Triple Network Resting State Connectivity Predicts Distress Tolerance and Is Associated with Cocaine Use
Abstract
1. Introduction
2. Method
2.1. Participants
2.2. General Procedure
2.3. Measures
2.3.1. Demographic Information and Sample Characteristics
2.3.2. Distress Tolerance
2.4. fMRI Data Acquisition and Analysis
2.4.1. Image Acquisition
2.4.2. Preprocessing
2.4.3. Resting State Functional Connectivity (rsFC)
2.4.4. Within Network Connectivity
2.4.5. Between Network Connectivity
2.5. Data Analysis
2.5.1. Primary Aim: rsFC, Group, and DT
2.5.2. Exploratory Aims
3. Results
3.1. Group Differences in Participant Characteristics and Resting State Functional Connectivity
3.2. Distress Tolerance Task Effects and Identification of Covariates
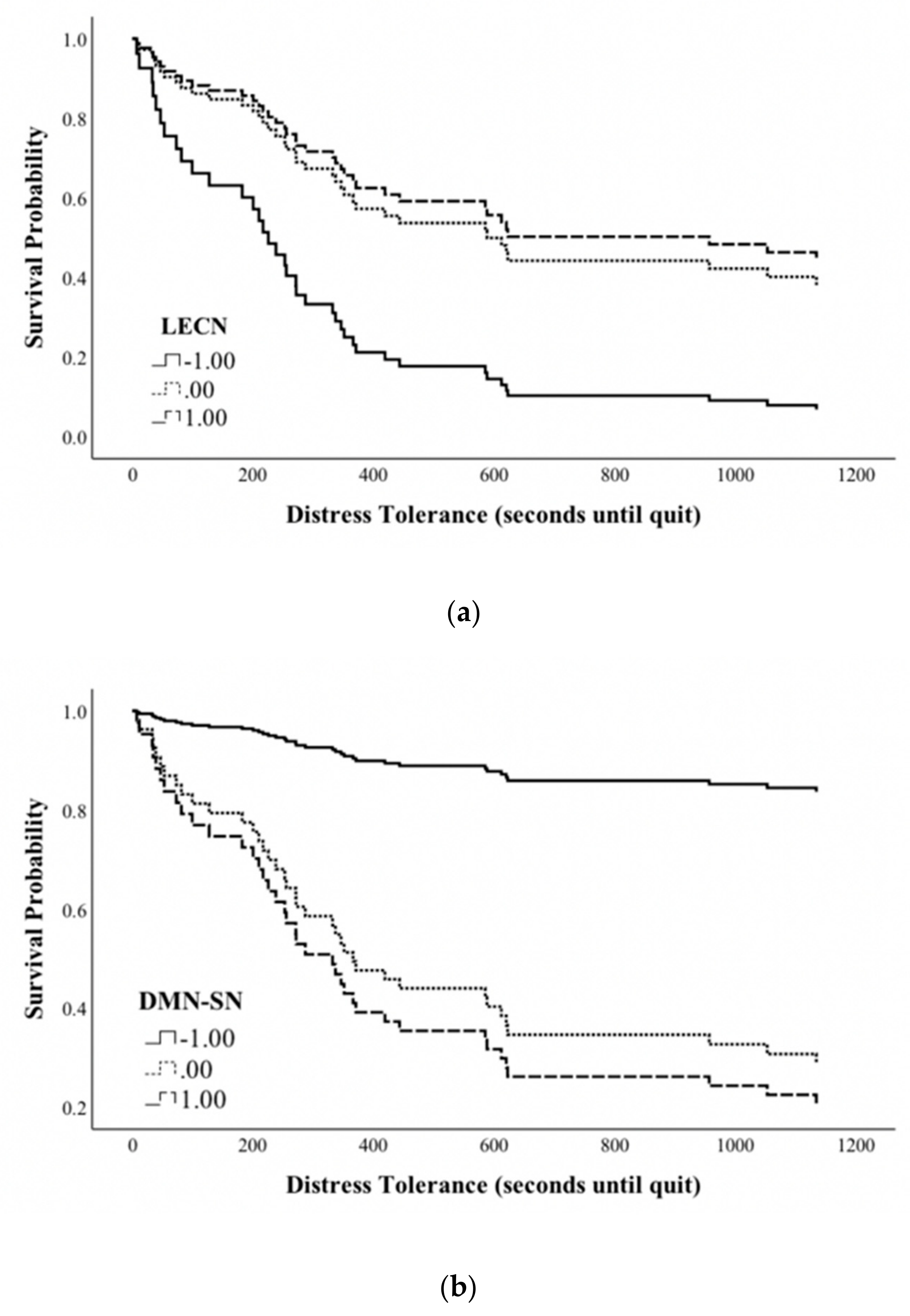
3.3. Within Network rsFC and DT
3.4. Between Network rSFC and Distress Tolerance
3.5. Unique Contribution of Within and between Network rsFC on DT
3.6. Cocaine Use Severity, rsFC, and Distress Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quinn, E.P.; Brandon, T.H.; Copeland, A.L. Is task persistence related to smoking and substance abuse? The application of learned industriousness theory to addictive behaviors. Exp. Clin. Psychopharmacol. 1996, 4, 186–190. [Google Scholar] [CrossRef]
- Brandon, T.H.; Herzog, T.A.; Juliano, L.M.; Irvin, J.E.; Lazev, A.B.; Simmons, V.N. Pretreatment task persistence predicts smoking cessation outcome. J. Abnorm. Psychol. 2003, 112, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Daughters, S.B.; Lejuez, C.W.; Bornovalova, M.A.; Kahler, C.W.; Strong, D.R.; Brown, R.A. Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. J. Abnorm. Psychol. 2005, 114, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Tull, M.T.; Gratz, K.L.; Coffey, S.F.; Weiss, N.H.; McDermott, M.J. Examining the interactive effect of posttraumatic stress disorder, distress tolerance, and gender on residential substance use disorder treatment retention. J. Soc. Psychol. Addict. Behav. 2013, 27, 763–773. [Google Scholar] [CrossRef]
- Brown, R.A.; Lejuez, C.W.; Kahler, C.W.; Strong, D.R. Distress tolerance and duration of past smoking cessation attempts. J. Abnorm. Psychol. 2002, 111, 180–185. [Google Scholar] [CrossRef]
- Cameron, A.J.; Reed, K.M.P.; Ninnemann, A. Reactivity to negative affect in smokers: The role of implicit associations and distress tolerance in smoking cessation. Addict. Behav. 2013, 38, 2905–2912. [Google Scholar] [CrossRef]
- Daughters, S.B.; Lejuez, C.W.; Kahler, C.W.; Strong, D.R.; Brown, R.A. Psychological distress tolerance and duration of most recent abstinence attempt among residential treatment-seeking substance abusers. Psychol. Addict. Behav. 2005, 19, 208–211. [Google Scholar] [CrossRef]
- Strong, D.R.; Brown, R.A.; Sims, M.; Herman, D.S.; Anderson, B.J.; Stein, M.D. Persistence on a stress-challenge task before initiating buprenorphine treatment was associated with successful transition from opioid use to early abstinence. J. Addict. Med. 2012, 6, 219–225. [Google Scholar] [CrossRef]
- Reese, E.D.; Conway, C.C.; Anand, D.; Bauer, D.J.; Daughters, S.B. Distress tolerance trajectories following substance use treatment. J. Consult. Clin. Psychol. 2019, 87, 645–656. [Google Scholar] [CrossRef]
- Baker, T.B.; Piper, M.E.; McCarthy, D.E.; Majeskie, M.R.; Fiore, M.C. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol. Rev. 2004, 111, 33–51. [Google Scholar] [CrossRef]
- Wise, R.A.; Koob, G.F. The development and maintenance of drug addiction. Neuropsychopharmacology 2014, 39, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Fedota, J.R.; Stein, E.A. Resting-state functional connectivity and nicotine addiction: Prospects for biomarker development. Ann. N. Y. Acad. Sci. 2015, 1349, 64–82. [Google Scholar] [CrossRef]
- Sutherland, M.T.; McHugh, M.J.; Pariyadath, V.; Stein, E.A. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage 2012, 62, 2281–2295. [Google Scholar] [CrossRef]
- Sutherland, M.T.; Stein, E.A. Functional neurocircuits and neuroimaging biomarkers of tobacco use disorder. Trends Mol. Med. 2018, 24, 129–143. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Hu, Y.; Gu, H.; Salmeron, B.J.; Adinoff, B.; Stein, E.A.; Yang, Y. Salience and default mode network dysregulation in chronic cocaine users predict treatment outcome. Brain 2017, 140, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Weiland, B.J.; Sabbineni, A.; Calhoun, V.D.; Welsh, R.C.; Bryan, A.D.; Jung, R.E.; Mayer, A.R.; Hutchison, K.E. Reduced Left Executive Control Network Functional Connectivity Is Associated with Alcohol Use Disorders. Alcohol. Clin. Exp. Res. 2014, 38, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cortes, C.R.; Mathur, K.; Tomasi, D.; Momenan, R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: A resting-state study. Addict. Biol. 2017, 22, 206–217. [Google Scholar] [CrossRef]
- Camchong, J.; Stenger, V.A.; Fein, G. Resting state synchrony in long-term abstinent alcoholics with versus without comorbid drug dependence. Drug Alcohol Depend. 2013, 131, 56–65. [Google Scholar] [CrossRef]
- McHugh, M.J.; Gu, H.; Yang, Y.; Adinoff, B.; Stein, E.A. Executive control network connectivity strength protects against relapse to cocaine use. Addict. Biol. 2017, 22, 1790–1801. [Google Scholar] [CrossRef] [PubMed]
- Lerman, C.; Gu, H.; Loughead, J.; Ruparel, K.; Yang, Y.; Stein, E.A. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 2014, 71, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, J.; Wang, W.; Wang, Y.; Li, W.; Chen, J.; Zhu, J.; Yan, X.; Li, Y.; Li, Z.; et al. Disrupted coupling of large-scale networks is associated with relapse behaviour in heroin-dependent men. J. Psychiatry Neurosci. 2018, 43, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Daughters, S.B.; Ross, T.J.; Bell, R.P.; Yi, J.Y.; Ryan, J.; Stein, E.A. Distress tolerance among substance users is associated with functional connectivity between prefrontal regions during a distress tolerance task. Addict. Biol. 2017, 22, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Gibbon, M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In Comprehensive Handbook of Psychological Assessment, Volume 2: Personality Assessment; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2004; pp. 134–143. ISBN 978-0-471-41612-8. [Google Scholar]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerstrom, K.-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Wechsler, D. Psychological Corporation WASI-II Wechsler Abbreviated Scale of Intelligence, 2nd ed.; The Psychological Corporation: San Antonio, TX, USA, 2011. [Google Scholar]
- Sobell, L.C.; Sobell, M.B. Timeline Follow-Back. In Measuring Alcohol Consumption: Psychosocial and Biochemical Methods; Litten, R.Z., Allen, J.P., Eds.; Humana Press: Totowa, NJ, USA, 1992; pp. 41–72. ISBN 978-1-4612-0357-5. [Google Scholar]
- Strong, D.R.; Lejuez, C.W.; Daughters, S.; Marinello, M.; Kahler, C.W.; Brown, R.A. The Computerized Mirror Tracing Task, 1st ed. Unpublished work. 2003.
- Deichmann, R.; Gottfried, J.A.; Hutton, C.; Turner, R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage 2003, 19, 430–441. [Google Scholar] [CrossRef]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Shirer, W.R.; Ryali, S.; Rykhlevskaia, E.; Menon, V.; Greicius, M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 2012, 22, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R.; Sinha, R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal–limbic dysfunction in psycho-stimulant addiction. Neurosci. Biobehav. Rev. 2008, 32, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, C.A.; Canterberry, M. The use of brain imaging to elucidate neural circuit changes in cocaine addiction. Subst. Abuse Rehabil. 2012, 3, 115–128. [Google Scholar] [CrossRef][Green Version]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Garrison, K.A.; Potenza, M.N. Neuroimaging and biomarkers in addiction treatment. Curr. Psychiatry Rep. 2014, 16, 513. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Chen, M.C.; Gotlib, I.H. Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiol. Dis. 2013, 52, 4–11. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Tomasi, D.; Rajaram, S.; Cottone, L.A.; Zhang, L.; Maloney, T.; Telang, F.; Alia-Klein, N.; Volkow, N.D. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience 2007, 144, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Liu, Y.; Fu, X.-M.; Li, N.; Wang, C.-X.; Zhang, H.; Qian, R.-B.; Xu, H.-S.; Hu, X.; Zhang, D.-R. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS ONE 2011, 6, e16560. [Google Scholar] [CrossRef] [PubMed]
- Center for Behavioral Health Statistics and Quality. Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health; SAMHSA: Rockville, MD, USA, 2015. [Google Scholar]
- Konkel, L. Racial and Ethnic Disparities in Research Studies: The challenge of creating more diverse cohorts. Environ. Health Perspect. 2015, 123, A297–A302. [Google Scholar] [CrossRef] [PubMed]
| Total Sample (n = 57) | HC (n = 28) | CU (n = 29) | Statistic | |
|---|---|---|---|---|
| Age (Mean, SD) | 40.46 (8.44) | 39.57 (8.90) | 41.31 (8.04) | t(55) = −0.77 |
| Gender (Nmale, %) | 44 (77.20) | 17 (60.70) | 27 (93.10) | χ2(1) = 8.49 ** |
| Race (NBlack/A.A. %) | 44 (77.20) | 21 (75.00) | 23 (79.30) | χ2(1) = 0.15 |
| IQ (Mean, SD) | 103.63 (12.36) | 106.29 (12.75) | 101.56 (11.88) | t (46) = 1.33 |
| Anxiety Symptoms (Mean, SD) | 2.88 (5.21) | 1.72 (3.14) | 4.04 (6.53) | t(54) = −1.69 |
| Depression Symptoms (Mean, SD) | 4.38 (6.46) | 2.21 (3.56) | 6.54 (7.91) | t(54) = −2.63 * |
| Within rsFC (Mean, SD) | ||||
| DMN | 0.45 (0.05) | 0.44 (0.04) | 0.47 (0.03) | t(55) = −2.26 * |
| SN | 0.41 (0.04) | 0.41 (0.04) | 0.41 (0.05) | t(55) = 0.26 |
| RECN | 1.23 (0.44) | 1.30 (0.42) | 1.17 (0.45) | t(55) = 1.13 |
| LECN | 0.75 (0.49) | 0.89 (0.46) | 0.62 (0.49) | t(55) = 2.13 * |
| Between rsFC (Mean, SD) | ||||
| L.RAI | −0.96 (0.39) | −1.04 (0.40) | −0.88 (0.36) | t(55) = −1.56 |
| R.RAI | −0.74 (0.32) | −0.69 (0.30) | −0.79 (0.33) | t(55) = 1.26 |
| DMN-SN | 0.73 (0.10) | 0.74 (0.09) | 0.72 (0.11) | t(55) = 0.48 |
| LECN-DMN | 0.44 (0.18) | 0.48 (0.13) | 0.40 (0.22) | t(55) = 1.73 |
| RECN-DMN | −0.07 (0.23) | −0.12 (0.24) | −0.02 (0.22) | t(55) = −1.63 |
| LECN-SN | 0.00 (0.23) | −0.06 (0.23) | 0.06 (0.22) | t(55) = −2.05 * |
| RECN-SN | 0.20 (0.23) | 0.27 (0.19) | 0.14 (0.24) | t(55) = 2.18 * |
| Total Sample (n = 57) | HC (n = 28) | CU (n = 29) | Statistic | |
|---|---|---|---|---|
| Task Order (MT first N, %) | 25 (43.90) | 13 (46.40) | 12 (41.40) | χ2(1) = 0.15 |
| Task 1: | ||||
| Performance † (Mean, SD) | 0.19 (1.11) | 0.14 (0.97) | 0.25 (1.27) | F(1,46) = 0.11 |
| Motivation (Mean, SD) | 8.38 (1.83) | 8.45 (1.84) | 8.31 (1.85 | F(1,54) = 0.06 |
| Distress (Mean, SD) | aF(1,53) = 31.60 *** | |||
| Pre-Easy | 10.83 (16.66) | 8.25 (14.39) | 13.32 (18.50) | bF(1,53) = 1.31 |
| Pre-DT | 26.36 (25.47) | 22.73 (22.80) | 29.87 (27.76) | cF(1,53) = 0.21 |
| Task 2: | ||||
| Performance† (Mean, SD) | −0.20 (0.83) | −0.13 (0.82) | −0.27 (0.85) | F(1,44) = 0.27 |
| Motivation (Mean, SD) | 8.25 (1.70) | 7.96 (1.79) | 8.54 (1.60) | F(1,52) = 1.48 |
| Distress (Mean, SD) | aF(1,52) = 18.76 *** | |||
| Pre-Easy | 18.94 (24.26) | 14.84 (21.85) | 22.90 (26.14) | bF(1,52) = 1.96 |
| Pre-DT | 30.02 (27.64) | 24.44 (26.45) | 35.22 (28.16) | cF(1,52) = 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reese, E.D.; Yi, J.Y.; McKay, K.G.; Stein, E.A.; Ross, T.J.; Daughters, S.B. Triple Network Resting State Connectivity Predicts Distress Tolerance and Is Associated with Cocaine Use. J. Clin. Med. 2019, 8, 2135. https://doi.org/10.3390/jcm8122135
Reese ED, Yi JY, McKay KG, Stein EA, Ross TJ, Daughters SB. Triple Network Resting State Connectivity Predicts Distress Tolerance and Is Associated with Cocaine Use. Journal of Clinical Medicine. 2019; 8(12):2135. https://doi.org/10.3390/jcm8122135
Chicago/Turabian StyleReese, Elizabeth D., Jennifer Y. Yi, Katlyn G. McKay, Elliot A. Stein, Thomas J. Ross, and Stacey B. Daughters. 2019. "Triple Network Resting State Connectivity Predicts Distress Tolerance and Is Associated with Cocaine Use" Journal of Clinical Medicine 8, no. 12: 2135. https://doi.org/10.3390/jcm8122135
APA StyleReese, E. D., Yi, J. Y., McKay, K. G., Stein, E. A., Ross, T. J., & Daughters, S. B. (2019). Triple Network Resting State Connectivity Predicts Distress Tolerance and Is Associated with Cocaine Use. Journal of Clinical Medicine, 8(12), 2135. https://doi.org/10.3390/jcm8122135





