Maximum Glomerular Diameter and Oxford MEST-C Score in IgA Nephropathy: The Significance of Time-Series Changes in Pseudo-R2 Values in Relation to Renal Outcomes
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Pathological Analyses
2.3. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
3.2. Patients’ Pathological Features
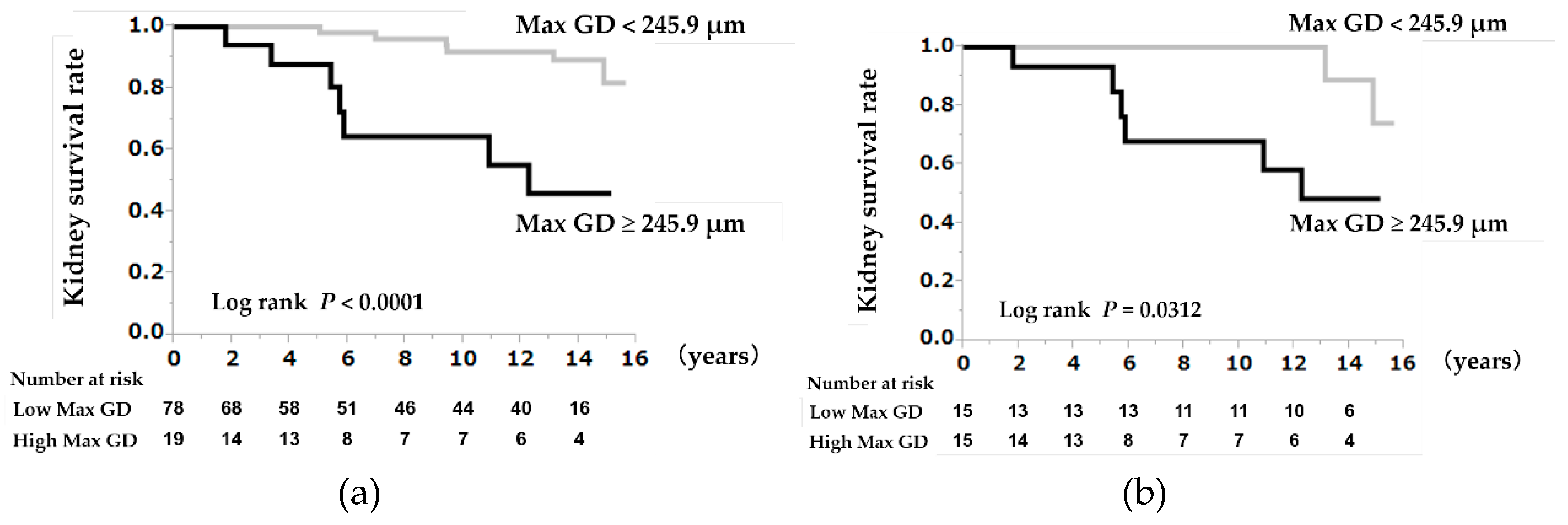
3.3. A High Max GD as a Prognostic Indicator
3.4. Clinical and Pathological Findings According to the Max GD Value
3.5. A High Max GD as a Prognostic Indicator in the Propensity Score-Matched Cohorts
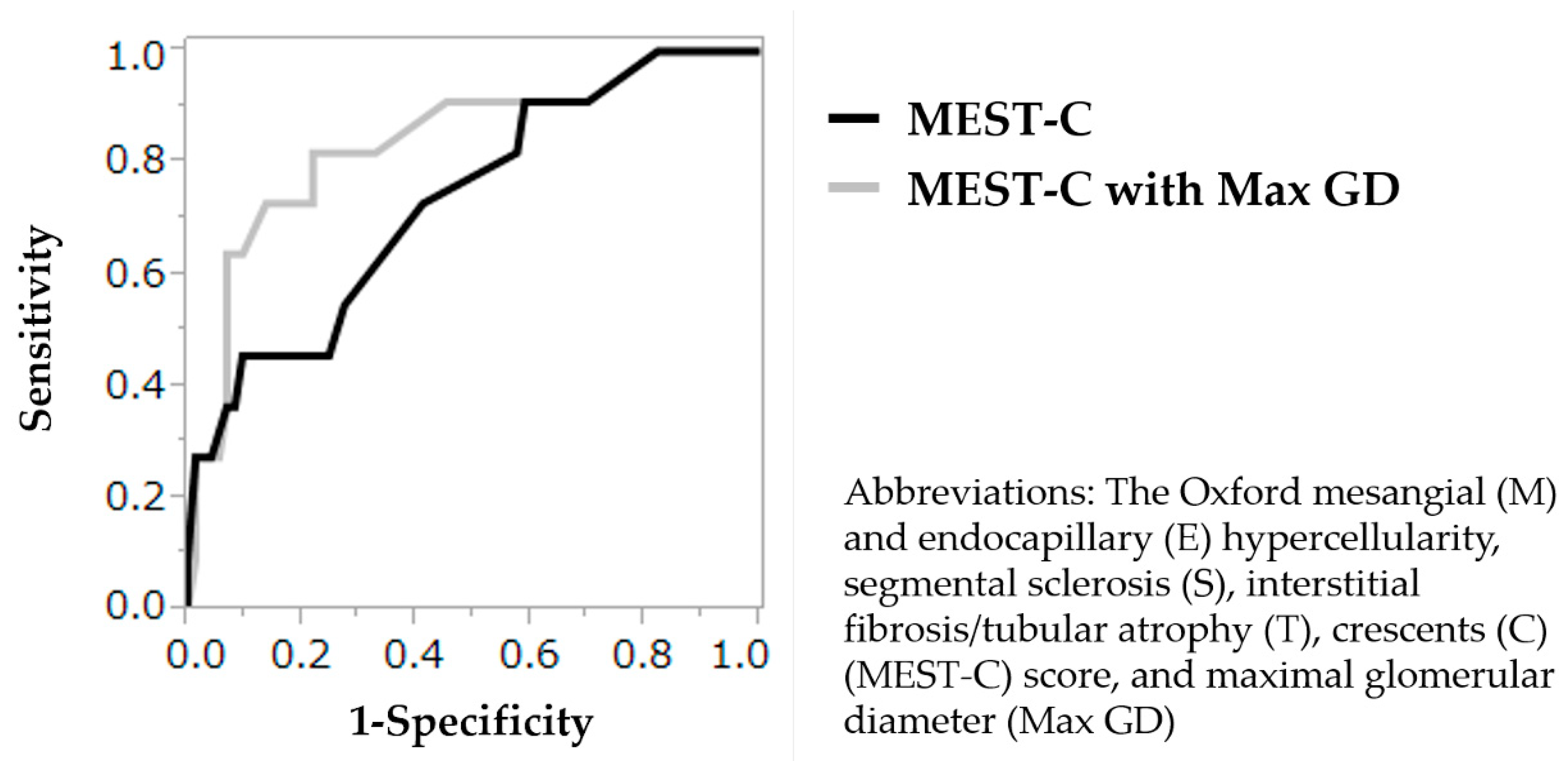
3.6. Validation of the Prognostic Values of the MEST-C Score and Max GD
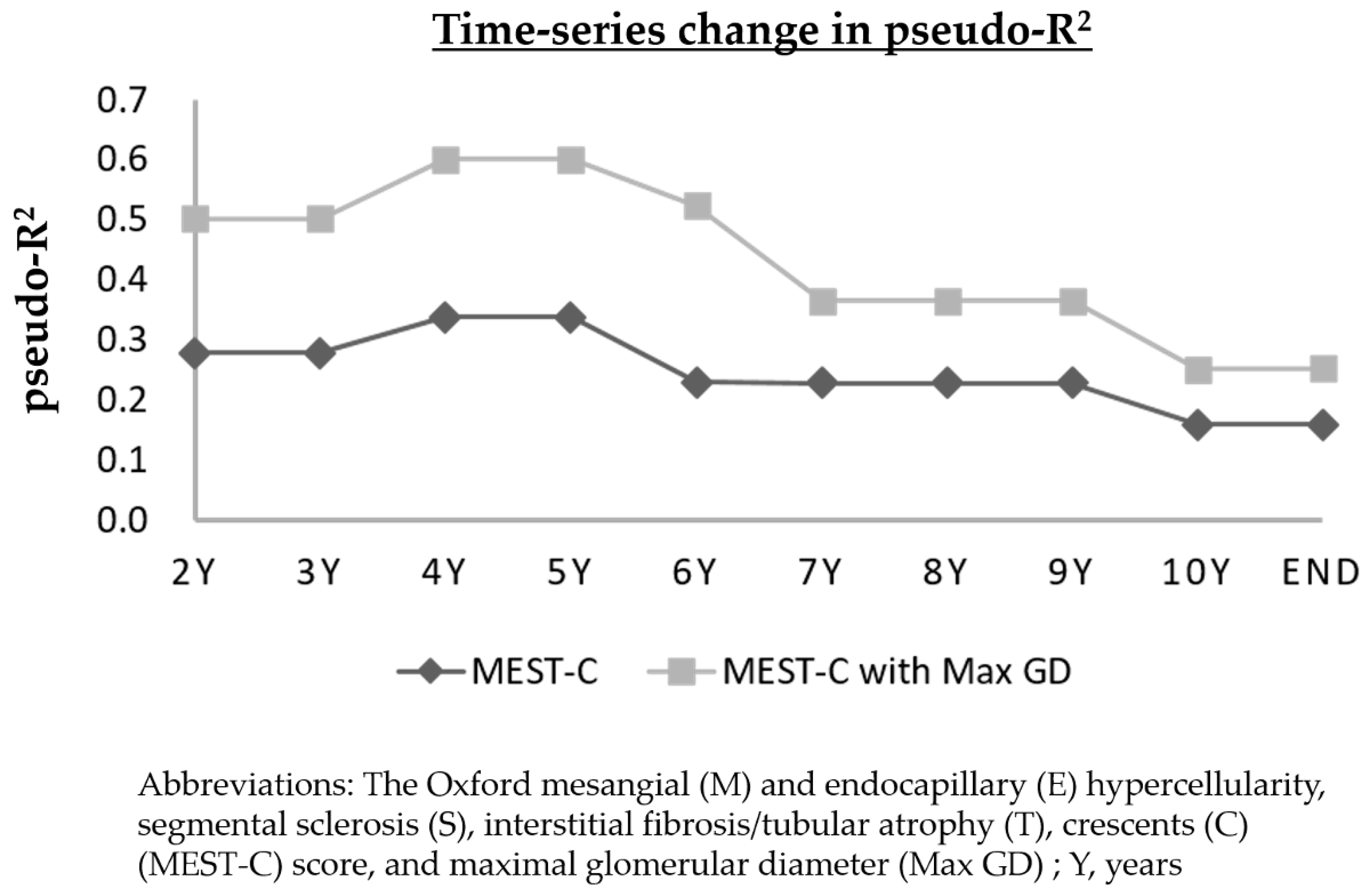
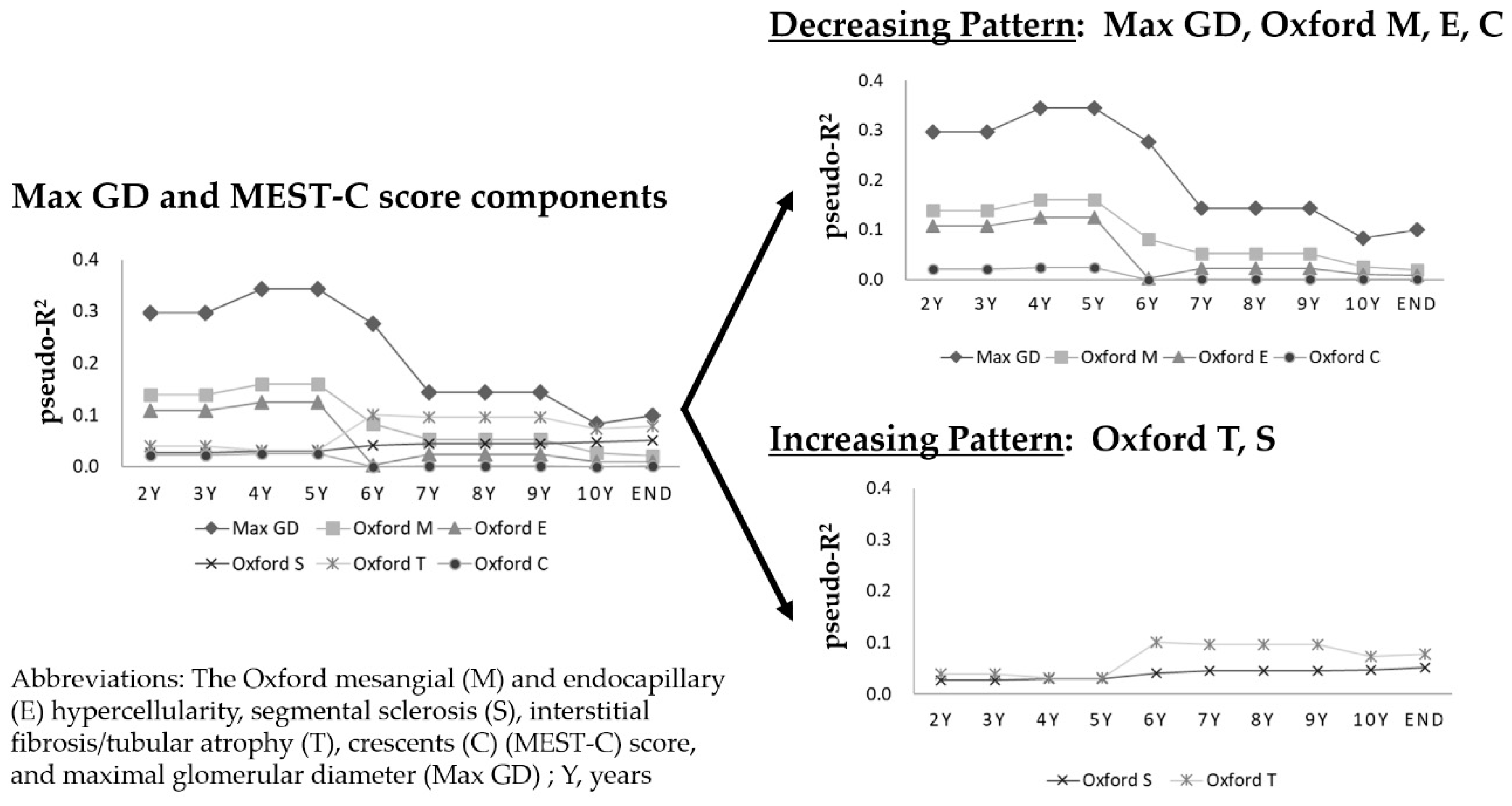
3.7. Time-Series Change in Pseudo-R2 Values of the Prognostic Efficacy in Relation to Renal Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Maisonneuve, P.; Agodoa, L.; Gellert, R.; Stewart, J.H.; Buccianti, G.; Lowenfels, A.B.; Wolfe, R.A.; Jones, E.; Disney, A.P.S.; Briggs, D.; et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: Results from an international comparative study. Am. J. Kidney Dis. 2000, 35, 157–165. [Google Scholar] [CrossRef]
- Roberts, I.S.; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; Bonsib, S.; Bruijn, J.A.; Cattran, D.C.; et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Cattran, D.C.; Coppo, R.; Cook, H.T.; Feehally, J.; Roberts, I.S.D.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009, 76, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Espino-Hernandez, G.; Reich, H.N.; Coppo, R.; Roberts, I.S.; Feehally, J.; Herzenberg, A.M.; Cattran, D.C. The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int. 2016, 89, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lv, J.; Wang, W.; Li, G.; Liu, Z.; Chen, H.; Xu, F.; Sun, J.; Ouyang, Y.; Zhang, X.; et al. Kidney Failure Risk Prediction Equations in IgA Nephropathy: A Multicenter Risk Assessment Study in Chinese Patients. Am. J. Kidney Dis. 2018, 72, 371–380. [Google Scholar] [CrossRef]
- Coppo, R.; Troyanov, S.; Bellur, S.; Cattran, D.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Morando, L.; Camilla, R.; Tesar, V.; et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014, 86, 828–836. [Google Scholar] [CrossRef]
- Herzenberg, A.M.; Fogo, A.B.; Reich, H.N.; Troyanov, S.; Bavbek, N.; Massat, A.E.; Hunley, T.E.; Hladunewich, M.A.; Julian, B.A.; Fervenza, F.C.; et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int. 2011, 80, 310–317. [Google Scholar] [CrossRef]
- Moriyama, T.; Nakayama, K.; Iwasaki, C.; Ochi, A.; Tsuruta, Y.; Itabashi, M.; Tsukada, M.; Takei, T.; Uchida, K.; Nitta, K. Severity of nephrotic IgA nephropathy according to the Oxford classification. Int. Urol. Nephrol. 2012, 44, 1177–1184. [Google Scholar] [CrossRef]
- Shi, S.F.; Wang, S.X.; Jiang, L.; Lv, J.G.; Liu, L.J.; Chen, Y.Q.; Zhu, S.N.; Liu, G.; Zou, W.Z.; Zhang, H.; et al. Pathologic Predictors of Renal Outcome and Therapeutic Efficacy in IgA Nephropathy: Validation of the Oxford Classification. Clin. J. Am. Soc. Nephrol. 2011, 6, 2175–2184. [Google Scholar] [CrossRef]
- Barbour, S.; Reich, H. An update on predicting renal progression in IgA nephropathy. Curr. Opin. Nephrol. Hypertens. 2018, 27, 214–220. [Google Scholar] [CrossRef]
- Trimarchi, H.; Barratt, J.; Cattran, D.C.; Cook, H.T.; Coppo, R.; Haas, M.; Liu, Z.H.; Roberts, I.S.; Yuzawa, Y.; Zhang, H.; et al. Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017, 91, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ohara, M.; Honda, K.; Mochizuki, T.; Nitta, K. Maximal glomerular diameter as a 10-year prognostic indicator for IgA nephropathy. Nephrol. Dial. Transplant. 2011, 26, 3937–3943. [Google Scholar] [CrossRef] [PubMed]
- Lane, P.H.; Steffes, M.W.; Mauer, S.M. Estimation of glomerular volume: A comparison of four methods. Kidney Int. 1992, 41, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the yield of medical tests. JAMA 1982, 247, 2543–2546. [Google Scholar] [CrossRef] [PubMed]
- Hauber, A.B.; Gonzalez, J.M.; Groothuis-Oudshoorn, C.G.; Prior, T.; Marshall, D.A.; Cunningham, C.; IJzerman, M.J.; Bridges, J.F. Statistical Methods for the Analysis of Discrete Choice Experiments: A Report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health 2016, 19, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Hurvich, C.M.; Tsai, C.L. Model selection for extended quasi-likelihood models in small samples. Biometrics 1995, 51, 1077–1084. [Google Scholar] [CrossRef]
- Fries, J.W.; Sandstrom, D.J.; Meyer, T.W.; Rennke, H.G. Glomerular hypertrophy and epithelial cell injury modulate progressive glomerulosclerosis in the rat. Lab. Investig. 1989, 60, 205–218. [Google Scholar]
- Miller, P.L.; Rennke, H.G.; Meyer, T.W. Glomerular hypertrophy accelerates hypertensive glomerular injury in rats. Am. J. Physiol. 1991, 261, F459–F465. [Google Scholar] [CrossRef]
- Toyota, E.; Ogasawara, Y.; Fujimoto, K.; Kajita, T.; Shigeto, F.; Asano, T.; Watanabe, N.; Kajiya, F. Global heterogeneity of glomerular volume distribution in early diabetic nephropathy. Kidney Int. 2004, 66, 855–861. [Google Scholar] [CrossRef]
- Cahill, M.M.; Ryan, G.B.; Bertram, J.F. Biphasic glomerular hypertrophy in rats administered puromycin aminonucleoside. Kidney Int. 1996, 50, 768–775. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fogo, A.; Hawkins, E.P.; Berry, P.L.; Glick, A.D.; Chiang, M.L.; MacDonell, R.C., Jr.; Ichikawa, I. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990, 38, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Osterby, R.; Gundersen, H.J. Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia 1975, 11, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, G.; Bonfanti, R.; Meschi, F.; Bognetti, E.; Paesano, P.L.; Gianolli, L.; Querques, M.; Maestroni, A.; Calori, G.; Del Maschio, A.; et al. Persistent renal hypertrophy and faster decline of glomerular filtration rate precede the development of microalbuminuria in type 1 diabetes. Diabetes 2006, 55, 2620–2625. [Google Scholar] [CrossRef]
- Kataoka, H.; Mochizuki, T.; Nitta, K. Large Renal Corpuscle: Clinical Significance of Evaluation of the Largest Renal Corpuscle in Kidney Biopsy Specimens. Contrib. Nephrol. 2018, 195, 20–30. [Google Scholar]
- Yoshida, Y.; Kawamura, T.; Ikoma, M.; Fogo, A.; Ichikawa, I. Effects of antihypertensive drugs on glomerular morphology. Kidney Int. 1989, 36, 626–635. [Google Scholar] [CrossRef]
- Peter, A.I.; Naidu, E.C.; Akang, E.; Ogedengbe, O.O.; Offor, U.; Rambharose, S.; Kalhapure, R.; Chuturgoon, A.; Govender, T.; Azu, O.O. Investigating Organ Toxicity Profile of Tenofovir and Tenofovir Nanoparticle on the Liver and Kidney: Experimental Animal Study. Toxicol. Res. 2018, 34, 221–229. [Google Scholar] [CrossRef]
- Coppo, R.; D’Arrigo, G.; Tripepi, G.; Russo, M.L.; Roberts, I.S.D.; Bellur, S.; Cattran, D.; Cook, T.H.; Feehally, J.; Tesar, V.; et al. Is there long-term value of pathology scoring in immunoglobulin A nephropathy? A validation study of the Oxford Classification for IgA Nephropathy (VALIGA) update. Nephrol. Dial. Transplant. 2018. [Google Scholar] [CrossRef]
- Chakera, A.; MacEwen, C.; Bellur, S.S.; Chompuk, L.O.; Lunn, D.; Roberts, I.S.D. Prognostic value of endocapillary hypercellularity in IgA nephropathy patients with no immunosuppression. J. Nephrol. 2016, 29, 367–375. [Google Scholar] [CrossRef]
- Haas, M.; Verhave, J.C.; Liu, Z.H.; Alpers, C.E.; Barratt, J.; Becker, J.U.; Cattran, D.; Cook, H.T.; Coppo, R.; Feehally, J.; et al. A Multicenter Study of the Predictive Value of Crescents in IgA Nephropathy. JASN 2017, 28, 691–701. [Google Scholar] [CrossRef]
- Alamartine, E.; Sauron, C.; Laurent, B.; Sury, A.; Seffert, A.; Mariat, C. The use of the Oxford classification of IgA nephropathy to predict renal survival. Clin. J. Am. Soc. Nephrol. 2011, 6, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Choi, S.R.; Park, H.S.; Lee, J.Y.; Sun, I.O.; Hwang, H.S.; Chung, B.H.; Park, C.W.; Yang, C.W.; Kim, Y.S.; et al. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrol. Dial. Transplant. 2012, 27, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Shi, S.; Xu, D.; Zhang, H.; Troyanov, S.; Cattran, D.C.; Wang, H. Evaluation of the Oxford Classification of IgA nephropathy: A systematic review and meta-analysis. Am. J. Kidney Dis. 2013, 62, 891–899. [Google Scholar] [CrossRef] [PubMed]
| Variables | Entire Cohort | Max GD ≥245.9 μm | Max GD <245.9 μm | p-Value | Standardized Differences |
|---|---|---|---|---|---|
| n = 97 | n = 19 | n = 78 | |||
| Clinical Findings | |||||
| Age (years) | 34 ± 12.6 | 42.3 ± 15.3 | 32 ± 11.1 | 0.0013 | 0.771 |
| Sex (Male; n (%)) | 42 (43.3) | 11 (57.9) | 31 (39.7) | 0.1985 | 0.370 |
| BMI (kg/m2) | 22.2 ± 3.1 | 21.8 ± 2.4 | 22.2 ± 3.2 | 0.6172 | 0.141 |
| SBP (mmHg) | 122.6 ± 16.3 | 130.6 ± 21 | 120.7 ± 14.4 | 0.0163 | 0.550 |
| DBP (mmHg) | 76.1 ± 12.8 | 81.2 ± 14.3 | 74.8 ± 12.1 | 0.0494 | 0.483 |
| MBP (mmHg) | 91.6 ± 13 | 97.7 ± 15.8 | 90.1 ± 11.9 | 0.0224 | 0.543 |
| PP (mmHg) | 46.5 ± 10.9 | 49.4 ± 12.2 | 45.8 ± 10.6 | 0.2099 | 0.315 |
| Laboratory Findings | |||||
| Total protein (g/dL) | 6.63 ± 0.63 | 6.71 ± 0.84 | 6.61 ± 0.57 | 0.5423 | 0.139 |
| Serum albumin (g/dL) | 3.93 ± 0.41 | 3.84 ± 0.48 | 3.95 ± 0.39 | 0.3101 | 0.252 |
| Blood urea nitrogen (mg/dL) | 15.5 ± 6.1 | 17.8 ± 5.7 | 14.9 ± 6.1 | 0.0736 | 0.491 |
| Serum creatinine (mg/dL) | 0.90 ± 0.29 | 1.07 ± 0.31 | 0.86 ± 0.27 | 0.0041 | 0.722 |
| eGFR (mL/min/1.73 m2) | 71.2 ± 19.7 | 58 ± 18 | 74.4 ± 18.9 | 0.0009 | 0.889 |
| Uric acid (mg/dL) | 5.76 ± 1.73 | 6.29 ± 1.65 | 5.63 ± 1.73 | 0.1478 | 0.390 |
| Total cholesterol (mg/dL) | 192.1 ± 37.5 | 197.8 ± 31.8 | 190.7 ± 38.8 | 0.4653 | 0.200 |
| Triglyceride (mg/dL) | 119.2 ± 70 | 154.7 ± 94.9 | 110.6 ± 60.2 | 0.0130 | 0.555 |
| Hemoglobin A1c (NGSP) (%) | 5.39 ± 0.41 | 5.53 ± 0.30 | 5.35 ± 0.42 | 0.1220 | 0.493 |
| IgG (mg/dL) | 1131.6 ± 239.9 | 1158.4 ± 281.4 | 1125.1 ± 230.3 | 0.5900 | 0.130 |
| IgA (mg/dL) | 314.2 ± 110.9 | 387.4 ± 145.3 | 296.4 ± 93.6 | 0.0011 | 0.745 |
| IgM (mg/dL) | 119.7 ± 53.9 | 108.8 ± 53.1 | 122.4 ± 54.1 | 0.3270 | 0.254 |
| CH50 (mg/dL) | 39.5 ± 8.4 | 40.6 ± 8.8 | 39.2 ± 8.3 | 0.5137 | 0.164 |
| C3 (mg/dL) | 97.1 ± 16.8 | 102 ± 22.2 | 95.9 ± 15.2 | 0.1531 | 0.321 |
| C4 (mg/dL) | 22.8 ± 6.3 | 26.3 ± 5.7 | 22 ± 6.2 | 0.0067 | 0.722 |
| IgA/C3 ratio | 3.31 ± 1.13 | 3.86 ± 1.49 | 3.18 ± 0.99 | 0.0174 | 0.538 |
| U-Prot (g/day) | 0.72 (0–4.20) | 0.78 (0–4.20) | 0.70 (0–2.74) | 0.1496 | 0.427 |
| U-RBC (counts/HPF) | 20 (0–100) | 10 (1–100) | 20 (0–100) | 0.2638 | 0.153 |
| U-NAG (U/g·Cre) | 5.9 (1.7–25) | 5.9 (3.4–22.1) | 5.9 (1.7–25) | 0.2769 | 0.352 |
| U-β2MG (µg/g·Cre) | 100 (0–3464.4) | 302.2 (17.7–3464.4) | 98.4 (0–1223.2) | 0.0197 | 0.737 |
| Initial treatments | |||||
| Corticosteroids (n (%)) | 55 (57.3) | 10 (52.6) | 45 (58.4) | 0.7964 | 0.117 |
| Tonsillectomy (n (%)) | 24 (25) | 3 (15.8) | 21 (27.3) | 0.3852 | 0.282 |
| Corticosteroids combined with tonsillectomy (n (%)) | 18 (18.8) | 2 (10.5) | 16 (20.8) | 0.5120 | 0.286 |
| Immunosuppressants (n (%)) | 2 (2.1) | 2 (10.5) | 0 (0) | 0.0375 | 0.484 |
| Concomitant drugs | |||||
| Antihypertensive agents (n (%)) | 70 (72.9) | 16 (84.2) | 54 (70.1) | 0.2623 | 0.341 |
| ARB and or ACEI (n (%)) | 69 (71.9) | 16 (84.2) | 53 (68.8) | 0.2574 | 0.369 |
| CCB (n (%)) | 18 (18.8) | 7 (36.8) | 11 (14.3) | 0.0439 | 0.534 |
| Anti-platelet agents | 61 (63.5) | 13 (68.4) | 48 (62.3) | 0.7914 | 0.128 |
| Anti-coagulation | 5 (5.2) | 2 (10.5) | 3 (3.9) | 0.2561 | 0.257 |
| EPA (n (%)) | 38 (39.6) | 8 (42.1) | 30 (39) | 0.7996 | 0.063 |
| No therapy (n (%)) | 6 (6.3) | 0 (0) | 6 (7.8) | 0.5951 | 0.411 |
| Comorbidities | |||||
| Hypertension (n (%)) | 71 (74) | 16 (84.2) | 55 (71.4) | 0.3829 | 0.312 |
| Hyperuricemia (n (%)) | 39 (41.5) | 12 (63.2) | 27 (36) | 0.0394 | 0.565 |
| Hypertriglyceridemia (n (%)) | 38 (39.6) | 10 (52.6) | 28 (36.4) | 0.2040 | 0.330 |
| Hypercholesterolemia (n (%)) | 29 (30.2) | 8 (42.1) | 21 (27.3) | 0.2654 | 0.315 |
| Variables | Entire Cohort | Max GD ≥245.9 μm | Max GD <245.9 μm | p-Value | Standardized Differences |
|---|---|---|---|---|---|
| n = 97 | n = 19 | n = 78 | |||
| Number of glomeruli | 13 (5–46) | 13 (7–46) | 13 (5–36) | 0.1988 | 0.441 |
| Global sclerosis (%) | 11.1 (0–75) | 13.9 (0–50) | 11.1 (0–75) | 0.1160 | 0.339 |
| Segmental sclerosis or adhesion (%) | 12.5 (0–83.3) | 20 (0–44.4) | 12.5 (0–83.3) | 0.2223 | 0.176 |
| Segmental sclerosis (%) | 0 (0–37.5) | 0 (0–28.6) | 0 (0–37.5) | 0.8250 | 0.020 |
| Adhesion (%) | 12.5 (0–83.3) | 16.7 (0–44.4) | 12.5 (0–83.3) | 0.8172 | 0.036 |
| Crescent (%) | 8.3 (0–55.6) | 14.3 (0–44.4) | 7.1 (0–55.6) | 0.3351 | 0.139 |
| Cellular or Fibro-cellular (%) | 3.9 (0–55.6) | 8.3 (0–33.3) | 0 (0–55.6) | 0.2016 | 0.179 |
| Fibrous (%) | 0 (0–24) | 0 (0–11.1) | 0 (0–24) | 0.4035 | 0.155 |
| Mesangial cell proliferation (0–3) | 1 (0–3) | 2 (0–2) | 1 (0–3) | 0.0598 | 0.396 |
| Mesangial matrix expansion (0–3) | 1 (0–3) | 1.5 (0–2) | 1 (0–3) | 0.5536 | 0.070 |
| Interstitial fibrosis (%) | 14.5 ± 11.5 | 20.1 ± 10.3 | 13.2 ± 11.4 | 0.0172 | 0.635 |
| Interstitial fibrosis (0–3) | 1 (0–3) | 1 (1–2) | 1 (0–3) | 0.0030 | 0.816 |
| Interstitial inflammation (0–3) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.2011 | 0.300 |
| Arteriosclerosis (0–3) | 0 (0–2) | 1 (0–2) | 0 (0–2) | 0.0324 | 0.597 |
| Arteriolar hyalinosis (0–3) | 0 (0–3) | 1 (0–3) | 0 (0–3) | 0.0268 | 0.580 |
| Max GD (µm) | 218.3 ± 27 | 258.4 ± 8.5 | 208.6 ± 20 | <0.0001 | 3.241 |
| Oxford Classification (n = 84) | |||||
| M1 | 40 (47.6) | 11 (64.7) | 29 (43.3) | 0.1735 | 0.440 |
| E1 | 47 (55.9) | 10 (58.8) | 37 (55.2) | 1 | 0.073 |
| S1 | 73 (86.9) | 16 (94.1) | 57 (85.1) | 0.4481 | 0.298 |
| T1 | 14 (16.7) | 5 (29.4) | 9 (13.4) | 0.1458 | 0.398 |
| T2 | 2 (2.4) | 0 (0) | 2 (3) | 1 | 0.249 |
| C1 | 39 (46.4) | 10 (58.8) | 29 (43.3) | 0.2861 | 0.314 |
| C2 | 9 (10.7) | 2 (11.8) | 7 (10.5) | 1 | 0.041 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Clinical and Laboratory Findings | ||||
| Age (10-year increase) | 2.01 (1.29–3.07) | 0.0027 | 0.73 (0.18–2.99) | 0.6513 |
| Men (vs. women) | 1.84 (0.61–5.73) | 0.2741 | 4.86 (0.62–41.58) | 0.1250 |
| BMI (1 kg/m2 increase) | 1.10 (0.94–1.25) | 0.2181 | - | - |
| MBP (10 mmHg increase) | 1.82 (1.18–2.86) | 0.0068 | 0.56 (0.17–1.65) | 0.2798 |
| eGFR (10 mL/min/1.73 m2 increase) | 0.45 (0.31–0.64) | <0.0001 | 0.43 (0.19–0.81) | 0.0085 |
| Hemoglobin (1 g/dL increase) | 0.95 (0.67–1.32) | 0.7849 | - | - |
| Serum albumin (1 g/dL increase) | 0.18 (0.06–0.57) | 0.0041 | 0.02 (0–0.49) | 0.0175 |
| U-Prot (g/day) | 1.86 (1.16–2.79) | 0.0117 | 0.44 (0.12–1.68) | 0.2128 |
| Hypercholesterolemia (vs. no) | 3.68 (1.22–12.21) | 0.0206 | 1.50 (0.03–74.43) | 0.8424 |
| Hypertriglyceridemia (vs. no) | 3.78 (1.23–13.99) | 0.0199 | 7.90 (0.16–568.61) | 0.3407 |
| Hyperuricemia (vs. no) | 6.94 (2.11–31.01) | 0.0011 | 2.71 (0.27–45.47) | 0.4123 |
| Initial treatments | ||||
| Corticosteroids (vs. no) | 0.54 (0.18–1.64) | 0.2763 | - | - |
| Tonsillectomy (vs. no) | 0.36 (0.06–1.35) | 0.1411 | - | - |
| Corticosteroids combined with tonsillectomy (vs. no) | 0.60 (0.09–2.24) | 0.4851 | - | - |
| Immunosuppressants (vs. no) | 10.25 (0.53–64.22) | 0.1009 | - | - |
| Histological findings | ||||
| Global sclerosis (%) | 1.04 (1.01–1.07) | 0.0227 | 0.93 (0.85–0.99) | 0.0434 |
| Segmental sclerosis or adhesion (%) | 1.01 (0.98–1.04) | 0.5240 | - | - |
| Segmental sclerosis (%) | 1.03 (0.97–1.07) | 0.2967 | - | - |
| Adhesion (%) | 0.99 (0.95–1.02) | 0.5415 | - | - |
| Crescent (%) | 1 (0.95–1.03) | 0.9028 | - | - |
| Cellular or Fibro-cellular (%) | 0.99 (0.94–1.04) | 0.8018 | - | - |
| Fibrous (%) | 0.99 (0.86–1.08) | 0.8393 | - | - |
| Mesangial cell proliferation (0–3) | 2.26 (0.84–6.28) | 0.1086 | - | - |
| Mesangial matrix expansion (0–3) | 3.07 (1.29–7.29) | 0.0119 | 0.38 (0.04–2.95) | 0.3684 |
| Interstitial fibrosis (0–3) | 6.06 (2.46–16.17) | <0.0001 | 10.97 (1.36–129.18) | 0.0242 |
| Interstitial inflammation (0–3) | 1.94 (0.80–4.70) | 0.1431 | - | - |
| Arteriosclerosis (0–3) | 2.40 (1.25–4.84) | 0.0085 | 3.10 (0.70–16.38) | 0.1354 |
| Arteriolar hyalinosis (0–3) | 2.78 (1.34–6.07) | 0.0064 | 0.79 (0.08–7.67) | 0.8348 |
| Max GD (10 µm increase) | 1.37 (1.08–1.67) | 0.0069 | 1.51 (1.08–2.36) | 0.0192 |
| Oxford Classification (n = 84) | ||||
| M0/M1 | 2.24 (0.75–7.42) | 0.1498 | NA | - |
| E0/E1 | 0.66 (0.20–1.99) | 0.4662 | NA | - |
| S0/S1 | 1.33 (0.41–5.96) | 0.6566 | NA | - |
| T0/T1/T2 | 4.54 (1.74–11.20) | 0.0031 | NA | - |
| C0/C1/C2 | 1.21 (0.50–2.72) | 0.6636 | NA | - |
| Years | Max GD | Oxford M | Oxford E | Oxford S | Oxford T | Oxford C | Oxford MEST-C | Oxford MEST-C with Max GD |
|---|---|---|---|---|---|---|---|---|
| 2Y | 0.2966 | 0.1380 | 0.1079 | 0.0260 | 0.0392 | 0.0212 | 0.2778 | 0.5017 |
| 3Y | 0.2966 | 0.1380 | 0.1079 | 0.0260 | 0.0392 | 0.0212 | 0.2778 | 0.5017 |
| 4Y | 0.3438 | 0.1598 | 0.1249 | 0.0301 | 0.0307 | 0.0246 | 0.3384 | 0.6011 |
| 5Y | 0.3438 | 0.1598 | 0.1249 | 0.0301 | 0.0307 | 0.0246 | 0.3384 | 0.6011 |
| 6Y | 0.2758 | 0.0819 | 0.0021 | 0.0405 | 0.1001 | 0.0000 | 0.2299 | 0.5242 |
| 7Y | 0.1432 | 0.0520 | 0.0230 | 0.0448 | 0.0962 | 0.0011 | 0.2279 | 0.3655 |
| 8Y | 0.1432 | 0.0520 | 0.0230 | 0.0448 | 0.0962 | 0.0011 | 0.2279 | 0.3655 |
| 9Y | 0.1432 | 0.0520 | 0.0230 | 0.0448 | 0.0962 | 0.0011 | 0.2279 | 0.3655 |
| 10Y | 0.0828 | 0.0259 | 0.0094 | 0.0469 | 0.0727 | 0.0001 | 0.1592 | 0.2509 |
| End | 0.0994 | 0.0201 | 0.0086 | 0.0510 | 0.0772 | 0.0011 | 0.1593 | 0.2523 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, H.; Moriyama, T.; Manabe, S.; Kawachi, K.; Ushio, Y.; Watanabe, S.; Akihisa, T.; Makabe, S.; Sato, M.; Iwasa, N.; et al. Maximum Glomerular Diameter and Oxford MEST-C Score in IgA Nephropathy: The Significance of Time-Series Changes in Pseudo-R2 Values in Relation to Renal Outcomes. J. Clin. Med. 2019, 8, 2105. https://doi.org/10.3390/jcm8122105
Kataoka H, Moriyama T, Manabe S, Kawachi K, Ushio Y, Watanabe S, Akihisa T, Makabe S, Sato M, Iwasa N, et al. Maximum Glomerular Diameter and Oxford MEST-C Score in IgA Nephropathy: The Significance of Time-Series Changes in Pseudo-R2 Values in Relation to Renal Outcomes. Journal of Clinical Medicine. 2019; 8(12):2105. https://doi.org/10.3390/jcm8122105
Chicago/Turabian StyleKataoka, Hiroshi, Takahito Moriyama, Shun Manabe, Keiko Kawachi, Yusuke Ushio, Saki Watanabe, Taro Akihisa, Shiho Makabe, Masayo Sato, Naomi Iwasa, and et al. 2019. "Maximum Glomerular Diameter and Oxford MEST-C Score in IgA Nephropathy: The Significance of Time-Series Changes in Pseudo-R2 Values in Relation to Renal Outcomes" Journal of Clinical Medicine 8, no. 12: 2105. https://doi.org/10.3390/jcm8122105
APA StyleKataoka, H., Moriyama, T., Manabe, S., Kawachi, K., Ushio, Y., Watanabe, S., Akihisa, T., Makabe, S., Sato, M., Iwasa, N., Sawara, Y., Ohara, M., Taneda, S., Uchida, K., Honda, K., Mochizuki, T., Tsuchiya, K., & Nitta, K. (2019). Maximum Glomerular Diameter and Oxford MEST-C Score in IgA Nephropathy: The Significance of Time-Series Changes in Pseudo-R2 Values in Relation to Renal Outcomes. Journal of Clinical Medicine, 8(12), 2105. https://doi.org/10.3390/jcm8122105






