The Innate Immune Cell Profile of the Cornea Predicts the Onset of Ocular Surface Inflammatory Disorders
Abstract
1. Introduction
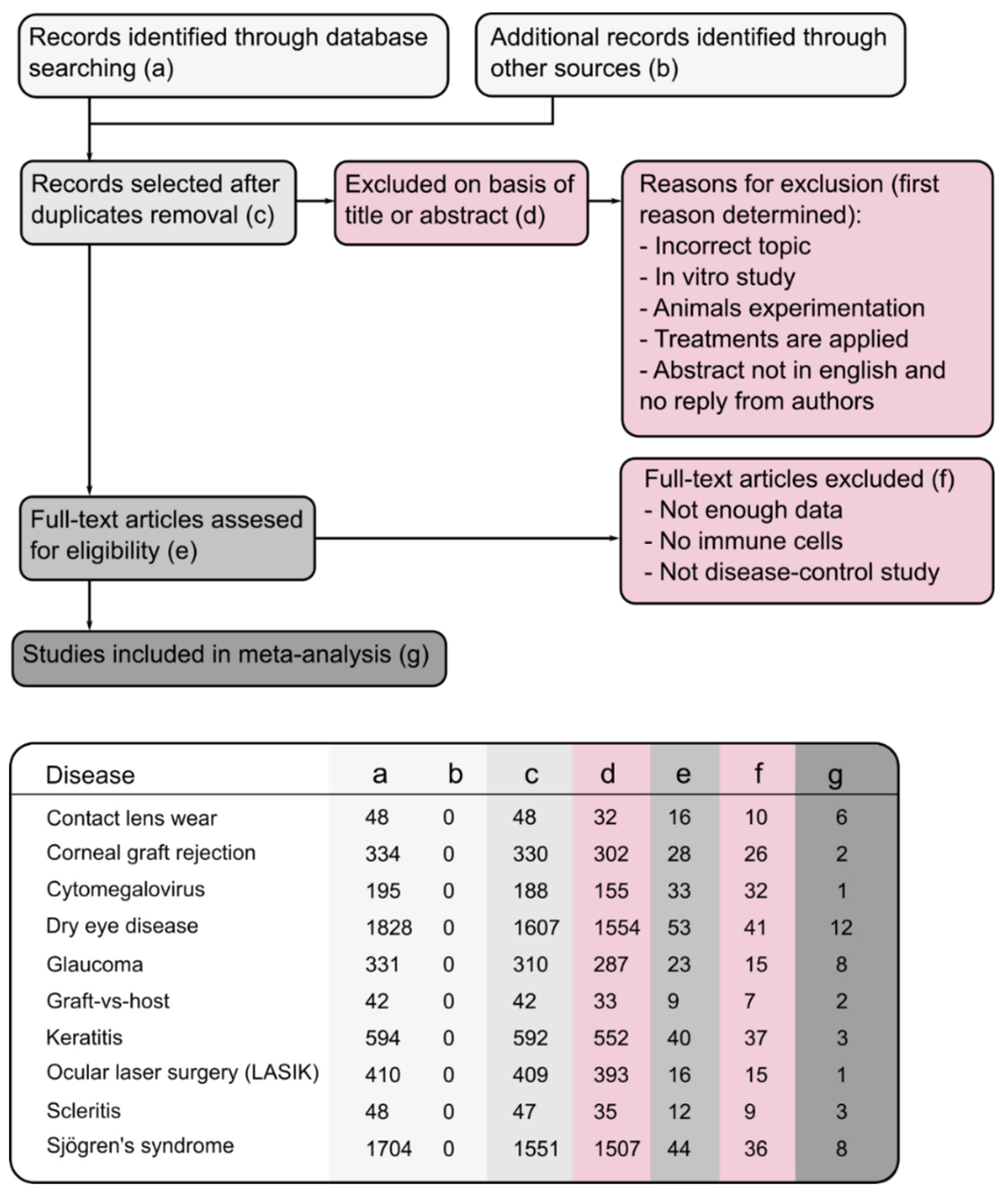
2. Method
2.1. Study Retrieval and Selection
2.2. Statistical Analysis
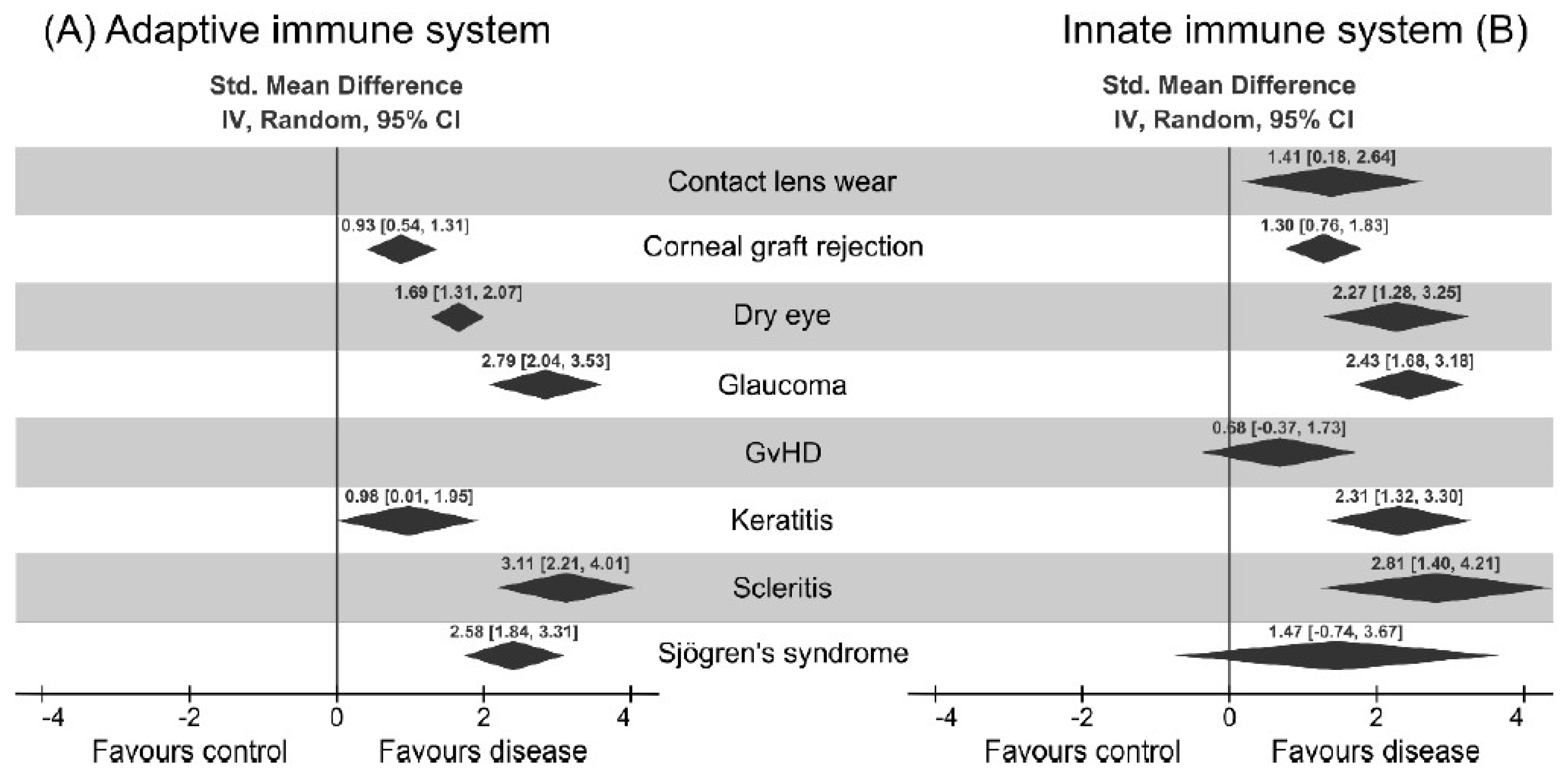
3. Results
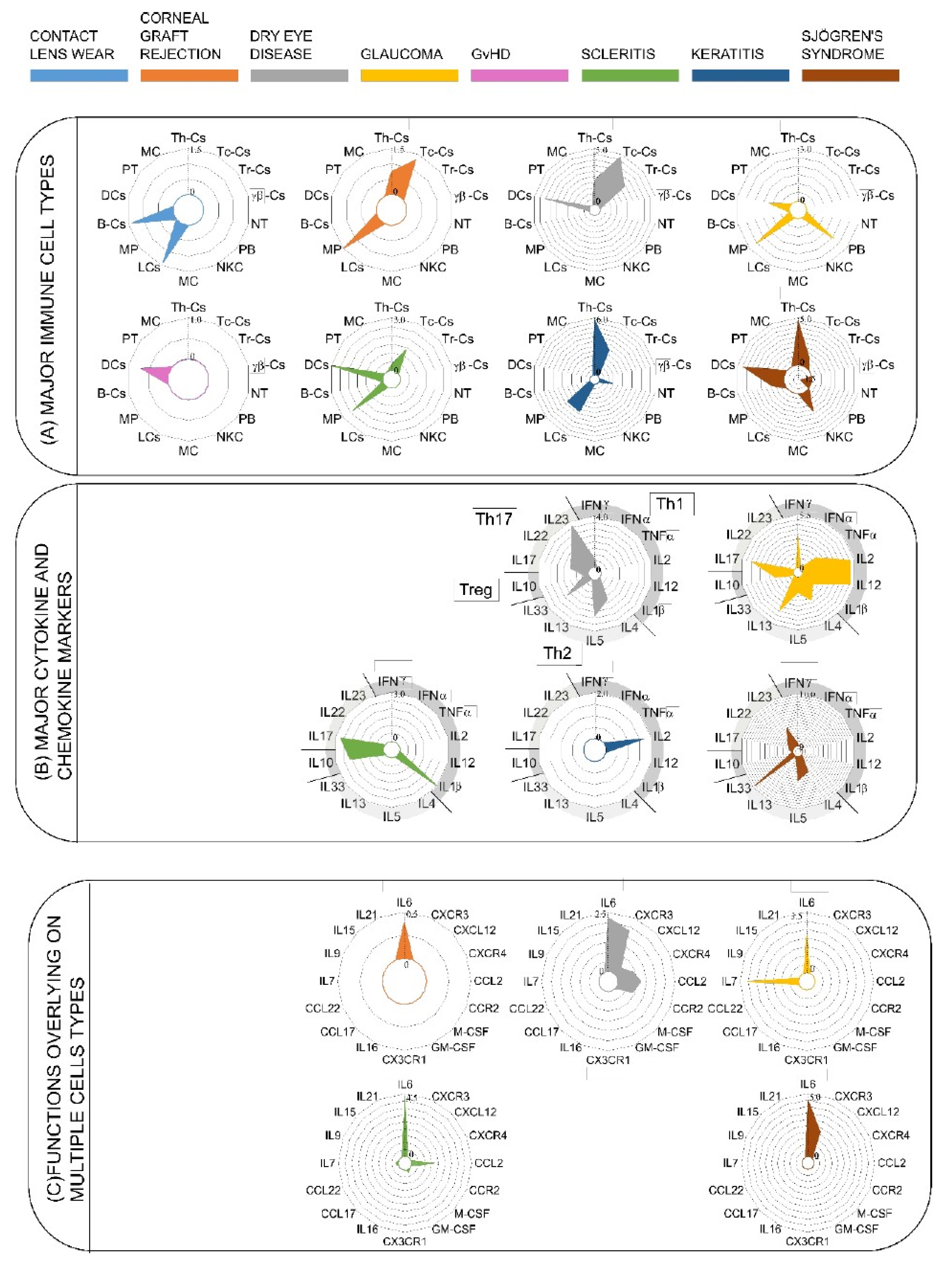
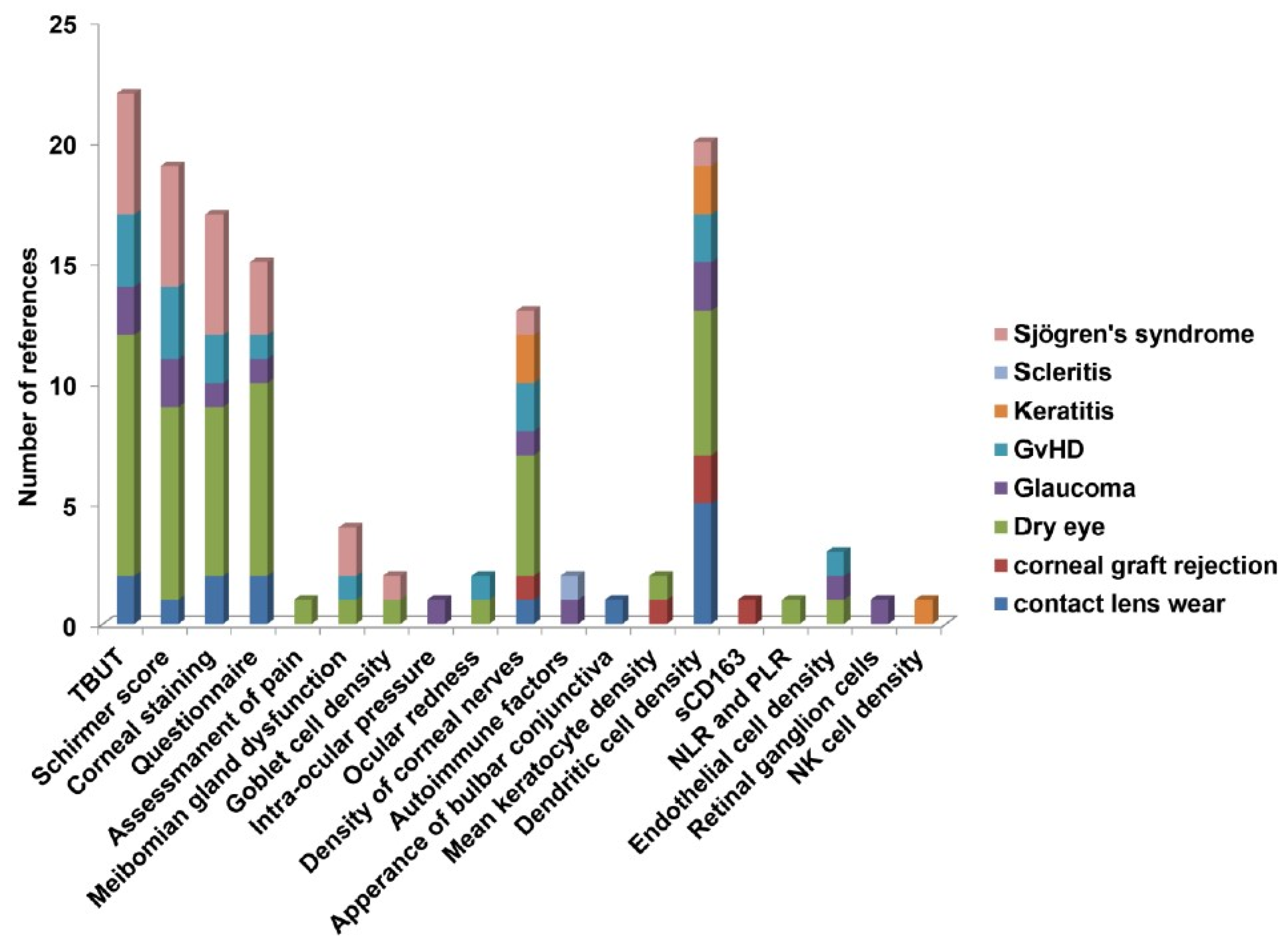
3.1. Dendritic Cells are the Major Immune Cells Responsible for Ocular Surface Diseases
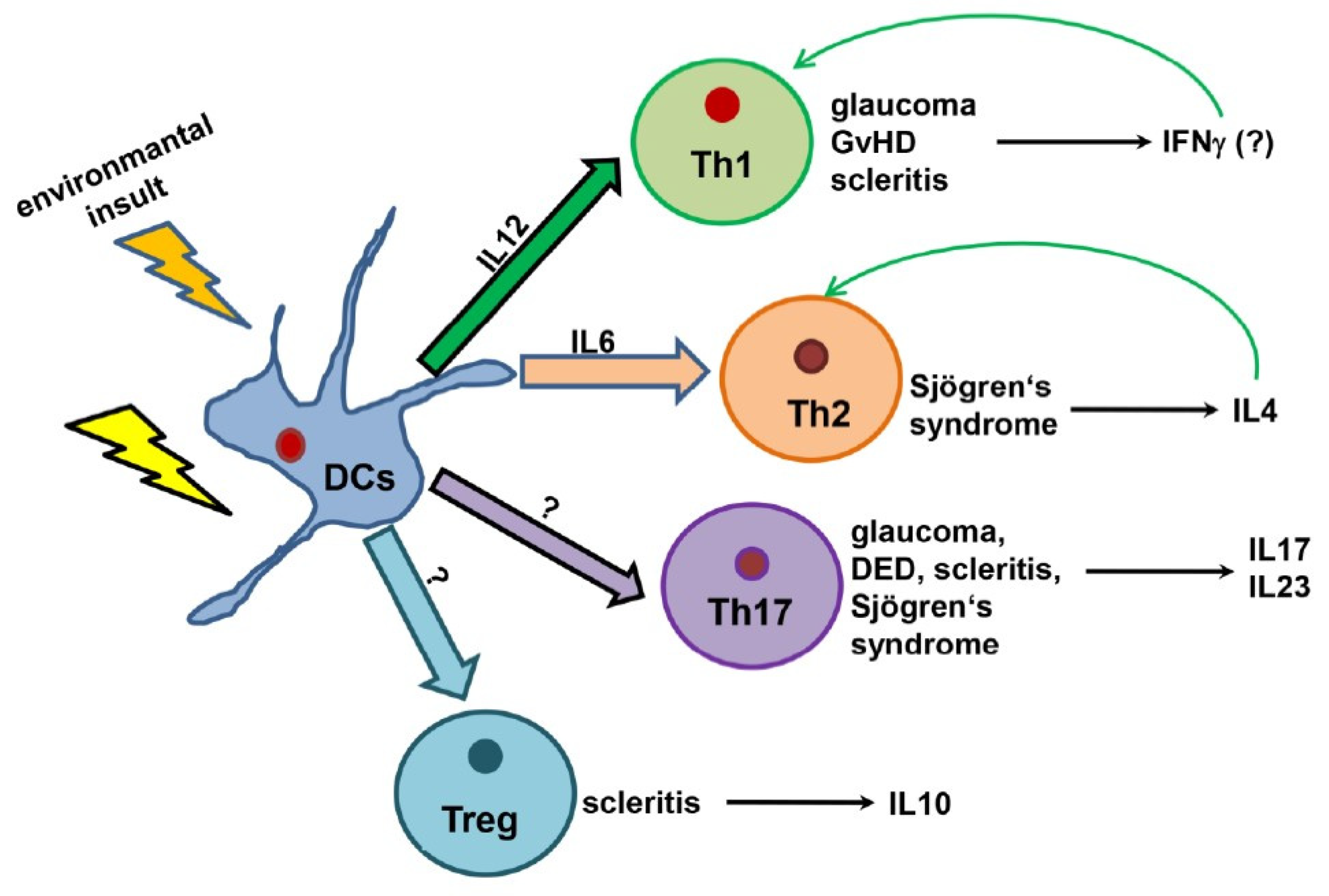
3.2. Upregulation of Cytokines Mediating Th1, Th17, and Treg Activation
3.3. Clinical Factors Associated with Ocular Surface Diseases
4. Discussion
Pitfalls and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia, D.M.; Reis de Oliveira Modulo, C.M.; Faustino JBarbosa, A.P.; Alves, M.; Rocha, E.M. Is Sjögren’s syndrome dry eye similar to dry eye caused by other etiologies? Discriminating different diseases by dry eye tests. PLoS ONE 2018, 13, e0208420. [Google Scholar] [CrossRef] [PubMed]
- Milner, M.S.; Beckman, K.A.; Luchs, J.I.; Allen, Q.B.; Awdeh, R.M.; Berdahl, J.; Boland, T.S.; Buznego, C.; Gira, J.P.; Goldber, D.F.; et al. Dysfunctional tear syndrome: Dry eye diseases and associated tear film disorders—New strategies for diagnosis and treatments. Curr. Opin. Opthalmol. 2017, 3–47. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Asche, C.V.; Fairchild, C.J. The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 2011, 30, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Lan, W.; Lim, R.R.; Chaurasia, S.S. S100A proteins as molecular targets in the ocular surface inflammatory diseases. Ocul. Surf. 2014, 12, 23–31. [Google Scholar] [CrossRef]
- Shih, K.C.; Lam, K.S.; Tong, L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes 2017, 7, e251. [Google Scholar] [CrossRef]
- Bose, T.; Lee, R.; Hou, A.; Tong, L.; Chandy, K.G. Tissue resident memory T cells in the human conjunctiva and immune signatures in human dry eye disease. Sci. Rep. 2017, 7, 45312. [Google Scholar] [CrossRef]
- Walsh, K.P.; Mills, K.H. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013, 34, 521–530. [Google Scholar] [CrossRef]
- Katz, S.I.; Tamaki, K.; Sachs, D.H. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature 1979, 282, 324–326. [Google Scholar] [CrossRef]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef]
- Miller, C.J.; Shattock, R.J. Target cells in vaginal HIV transmission. Microbes Infect. 2003, 5, 59–67. [Google Scholar] [CrossRef]
- Valladeau, J.; Saeland, S. Cutaneous dendritic cells. Semin. Immunol. 2005, 17, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Stoitzner, P.; Tripp, C.H.; Eberhart, A.; Price, K.M.; Jung, J.Y.; Bursch, L.; Ronchese, F.; Romani, N. Langerhans cells cross-present antigen derived from skin. Proc. Natl. Acad. Sci. USA 2006, 103, 7783–7788. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, D.; Haak, S.; Sisirak, V.; Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 2013, 13, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Pritchard, N.; Efron, N. Changes in corneal Langerhans cell density during the first few hours of contact lens wear. Cont. Lens Anterior Eye 2016, 39, 307–310. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Colorado, L.H.; Pritchard, N.; Efron, N. Longitudinal changes in Langerhans cell density of the cornea and conjunctiva in contact lens-induced dry eye. Clin. Exp. Optom. 2017, 100, 33–40. [Google Scholar] [CrossRef]
- Efron, N.; Al-Dossari, M.; Pritchard, N. Confocal microscopy of the bulbar conjunctiva in contact lens wear. Cornea 2010, 29, 43–52. [Google Scholar] [CrossRef]
- Lopez-De La Rosa, A.; Arroyo-Del Arroyo, C.; Canadas, P.; Lopez-Miguel, A.; Calonge, M.; Enriquez-De-Salamanca, A.; Gonzalez-Garcia, M.J. Are Contact Lens Discomfort or Soft Contact Lens Material Properties Associated with Alterations in the Corneal Sub-Basal Nerve Plexus? Curr. Eye Res. 2018, 43, 487–492. [Google Scholar] [CrossRef]
- Sindt, C.W.; Grout, T.K.; Critser, D.B.; Kern, J.R.; Meadows, D.L. Dendritic immune cell densities in the central cornea associated with soft contact lens types and lens care solution types: A pilot study. Clin. Ophthalmol. 2012, 6, 511–519. [Google Scholar] [CrossRef]
- Zhivov, A.; Stave, J.; Vollmar, B.; Guthoff, R. In vivo confocal microscopic evaluation of langerhans cell density and distribution in the corneal epithelium of healthy volunteers and contact lens wearers. Cornea 2007, 26, 47–54. [Google Scholar] [CrossRef]
- Funding, M.; Vorum, H.; Nexo, E.; Moestrup, S.K.; Ehlers, N.; Moller, M.J. Soluble CD163 and interleukin-6 are increased in aqueous humour from patients with endothelial rejection of corneal grafts. Acta Ophthalmol. Scand. 2005, 83, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Kuffova, L.; Holan, V.; Lumsden, L.; Forrester, J.V.; Filipec, M. Cell subpopulations in failed human corneal grafts. Br. J. ophthalmol. 1999, 83, 1364–1369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, D.; Song, P.; Wang, S.; Sun, D.; Wang, Y.; Zhang, Y.; Gao, H. Laser Scanning In Vivo Confocal Microscopy of Clear Grafts after Penetrating Keratoplasty. BioMed Res. Int. 2016, 2016, 5159746. [Google Scholar] [CrossRef]
- Celik, T. Assessment of Neutrophil-to-Lymphocyte Ratio and Platelet-to—Lymphocyte Ratio in Patients with Dry Eye Disease. Ocul. Immunol. Inflamm. 2018, 26, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.W.; Mittal, S.K.; Hwang, H.S.; Chang, E.J.; Lee, J.H.; Seo, Y.; Yeo, A.; Noh, H.; Lee, H.S.; Chauhan, S.K. Lacrimal gland-derived IL-22 regulates IL-17-mediated ocular mucosal inflammation. Mucosal Immunol. 2017, 10, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Rahimi Darabad, R.; Cruzat, A.; Hajrasouliha, A.R.; Witkin, D.; Wong, N.; Dana, R.; Hamrah, P. Corneal Epithelial Immune Dendritic Cell Alterations in Subtypes of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7179–7185. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Saboo, U.S.; Abud, T.B.; Dohlman, T.H.; Arnoldner, M.A.; Hamrah, P.; Dana, R. Reduced Corneal Endothelial Cell Density in Patients with Dry Eye Disease. Am. J. Ophthalmol. 2015, 159, 1022–1026.e2. [Google Scholar] [CrossRef]
- Liu, R.; Gao, C.; Chen, H.; Li, Y.; Jin, Y.; Qi, H. Analysis of Th17-associated cytokines and clinical correlations in patients with dry eye disease. PLoS ONE 2017, 12, e0173301. [Google Scholar] [CrossRef]
- Luo, G.; Xin, Y.; Qin, D.; Yan, A.; Zhou, Z.; Liu, Z. Correlation of interleukin-33 with Th cytokines and clinical severity of dry eye disease. Indian J. Ophthalmol. 2018, 66, 39–43. [Google Scholar] [CrossRef]
- Nicolle, P.; Liang, H.; Reboussin, E.; Rabut, G.; Warcoin, E.; Brignole-Baudouin, F.; Melik-Parsadaniantz, S.; Baudouin, C.; Labbe, A.; Reaux-Le Goazigo, A. Proinflammatory Markers, Chemokines, and Enkephalin in Patients Suffering from Dry Eye Disease. Int. J. Mol. Sci. 2018, 19, 1221. [Google Scholar] [CrossRef]
- Shetty, R.; Sethu, S.; Deshmukh, R.; Deshpande, K.; Ghosh, A.; Agarwal, A.; Shroff, R. Corneal Dendritic Cell Density Is Associated with Subbasal Nerve Plexus Features, Ocular Surface Disease Index, and Serum Vitamin D in Evaporative Dry Eye Disease. BioMed Res. Int. 2016, 2016, 4369750. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, S.; Liu, Y.; Zhu, T.; Wang, K.; Ren, T.; Wu, Z.; Xu, H.; Zhu, L. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye (Lond.) 2014, 28, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Tepelus, T.C.; Chiu, G.B.; Huang, J.; Huang, P.; Sadda, S.R.; Irvine, J.; Lee, O.L. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: A preliminary study. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Park, C.S.; You, I.C.; Choi, H.J.; Lee, K.H.; Im, S.K.; Park, H.Y.; Pflugfelder, J.C. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Investig. Ophthalmol. Vis. Sci. 2010, 51, 643–650. [Google Scholar] [CrossRef]
- Baghdasaryan, E.; Tepelus, T.C.; Vickers, L.A.; Huang, P.; Chopra, V.; Sadda, S.R.; Lee, O.L. Assessment of Corneal Changes Associated with Topical Antiglaucoma Therapy Using in vivo Confocal Microscopy. Ophthalmic Res. 2019, 61, 51–59. [Google Scholar] [CrossRef]
- Gramlich, O.W.; Beck, S.; von Thus Und Hohenstein-Blaul, N.; Boehm, N.; Ziegler, A.; Vetter, J.M.; Pfeiffer, N.; Grus, F.H. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS ONE 2013, 8, e57557. [Google Scholar] [CrossRef]
- Liang, H.; Baudouin, C.; Hamard, P.; Creuzot-Garcher, C.; Warnet, J.M.; Brignole-Baudouin, F. Activation of TH1/TH2 pathways detected through the expression of CCR4 and CCR5 on the ocular surface of glaucomatous patients treated over the long term. J. Fr. Ophtalmol. 2006, 29, 121–126. [Google Scholar] [CrossRef]
- Malvitte, L.; Montange, T.; Vejux, A.; Baudouin, C.; Bron, A.M.; Creuzot-Garcher, C.; Lizard, G. Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs. Br. J. Ophthalmol. 2007, 91, 29–32. [Google Scholar] [CrossRef]
- Margeta, M.A.; Lad, E.M.; Proia, A.D. CD163+ macrophages infiltrate axon bundles of postmortem optic nerves with glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2449–2456. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Agnifili, L.; Fasanella, V.; Lappa, A.; Brescia, L.; Lanzini, M.; Oddone, F.; Perri, P.; Mastropasqua, L. In Vivo Distribution of Corneal Epithelial Dendritic Cells in Patients with Glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5996–6002. [Google Scholar] [CrossRef]
- Taurone, S.; Ripandelli, G.; Pacella, F.; Bianchi, E.; Plateroti, A.M.; De Vito, S.; Plateroti, P.; Grippaudo, F.R.; Cavallotti, C.; Artico, M. Potential regulatory molecules in the human trabecular meshwork of patients with glaucoma: Immunohistochemical profile of a number of inflammatory cytokines. Mol. Med. Rep. 2015, 11, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Huang, P.; Li, W.; Li, Y.; Zhang, S.S.; Zhang, C. T-helper1/T-helper2 cytokine imbalance in the iris of patients with glaucoma. PLoS ONE 2015, 10, e0122184. [Google Scholar] [CrossRef]
- He, J.; Ogawa, Y.; Mukai, S.; Saijo-Ban, Y.; Kamoi, M.; Uchino, M.; Yamane, M.; Ozawa, N.; Fukui, M.; Mori, T. In Vivo Confocal Microscopy Evaluation of Ocular Surface with Graft-Versus-Host Disease-Related Dry Eye Disease. Sci. Rep. 2017, 7, 10720. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Qazi, Y.; Arnoldner, M.A.; Suri, K.; Dana, R. In Vivo Confocal Microscopy in Dry Eye Disease Associated with Chronic Graft-Versus-Host Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4686–4691. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, A.; Witkin, D.; Baniasadi, N.; Zheng, L.; Ciolino, J.B.; Jurkunas, U.V.; Chodosh, J.; Pavan-Langston, D.; Dana, R.; Hamrah, P. Inflammation and the nervous system: The connection in the cornea in patients with infectious keratitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5136–5143. [Google Scholar] [CrossRef] [PubMed]
- Iskeleli, G.; Camcioglu, Y.; Akova, N.; Kiran, B.; Bahar, H.; Deniz, G. Lymphocyte subgroups and natural killer cell activity in recurrent herpetic stromal keratitis. Eye Contact Lens 2008, 34, 169–173. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Calvacanti, B.M.; Cruzat, A.; Qazi, Y.; Ishikawa, S.; Osuka, A.; Lederer, J.; Hamrah, P. Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7457–7466. [Google Scholar] [CrossRef]
- Bernauer, W.; Watson, P.G.; Daicker, B.; Lightman, S. Cells perpetuating the inflammatory response in scleritis. Br. J. Ophthalmol. 1994, 78, 381–385. [Google Scholar] [CrossRef]
- Fong, L.P.; Sainz de la Maza, M.; Rice, B.A.; Kupferman, A.E.; Foster, C.S. Immunopathology of scleritis. Ophthalmology 1991, 98, 472–479. [Google Scholar] [CrossRef]
- Sainz de la Maza, M.; Foster, C.S. Necrotizing scleritis after ocular surgery. A clinicopathologic study. Ophthalmology 1991, 98, 1720–1726. [Google Scholar] [CrossRef]
- Fujihara, T.; Fujita, H.; Tsubota, K.; Saito, K.; Tsuzaka, K.; Abe, T.; Takeuchi, T. Preferential localization of CD8+ alpha E beta 7+ T cells around acinar epithelial cells with apoptosis in patients with Sjogren’s syndrome. J. Immunol. 1999, 163, 2226–2235. [Google Scholar] [PubMed]
- Pflugfelder, S.C.; Bian, F.; Gumus, K.; Farley, W.; Stern, M.E.; De Paiva, C.S. Severity of Sjogren’s Syndrome Keratoconjunctivitis Sicca Increases with Increased Percentage of Conjunctival Antigen—Presenting Cells. Int. J. Mol. Sci. 2018, 19, 2760. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.P.; Nighingale, P.; Southworth, S.; Denniston, A.K.; Tomlinus, P.J.; Turner, S.; Hamburger, J.; Bowman, S.J.; Curnow, S.J.; Rauz, S. Conjunctival Neutrophils Predict Progressive Scarring in Ocular Mucous Membrane Pemphigoid. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5457–5469. [Google Scholar] [CrossRef] [PubMed]
- Harden, J.L.; Egilmez, N.K. Indolamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol. Investig. 2012, 41, 738–764. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; McGovern, N.; Haniffa, M. Human dendritic cell subsets. Immunology 2013, 140, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Human dendritic cell subsets in vaccination. Curr. Opin. Immunol. 2013, 25, 396–402. [Google Scholar] [CrossRef]
- Segura, E.; Amigorena, S. Cross-presentation by human dendritic cell subsets. Immunol. Lett. 2014, 158, 73–78. [Google Scholar] [CrossRef]
- Segura, E.; Valladeau-Guilemond, J.; Donnadieu, M.-H.; Sastre-Garau, X.; Soumelis, V.; Amigorena, S. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 2012, 209, 653–660. [Google Scholar] [CrossRef]
- Durand, M.; Segura, E. The known unknowns of the human dendritic cell network. Front. Immunol. 2015, 6, 129. [Google Scholar] [CrossRef]
- Granot, T.; Senda, T.; Carpenter, D.J.; Matsuoka, N.; Weiner, J.; Gordon, C.L.; Miron, M.; Kumar, B.V.; Griesemer, A.; Ho, S.H. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity 2017, 46, 504–515. [Google Scholar] [CrossRef]
- Rhodes, J.W.; Tong, O.; Harman, A.N.; Turville, S.G. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front. Immunol. 2019, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, J.M.; Yuan, R.; Yi, X.; Li, L.; Gong, W.; Yang, T.; Li, L.; Su, S. Tissue-resident dendritic cells and diseases involving dendritic cell malfunction. Int. Immunopharmacol. 2016, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Enamorado, M.; Khouili, S.C.; Iborra, S.; Sancho, D. Genealogy, Dendritic Cell Priming, and Differentiation of Tissue-Resident Memory CD8(+) T Cells. Front. Immunol. 2018, 9, 1751. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N. Spreading the load: Antigen transfer between migratory and lymph node-resident dendritic cells promotes T-cell priming. Eur. J. Immunol. 2017, 47, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Mingozzi, F.; Broggi, A.; Barresi, S.; Zolezzi, F.; Bayry, J.; Raimondi, G.; Zanoni, I.; Granucci, F. Migratory, and not lymphoid-resident, dendritic cells maintain peripheral self-tolerance and prevent autoimmunity via induction of iTreg cells. Blood 2012, 120, 1237–1245. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, T.H. Fundamental role of dendritic cells in inducing Th2 responses. Korean J. Intern. Med. 2018, 33, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Agalioti, T.; Villablanca, E.J.; Huber, S.; Gagliani, N. TH17cell plasticity: The role of dendritic cells and molecular mechanisms. J. Autoimmune 2018, 87, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, S.; Rouse, B.T. Interplay of Regulatory T Cell and Th17 Cells during Infectious Diseases in Humans and Animals. Front. Immunol. 2017, 8, 341. [Google Scholar] [CrossRef]
- Eisenstein, E.M.; Williams, C.B. The T(reg)/Th17 cell balance: A new paradigm for autoimmunity. Pediatr. Res. 2009, 65, 26R–31R. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, A.; Wise, L.; Hibma, M. The role of Langerhans cells in pathologies of the skin. Immunol. Cell Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomar, A.P.d.; Montolío, A.; Cegoñino, J.; Dhanda, S.K.; Lio, C.T.; Bose, T. The Innate Immune Cell Profile of the Cornea Predicts the Onset of Ocular Surface Inflammatory Disorders. J. Clin. Med. 2019, 8, 2110. https://doi.org/10.3390/jcm8122110
Palomar APd, Montolío A, Cegoñino J, Dhanda SK, Lio CT, Bose T. The Innate Immune Cell Profile of the Cornea Predicts the Onset of Ocular Surface Inflammatory Disorders. Journal of Clinical Medicine. 2019; 8(12):2110. https://doi.org/10.3390/jcm8122110
Chicago/Turabian StylePalomar, Amaya Pérez del, Alberto Montolío, José Cegoñino, Sandeep Kumar Dhanda, Chit Tong Lio, and Tanima Bose. 2019. "The Innate Immune Cell Profile of the Cornea Predicts the Onset of Ocular Surface Inflammatory Disorders" Journal of Clinical Medicine 8, no. 12: 2110. https://doi.org/10.3390/jcm8122110
APA StylePalomar, A. P. d., Montolío, A., Cegoñino, J., Dhanda, S. K., Lio, C. T., & Bose, T. (2019). The Innate Immune Cell Profile of the Cornea Predicts the Onset of Ocular Surface Inflammatory Disorders. Journal of Clinical Medicine, 8(12), 2110. https://doi.org/10.3390/jcm8122110





