Influence of Antisynthetase Antibodies Specificities on Antisynthetase Syndrome Clinical Spectrum Time Course
Abstract
:1. Background
2. Methods
2.1. Patients
2.2. Manifestation’s Definition
2.2.1. Triad Findings
2.2.2. Accompanying Findings
2.3. Laboratory Tests
2.4. Statistical Analysis
3. Results
3.1. ARS Groups’ Characteristics
3.1.1. Anti-Jo1 ARS
3.1.2. Anti-PL7 ARS
3.1.3. Anti-PL12 ARS
3.1.4. Anti-EJ ARS
3.1.5. Anti-OJ ARS
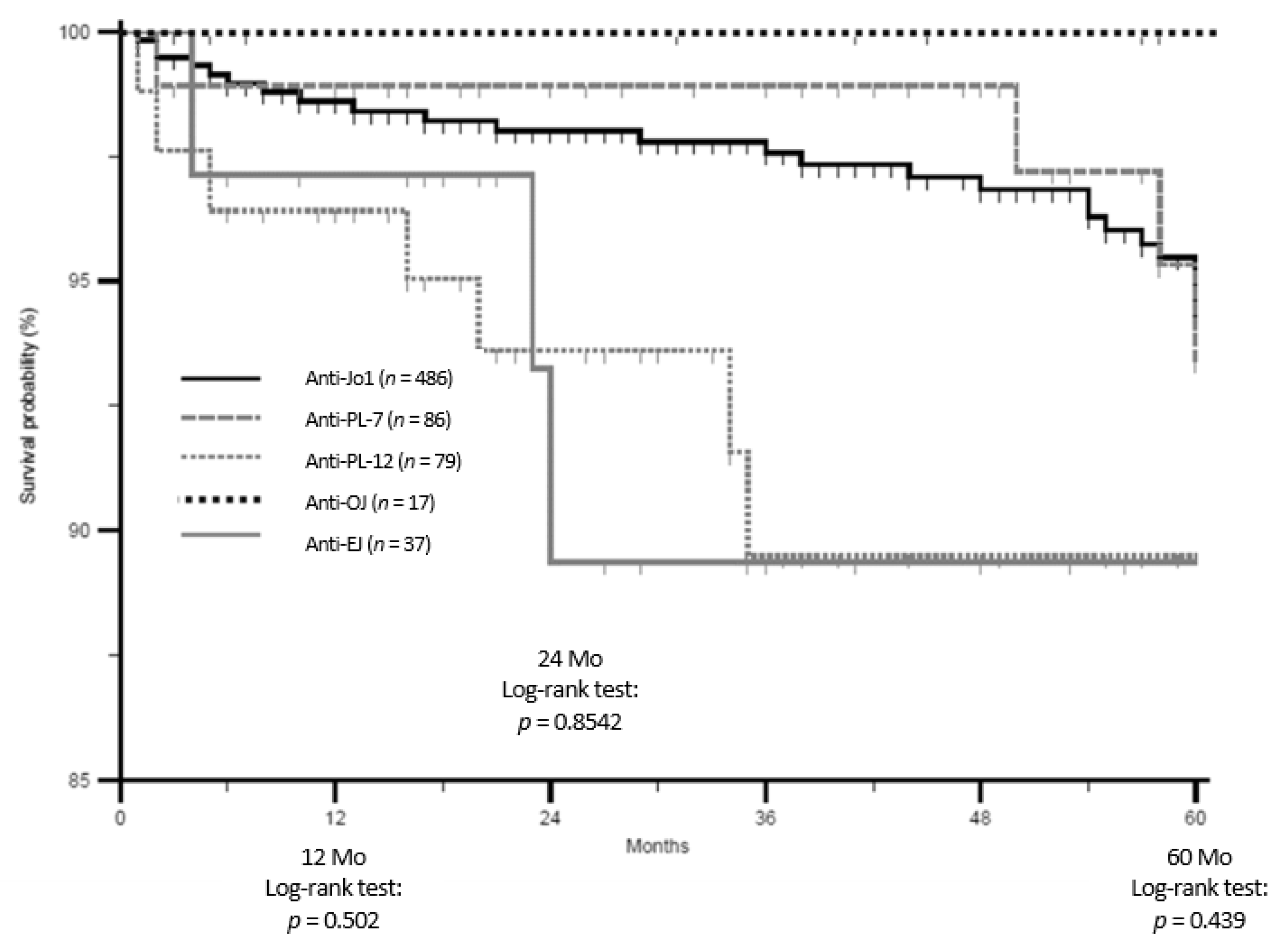
3.2. Groups Comparisons: Main Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Imbert-Masseau, A.; Hamidou, M.; Agard, C.; Grolleau, J.Y.; Chérin, P. Antisynthetase syndrome. Jt. Bone Spine 2003, 70, 161–168. [Google Scholar] [CrossRef]
- Monti, S.; Montecucco, C.; Cavagna, L. Clinical spectrum of anti-Jo-1-associated disease. Curr. Opin. Rheumatol. 2017, 29, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, L.; Nuño, L.; Scirè, C.A.; Govoni, M.; Longo, F.J.L.; Franceschini, F.; Neri, R.; Castañeda, S.; Giraldo, W.A.S.; Caporali, R.; et al. Serum Jo-1 autoantibody and isolated arthritis in the antisynthetase syndrome: Review of the literature and report of the experience of AENEAS collaborative group. Clin. Rev. Allergy Immunol. 2017, 52, 71–80. [Google Scholar] [CrossRef]
- Lefèvre, G.; Meyer, A.; Launay, D.; Machelart, I.; DeBandt, M.; Michaud, J.; Tournadre, A.; Godmer, P.; Kahn, J.E.; Behra-Marsac, A.; et al. Seronegative polyarthritis revealing antisynthetase syndrome: A multicentre study of 40 patients. Rheumatology 2015, 54, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Lefevre, G.; Bierry, G.; Duval, A.; Ottaviani, S.; Meyer, O.; Tournadre, A.; Le Goff, B.; Messer, L.; Buchdahl, A.L.; et al. In antisynthetase syndrome, ACPA are associated with severe and erosive arthritis: An overlapping rheumatoid arthritis and antisynthetase syndrome. Medicine 2015, 94, e523. [Google Scholar] [CrossRef]
- González-Gay, M.A.; Montecucco, C.; Selva-O’Callaghan, A.; Trallero-Araguas, E.; Molberg, O.; Andersson, H.; Rojas-Serrano, J.; Perez-Roman, D.I.; Bauhammer, J.; Fiehn, C.; et al. Timing of onset affects arthritis presentation pattern in antisyntethase syndrome. Clin. Exp. Rheumatol. 2018, 36, 44–49. [Google Scholar]
- Cavagna, L.; Nuno, L.; Scire, C.A.; Govoni, M.; Longo, F.J.L.; Franceschini, F.; Neri, R.; Castaneda, S.; Giraldo, W.A.S.; Caporali, R.; et al. Clinical spectrum time course in anti Jo-1 positive antisynthetase syndrome: Results from an international retrospective multicenter study. Medicine 2015, 94, e1144. [Google Scholar] [CrossRef]
- Hervier, B.; Devilliers, H.; Stanciu, R.; Meyer, A.; Uzunhan, Y.; Masseau, A.; Dubucquoi, S.; Hatron, P.Y.; Musset, L.; Wallaert, B.; et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: Phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun. Rev. 2012, 12, 210–217. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Huapaya, J.A.; Albayda, J.; Paik, J.J.; Johnson, C.; Silhan, L.; Christopher-Stine, L.; Mammen, A.L.; Danoff, S.K. A longitudinal cohort study of the anti-synthetase syndrome: Increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology 2017, 56, 999–1007. [Google Scholar] [CrossRef]
- Trallero-Araguás, E.; Grau-Junyent, J.M.; Labirua-Iturburu, A.; García-Hernández, F.J.; Monteagudo-Jiménez, M.; Fraile-Rodriguez, G.; Les-Bujanda, I.; Rodriguez-Carballeira, M.; Sáez-Comet, L.; Selva-O’Callaghan, A.; et al. Clinical manifestations and long-term outcome of anti-Jo1 antisynthetase patients in a large cohort of Spanish patients from the GEAS-IIM group. Semin. Arthritis Rheum. 2016, 46, 225–231. [Google Scholar] [CrossRef]
- Aggarwal, R.; Dhillon, N.; Fertig, N.; Koontz, D.; Qi, Z.; Oddis, C.V. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: Role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J. Rheumatol. 2017, 44, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Cavagna, L.; González-Gay, M.A. New criteria needed for antisynthetase syndrome. JAMA Neurol. 2018, 75, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, L.; Castañeda, S.; Sciré, C.; Gonzalez-Gay, M.A. AENEAS Collaborative Group Members. Antisynthetase syndrome or what else? Different perspectives indicate the need for new classification criteria. Ann. Rheum. Dis. 2018, 77, e50. [Google Scholar] [PubMed]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; Du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Scirè, C.A.; Gonzalez-Gay, M.A.; Selva-O’Callaghan, A.; Cavagna, L. Clinical spectrum time course of interstitial pneumonia with autoimmune features in patients positive for antisynthetase antibodies. Respir. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 2017, 69, 2271–2282. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Fujimoto, M.; Matsushita, T.; Kaji, K.; Komura, K.; Hasegawa, M.; Kodera, M.; Muroi, E.; Fujikawa, K.; Seishima, M.; et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: Heterogeneity within the syndrome. PLoS ONE 2013, 8, e60442. [Google Scholar] [CrossRef]
- Cavagna, L.; Caporali, R.; Abdì-Alì, L.; Dore, R.; Meloni, F.; Montecucco, C. Cyclosporine in anti-Jo1-positive patients with corticosteroid-refractory interstitial lung disease. J. Rheumatol. 2013, 40, 484–492. [Google Scholar] [CrossRef]
- Marie, I.; Josse, S.; Decaux, O.; Dominique, S.; Diot, E.; Landron, C.; Roblot, P.; Jouneau, S.; Hatron, P.Y.; Tiev, K.P.; et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun. Rev. 2012, 11, 739–745. [Google Scholar] [CrossRef]
- Aggarwal, R.; Cassidy, E.; Fertig, N.; Koontz, D.C.; Lucas, M.; Ascherman, D.P.; Oddis, C.V. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann. Rheum. Dis. 2014, 73, 227–232. [Google Scholar] [CrossRef]
- Shi, J.; Li, S.; Yang, H.; Zhang, Y.; Peng, Q.; Lu, X.; Wang, G. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J. Rheumatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emerg. Med. J. 2003, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana, I.; Fredi, M.; Ceribelli, A.; Mordenti, C.; Ferrari, F.; Carabellese, N.; Tincani, A.; Satoh, M.; Franceschini, F. Testing for myositis specific autoantibodies: Comparison between line blot and immunoprecipitation assays in 57 myositis sera. J. Immunol. Methods 2016, 433, 1–5. [Google Scholar] [CrossRef] [PubMed]
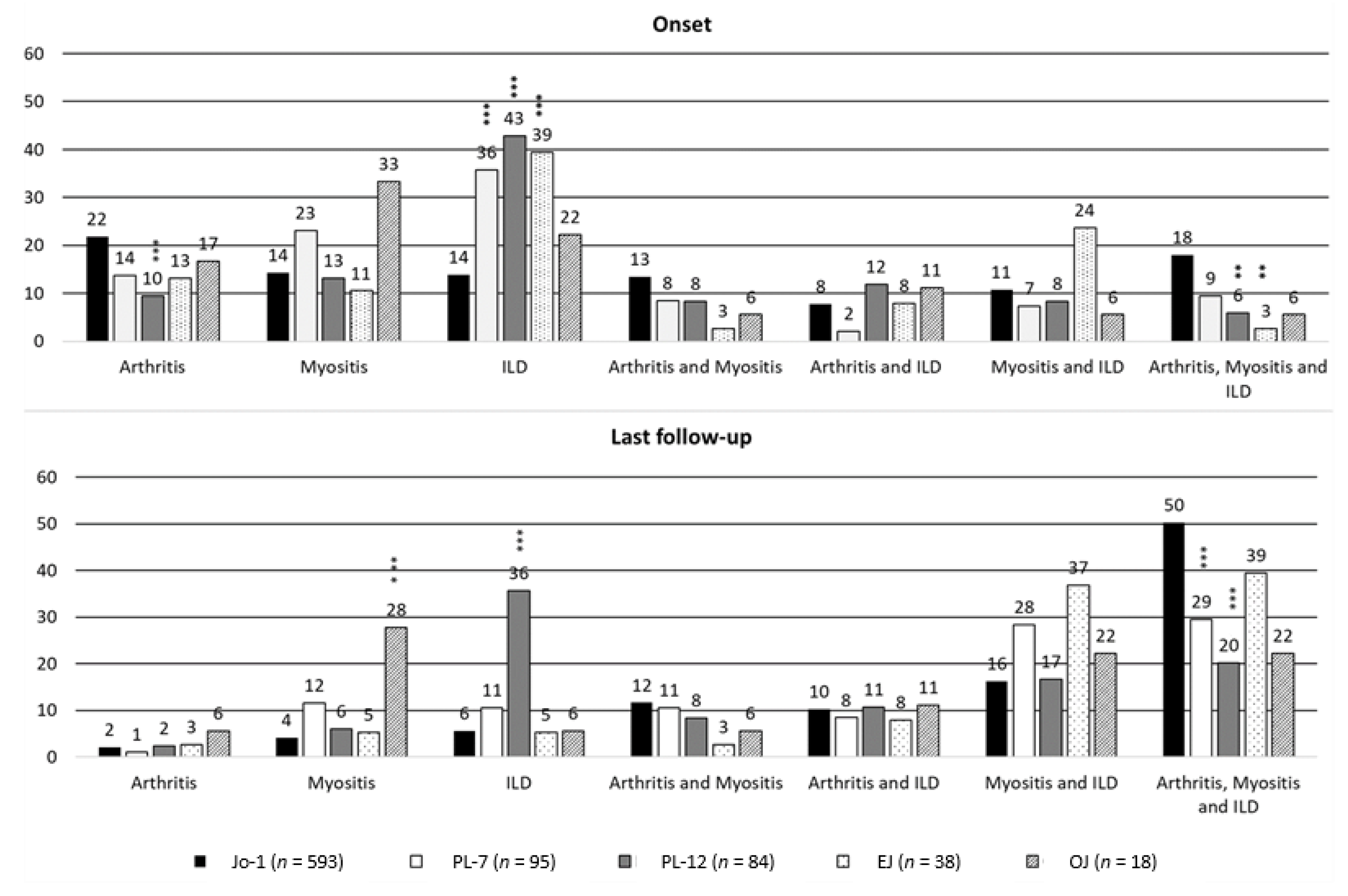
| Onset Characteristics | Anti-Jo-1 ARS (n = 593) | Anti-PL-7 ARS (n = 95) | Anti-PL-12 ARS (n = 84) | Anti-EJ ARS (n = 38) | Anti-OJ ARS (n = 18) | Test; p-Value; df |
|---|---|---|---|---|---|---|
| § Females (%) | 433 (73.1) | 71 (74.7) | 62 (73.8) | 29 (76.3) | 13 (72.2) | χ2 = 0.29; 0.99; 4 |
| Median age in years at disease onset (IQR) | 51.0 (41.0–61.0) | 53.0 (44.0–63.0) | 50.5 (42.5–61.5) | 54.5 (46.0–62.0) | 57.0 (47.0–67.0) | ^χ2 = 5.09; 0.28; 4 |
| Median diagostic delay in months (IQR) | 5.0 (2.0–15) | 12.0 (5.0–41.0) | 10.0 (4.0–26.0) | 6.0 (2.0–12.0) | 8.0 (2.0–58.0) | ^χ2 = 41.60; 0.0001; 4 |
| comparison vs Anti-Jo-1 ARS | reference | *p < 0.0001 | *p = 0.0002 | p = 0.95 | p = 0.10 | |
| ANA positive (%) | 350 (60.3) | 58 (64.4) | 49 (59.4) | 21 (60.00) | 7 (38.9) | χ2 = 4.14; 0.39; 4 |
| ANA negative (%) | 230 (39.7) | 32 (35.6) | 34 (41.0) | 14 (40.0) | 11 (61.1) | |
| Anti Ro positive (%) | 301 (51.3) | 50 (54.3) | 44 (59.2) | 19 (50.0) | 4 (22.2) | χ2 = 8.32; 0.08; 4 |
| Anti Ro negative (%) | 286 (48.7) | 42 (45.7) | 31 (40.8) | 19(50.0) | 14 (77.8) | |
| Arthritis (%) | 362 (61.1) | 32 (33.7) | 30 (35.7) | 10 (26.3) | 7 (38.9) | χ2 = 52.02; <0.0001; 4 |
| comparison vs Anti-Jo1-1 ARS | reference | *p < 0.0001 | *p < 0.0001 | *p < 0.0001 | p = 0.06 | |
| Symmetrical polyarthritis (%) | 253 (71.5) | 21 (65.6) | 20 (71.4) | 8 (80.0) | 2 (28.6) | Fisher exact test p = 0.20 |
| Oligoarticular/asymmetrical arthritis (%) | 101 (28.5) | 11 (34.4) | 8 (28.6) | 2 (20.0) | 5 (71.4) | |
| IgM-RF positive (%) | 91 (26.2) | 9 (32.1) | 5 (19.2) | 4 (40.0) | 3 (50.0) | Fisher exact test p = 0.39 |
| IgM-RF negative (%) | 256 (73.8) | 19 (67.9) | 21 (80.8) | 6 (60.0) | 3 (50.0) | |
| ACPA positive (%) | 34 (11.2) | 5 (20.8) | 1 (3.8) | 2 (25.0) | 0 (0) | Fisher exact test p = 0.20 |
| ACPA negative (%) | 270 (88.8) | 19 (79.2) | 25 (96.2) | 6 (75.0) | 6 (100) | |
| Myositis (%) | 336 (56.7) | 46 (48.4) | 30 (35.7) | 15 (39.5) | 9 (50.0) | χ2 = 16.87; 0.002; 4 |
| comparison vs Anti-Jo-1 ARS | reference | P = 0.13 | *p < 0.0001 | p = 0.04 | p = 0.57 | |
| Hypomyopathic onset (%) | 51 (15.2) | 12 (26.1) | 9 (30.0) | 3 (20.0) | 3 (33.3) | Fisher exact test p = 0.06 |
| Classic onset (%) | 284 (84.8) | 34 (73.9) | 21 (70.0) | 12 (80.0) | 6 (67.7) | |
| Interstitial Lung Disease (%) | 299 (50.4) | 52 (54.7) | 57 (69.0) | 28 (73.7) | 8 (44.4) | χ2 = 17.29; 0.002; 4 |
| comparison vs Anti-Jo-1 ARS | reference | p = 0.43 | *p = 0.001 | *p = 0.005 | p= 0.62 | |
| Acute onset (%) | 132 (44.6) | 25 (48.1) | 28 (48.3) | 20 (74.1) | 3 (37.5) | Fisher exact test p = 0.14 |
| Chronic onset (%) | 114 (38.5) | 18 (34.6) | 25 (43.1) | 6 (22.2) | 4 (50.0) | |
| Asymptomatic onset (%) | 50 (16.9) | 9 (17.3) | 5 (8.6) | 1 (3.7) | 1 (3.7) |
| Last Follow-Up Characteristics | Anti-Jo-1 ARS (n = 593) | Anti-PL-7 ARS (n= 95) | Anti-PL-12 ARS (n = 84) | Anti-EJ ARS (n = 38) | Anti-OJ ARS (n= 18) | Test; p-Value; df |
|---|---|---|---|---|---|---|
| Median disease duration in months (IQR) | 72 (30–136) | 61 (26–107) | 37.5 (20–73.5) | 35.5 (17–102) | 57.5 (9–118) | ^χ2 = 19.29; 0.0007; 4 |
| comparison vs Anti-Jo-1 ARS | reference | 0.320 | *<0.001 | 0.021 | 0.334 | |
| Arthritis (%) | 440 (74.2) | 47 (49.5) | 35 (41.7) | 20 (52.6) | 8 (44.4) | χ2 = 58.54; <0.001; 4 |
| comparison vs Anti-Jo-1 ARS | reference | *<0.001 | *<0.001 | *0.004 | *0.005 | |
| Symmetrical polyarthritis (%) | 294 (69.0) | 31 (66.0) | 23 (69.7) | 14 (77.8) | 3 (37.5) | χ2 = 4.50; 0.342; 4 |
| Oligoarticular/asymmetrical arthritis (%) | 132 (31.0) | 16 (34.0) | 10 (30.3) | 4 (22.2) | 5 (62.5) | |
| IgM-RF positive (%) | 107 (25.4) | 12 (28.6) | 5 (16.7) | 7 (36.8) | 3 (42.9) | Fisher exact test p = 0.392 |
| IgM-RF negative (%) | 314 (74.6) | 30 (71.4) | 25 (83.3) | 12 (63.2) | 4 (57.1) | |
| ACPA positive (%) | 37 (10.3) | 8 (22.2) | 2 (6.4) | 2 (15.4) | 0 (0.0) | Fisher exact test p = 0.185 |
| ACPA negative (%) | 323 (89.7) | 28 (77.8) | 29 (93.6) | 11 (84.6) | 7 (100.0) | |
| Patients with X-rays joint erosions (%) | 58 (15.3) | 4 (11.4) | 1 (4.3) | 2 (20.0) | 2 (28.6) | Fisher exact test p = 0.360 |
| Patients without X-rays joint erosions (%) | 320 (84.7) | 31 (85.6) | 22 (95.6) | 8 (80.0) | 5 (71.4) | |
| Myositis (%) | 487 (82.1) | 76 (80.0) | 43 (51.2) | 32 (84.2) | 14 (77.8) | χ2 = 42.93; <0.001; 4 |
| comparison vs Anti-Jo-1 ARS | reference | 0.62 | *<0.001 | 0.636 | 0.74 | |
| Hypomyopathic onset (%) | 97 (20.0) | 23 (30.7) | 12 (27.9) | 6 (18.7) | 5 (35.7) | χ2 = 7.01; 0.135; 4 |
| Classic onset (%) | 388 (80.0) | 52 (69.3) | 31 (72.1) | 26 (81.3) | 9 (64.3) | |
| Interstitial Lung Disease (%) | 486 (82.0) | 73 (76.8) | 70 (83.3) | 34 (89.5) | 11 (61.1) | χ2 = 8.16; 0.086; 4 |
| Acute onset (%) | 179 (37.3) | 28 (38.9) | 34 (48.6) | 21 (63.6) | 4 (36.4) | Fisher exact test p < 0.001 |
| Chronic onset (%) | 201 (41.9) | 32 (44.4) | 28 (40.0) | 11 (33.3) | 6 (54.6) | |
| Asymptomatic onset (%) | 100 (20.8) | 12 (16.7) | 8 (11.4) | 1 (3.0) | 1 (9.1) | |
| comparison vs Anti-Jo-1 ARS | reference | 0.713 | 0.09 | *p = 0.003 | p = 0.623 | |
| Incomplete ASSD with“ex-novo”triad findings (%) | 302 (62.1) | 50 (58.1) | 24 (30.4) | 23 (62.2) | 7 (41.2) | χ2 = 30.27; <0.001; 4 |
| Incomplete ASSD without“ex-novo”triad findings (%) | 184 (37.9) | 36 (41.9) | 55 (69.6) | 14 (37.8) | 10 (58.8) | |
| comparison vs Anti-Jo-1 ARS | reference | 0.482 | *<0.001 | 0.998 | 0.081 | |
| “ex-novo”arthritis (%) | 78 (33.8) | 15 (23.8) | 5 (9.3) | 10 (35.7) | 1 (9.1) | χ2 = 16.48; 0.002; 4 |
| no arthritis (%) | 153 (66.2) | 48 (76.2) | 49 (90.7) | 18 (64.3) | 10 (90.9) | |
| comparison vs Anti-Jo-1 ARS | reference | 0.13 | *<0.001 | 0.837 | *0.005 | |
| “ex-novo”myositis (%) | 151 (58.8) | 30 (61.2) | 13 (24.1) | 17 (73.9) | 5 (55.6) | χ2 = 26.43; <0.001; 4 |
| no myositis (%) | 106 (41.3) | 19 (38.8) | 41 (75.9) | 6 (26.1) | 4 (44.4) | |
| comparison vs Anti-Jo-1 ARS | reference | 0.75 | *<0.001 | 0.155 | p = 1.00 | |
| “ex-novo”Interstitial lung disease (%) | 187 (63.6) | 21 (48.8) | 12 (46.2) | 6 (60.0) | 3 (30.0) | χ2 = 9.63; 0.047; 4 |
| no Interstitial lung disease (%) | 107 (36.4) | 22 (51.2) | 14 (53.9) | 4 (40.0) | 7 (70.0) | |
| comparison vs Anti-Jo-1 ARS | reference | 0.063 | 0.079 | 0.816 | 0.031 | |
| Accompanying features (%) | 416 (70.9) | 70 (76.1) | 62 (77.5) | 27 (71.1) | 9 (50.0) | χ2 = 6.58; 0.160; 4 |
| No accompanying findings (%) | 171 (29.1) | 22 (23.9) | 18 (22.5) | 11 (29.0) | 9 (50.0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavagna, L.; Trallero-Araguás, E.; Meloni, F.; Cavazzana, I.; Rojas-Serrano, J.; Feist, E.; Zanframundo, G.; Morandi, V.; Meyer, A.; Pereira da Silva, J.A.; et al. Influence of Antisynthetase Antibodies Specificities on Antisynthetase Syndrome Clinical Spectrum Time Course. J. Clin. Med. 2019, 8, 2013. https://doi.org/10.3390/jcm8112013
Cavagna L, Trallero-Araguás E, Meloni F, Cavazzana I, Rojas-Serrano J, Feist E, Zanframundo G, Morandi V, Meyer A, Pereira da Silva JA, et al. Influence of Antisynthetase Antibodies Specificities on Antisynthetase Syndrome Clinical Spectrum Time Course. Journal of Clinical Medicine. 2019; 8(11):2013. https://doi.org/10.3390/jcm8112013
Chicago/Turabian StyleCavagna, Lorenzo, Ernesto Trallero-Araguás, Federica Meloni, Ilaria Cavazzana, Jorge Rojas-Serrano, Eugen Feist, Giovanni Zanframundo, Valentina Morandi, Alain Meyer, Jose Antonio Pereira da Silva, and et al. 2019. "Influence of Antisynthetase Antibodies Specificities on Antisynthetase Syndrome Clinical Spectrum Time Course" Journal of Clinical Medicine 8, no. 11: 2013. https://doi.org/10.3390/jcm8112013
APA StyleCavagna, L., Trallero-Araguás, E., Meloni, F., Cavazzana, I., Rojas-Serrano, J., Feist, E., Zanframundo, G., Morandi, V., Meyer, A., Pereira da Silva, J. A., Matos Costa, C. J., Molberg, O., Andersson, H., Codullo, V., Mosca, M., Barsotti, S., Neri, R., Scirè, C., Govoni, M., ... Gonzalez-Gay, M. A. (2019). Influence of Antisynthetase Antibodies Specificities on Antisynthetase Syndrome Clinical Spectrum Time Course. Journal of Clinical Medicine, 8(11), 2013. https://doi.org/10.3390/jcm8112013





















