. Int. J. Cardiol. 2013, 167, 2018–2023. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Hintermeier, A.; Kastner, J.; Kreiner, G.; Maurer, G.; Kratochwil, C. Distribution of clinical events across platelet aggregation values in all-comers treated with prasugrel and ticagrelor. Vascul. Pharmacol. 2016, 79, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.M.; Jilma, B. Why have studies of tailored anti-platelet therapy failed so far? Thromb. Haemost. 2013, 110, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Kubisa, M.J.; Jezewski, M.P.; Gasecka, A.; Siller-Matula, J.M.; Postuła, M. Ticagrelor—Toward more efficient platelet inhibition and beyond. Ther. Clin. Risk Manag. 2018, 14, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Brugaletta, S.; Gomez-Lara, J.; Caballero, J.; Ortega-Paz, L.; Teruel, L. TIcaGrEloR and Absorb bioresorbable vascular scaffold implantation for recovery of vascular function after successful chronic total occlusion recanalization (TIGER-BVS trial): Rationale and study design. Catheter. Cardiovasc. Interv. 2018, 91, 1–6. [Google Scholar] [CrossRef]

| Device | Material | Year of Receiving CE Marking | Drug Eluted | Strut Thickness (µm) | Minimal Resolution Time (months) |
|---|---|---|---|---|---|
| First generation | |||||
| ABSORB | PLLA | 2012 | everolimus | 156 | >36 |
| DESolve | PLLA | 2014 | novolimus | 150 | <24 |
| DESolve Cx plus | PLLA | 2017 | novolimus | 120 | <24 |
| DREAMS 1G | Magnesium alloy | 2015 | paclitaxel | 120 | 9–12 |
| Second generation | |||||
| Magmaris (DREAMS 2G) | Magnesium alloy | 2016 | sirolimus | 120 | 9–12 |
| Fantom | Tyrosine polycarbonate | 2017 | sirolimus | 125 | 36 |
| ART | PDLLA | 2015 | none | 170 | 6 |
| Study/Publication Date | Study Type | Follow-Up Time | No of Patients | No of Devices per Patient | Length of Devices (mm) | TLF (%) | ScT Definite/Probable (%) | MI (%) | TLR (%) | Cardiac Death (%) | Commercial Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABSORB (Abbott, Lake County, IL, USA) | |||||||||||
| ABSORB Cohort A [31]/Mar 2008 | Observational | 5 years | 30 | 1 | 12 or 18 | 3.4 | 0/0 | 3.4 | 10.3 | 0 | Abbott Vascular |
| ABSORB Japan [71]/Dec 2015 | Randomized | 1 year | 400 | 1–2 | 8, 12 or 18 | 4.2 | 1.5/1.5 | 3.4 | 2.6 | 0 | Abbott Vascular |
| ABSORB Cohort B [72]/Feb 2016 | Observational | 5 years | 101 | 1 | 18 | 14.0 | 0/0 | 3.0 | 11.0 | 0 | Abbott Vascular |
| PRAGUE-19 [73]/May 2016 | Observational | 3 years | 113 | 1 | <24 | 11.5 | 1.8/0.9 | 1.8 | 3.5 | 1.8 | Abbott Vascular |
| ABSORB II [17]/Nov 2016 | Randomized | 3 years | 335 | 1–2 | <48 | 10 | 3.0/3.0 | 8.0 | 7.0 | 1.0 | Abbott Vascular |
| ABSORB China [74]/Oct 2017 | Randomized | 3 years | 238 | 1–2 | <24 | 6.8 | 0.4/0.4 | 3.4 | 4.7 | 0.4 | Abbott Vascular |
| ABSORB III [67]/Oct 2017 | Randomized | 2 years | 1322 | 1–2 | <24 | 3.7 | 1.9 | 1.3 | 2.6 | 0.5 | Abbott Vascular |
| ABSORB III [66]/Dec 2017 | Randomized | 3 years | 1322 | 1–2 | <24 | 13.4 | 2.3 | 4.2 | 7.3 | 0.9 | Abbott Vascular |
| ABSORB IV [68]/Oct 2018 | Randomized | 1 year | 1296 | 1–3 | >24 | 5 | 1 | 5 | 2 | 0 | Abbott Vascular |
| AIDA [19]/Jun 2017 | Randomized | 2 years | 924 | 1–2 | N/A | 11.7 | 3.1/0.4 | 7.1 | 7 | 2 | Abbott Vascular |
| EVERBIO II [69]/Sep 2017 | Randomized | 2 years | 78 | N/A | N/A | 21 | 1.2 | 3 | 23 | 1.2 | Abbott Vascular, Biosensors International, Boston Scientific |
| TROFI II [70]/Nov 2018 | Randomized | 3 years | 95 | 1 | 8, 12, 18 or 28 | 5.3 | 2.1 | 3.2 | 4.2 | 2.1 | Abbott Vascular, Terumo |
| ISAR- ABSORB MI [75]/Dec 2018 | Randomized | 1 year | 173 | 1 | 1 | 7.0 | 1.2/0.6 | 0.6 | 4.8 | 2.3 | Abbott Vascular |
| DESolve NX (Elixir Medical Corporation, Milpitas, CA, USA) | |||||||||||
| DESolve First-in-Man trial [59]/Jan 2014 | Observational | 1 year | 15 | 1–2 | 14 or 18 | 6.7 | 0.8 | 6.7 | 6.7 | 6.7 | Elixir Medical |
| DESolve 2 years [56]/Mar 2016 | Observational | 2 years | 122 | 1 | 14 or 18 | 7.4 | 0.8 | 1.6 | 4.0 | 3.2 | Elixir Medical |
| DESolve Cx [76]/Oct 2017 | Observational | 6 months | 50 | 1 | 14, 18, 13 or 28 | 0 | 0 | 0 | 0 | 0 | Elixir Medical |
| DESolve PCMF Study [77]/Nov 2018 | Observational | 12 months | 102 | 1–2 | 14, 18 or 28 | 2,0 | 1.0/0 | 1,0 | 1,0 | 0 | Elixir Medical |
| DREAMS (Biotronik, Berlin, Germany) | |||||||||||
| BIOSOLVE-I [23]/Jun 2016 | Observational | 3 years | 46 | 1 | 16 | 6.6 | 0 | 2.2 | 4.3 | 0 | Biotronik AG |
| BIOSOLVE-II [22,24]/Sep 2016 | Observational | 2 years | 118 | 1–2 | ≤21 | 5.9 | 0 | 0.9 | 3.4 | 1.7 | Biotronik AG |
| BIOSOLVE-II and BIOSOLVE-III [24]/Jul 2017 | Observational | 6 months | 184 | 1–2 | ≤21 | 3.3 | 0 | 0.6 | 1.7 | 1.1 | Biotronik AG |
| Fantom (REVA Medical Inc., San Diego, CA, USA) | |||||||||||
| Fantom I [78]/Apr 2016 | Observational | 4 months | 7 | 1 | 18 | 0 | N/A | ||||
| Fantom II [79]/Sep 2017 | Observational | 6 months | 117 | 1 | 18 or 24 | 2.6 | 0.9 | 1.7 | 1.7 | 0 | REVA Medical |
| Study | Compared Devices (No of Patients in Groups) | TVF RR/HR (95% CI) | Ischemia Driven TLR RR/HR (95% CI) | Cardiac Death RR/HR (95% CI) | TVMI RR/HR (95% CI) | Device Thrombosis Probable/Definitive RR/HR (95% CI) |
|---|---|---|---|---|---|---|
| ABSORB Japan [71] | ABSORB BVS vs. Xience DES (266/134) | 1.15 [0.48, 2.72] p = 0.75 | 1.17 [0.31, 4.46] p = 1.00 | N/A | 1.51 [0.41, 5.47] p = 0.76 | 1.02 [0.19, 5.47] p = 1.00 |
| ABSORB II [17] | ABSORB BVS vs. Xience DES (335/166) | 2.11 [1.00, 4.44] p = 0.0425 | 1.65 [0.46, 5.92] p = 0.56 | 0.50 [0.10, 2.43] p = 0.56 | 5.70 [1.36, 23.87] p = 0.0061 | N/A p = 0.0331 |
| ABSORB China [74] | ABSORB BVS vs. Xience DES (236/235) | 1.00 [0.51, 1.94] p = 0.99 | 1.66 [0.61, 4.49] p = 0.31 | 0.33 [0.03, 3.17] p = 0.37 | 2.99 [0.61, 14.65] p = 0.28 | N/A P = 0.50 |
| ABSORB III [66] | ABSORB BVS vs. Xience DES (1322/686) | 1.41 [1.10, 1.81] p = 0.006 | 1.23 [0.85, 1.79] p = 0.27 | 1.17 [0.51, 2.69] p = 0.71 | 1.47 [1.02, 2.11] p = 0.03 | 3.12 [1.21, 8.05] p = 0.01 |
| ABSORB IV [68] | ABSORB BVS vs. Xience DES (1296/1308) | 1.35 [0.93, 1.97] p = 0.11 | 2.28 [0.99, 5.25] p = 0.0457 | N/A | 1.23 [0.84, 1.81] p = 0.29 | 4.05 [0.86, 19.06] p = 0.06 |
| AIDA [19] | ABSORB BVS vs. Xience DES (924/921) | 1.12 [0.85, 1.48] p = 0.43 | 1.17 [0.86, 1.58] p = 0.31 | 0.78 [0.42, 1.44] p = 0.43 | 1.60 [1.01, 2.53] p = 0.04 | 3.87 [1.78, 8.42] P < 0.001 |
| EVERBIO II [81] | ABSORB BVS vs. Promus Element and Biomatrix Flex DES (78/160) | p = 0.12 | p = 0.23 | p = 0.55 | p = 0.11 | N/A |
| TROFI II [70] | ABSORB BVS vs. Xience DES (95/96) | p = 0.465 | p = 0.678 | N/A | p = 0.327 | p = 0.55 |
| ISAR-Absorb II [75] | ABSORB BVS vs. EES (173/89) | 1.04 [0.39, 2.78] | 0.84 [0.27, 2.57] | 1.02 [0.19, 5.58] | 0.51 [0.03, 8.20] | 0.51 [0.07, 3.62] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeżewski, M.P.; Kubisa, M.J.; Eyileten, C.; De Rosa, S.; Christ, G.; Lesiak, M.; Indolfi, C.; Toma, A.; Siller-Matula, J.M.; Postuła, M. Bioresorbable Vascular Scaffolds—Dead End or Still a Rough Diamond? J. Clin. Med. 2019, 8, 2167. https://doi.org/10.3390/jcm8122167
Jeżewski MP, Kubisa MJ, Eyileten C, De Rosa S, Christ G, Lesiak M, Indolfi C, Toma A, Siller-Matula JM, Postuła M. Bioresorbable Vascular Scaffolds—Dead End or Still a Rough Diamond? Journal of Clinical Medicine. 2019; 8(12):2167. https://doi.org/10.3390/jcm8122167
Chicago/Turabian StyleJeżewski, Mateusz P., Michał J. Kubisa, Ceren Eyileten, Salvatore De Rosa, Günter Christ, Maciej Lesiak, Ciro Indolfi, Aurel Toma, Jolanta M. Siller-Matula, and Marek Postuła. 2019. "Bioresorbable Vascular Scaffolds—Dead End or Still a Rough Diamond?" Journal of Clinical Medicine 8, no. 12: 2167. https://doi.org/10.3390/jcm8122167
APA StyleJeżewski, M. P., Kubisa, M. J., Eyileten, C., De Rosa, S., Christ, G., Lesiak, M., Indolfi, C., Toma, A., Siller-Matula, J. M., & Postuła, M. (2019). Bioresorbable Vascular Scaffolds—Dead End or Still a Rough Diamond? Journal of Clinical Medicine, 8(12), 2167. https://doi.org/10.3390/jcm8122167






