Advanced MRI Assessment during Dendritic Cell Immunotherapy Added to Standard Treatment Against Glioblastoma
Abstract
:1. Introduction
2. Experimental Section
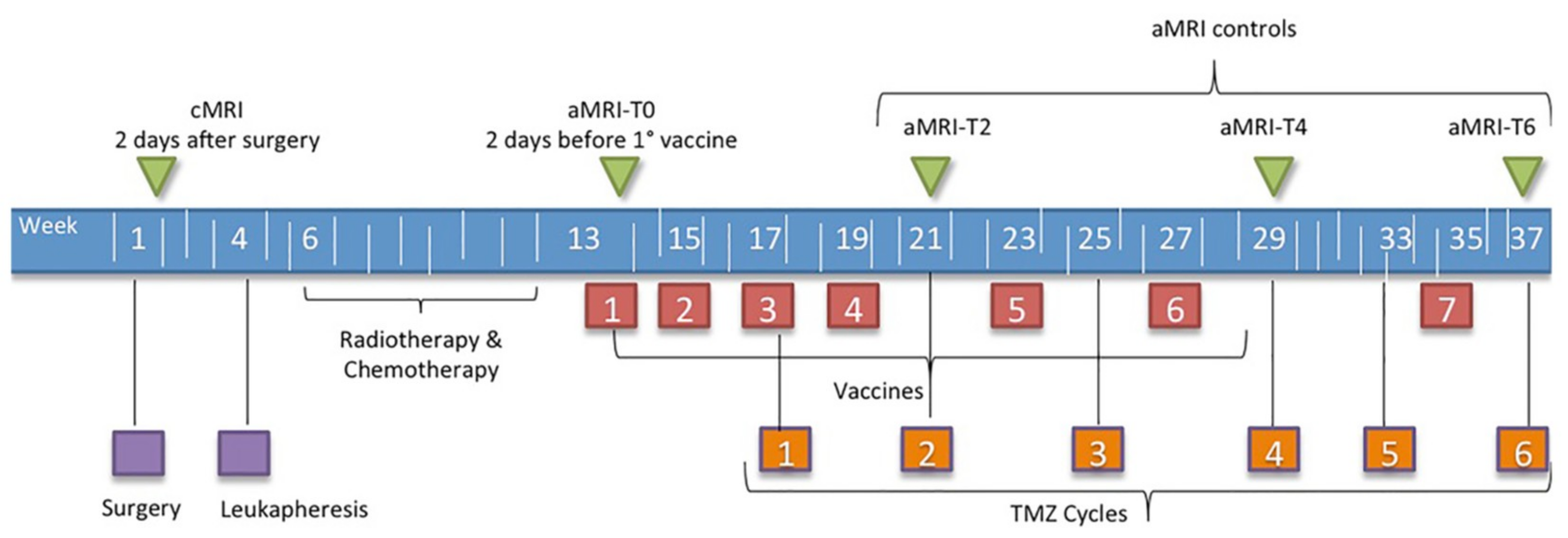
2.1. Patients and Treatment Strategies
2.2. MRI Acquisition
2.3. Post-Processing
2.3.1. Volume Estimation
2.3.2. Perfusion
2.3.3. Diffusion
2.4. RANO Criteria
2.5. Immune Monitoring
2.6. Statistical Analysis
3. Results
3.1. Clinical Data and Conventional MRI Assessment
3.2. Advanced MRI Response Assessment and Stratification of Survival
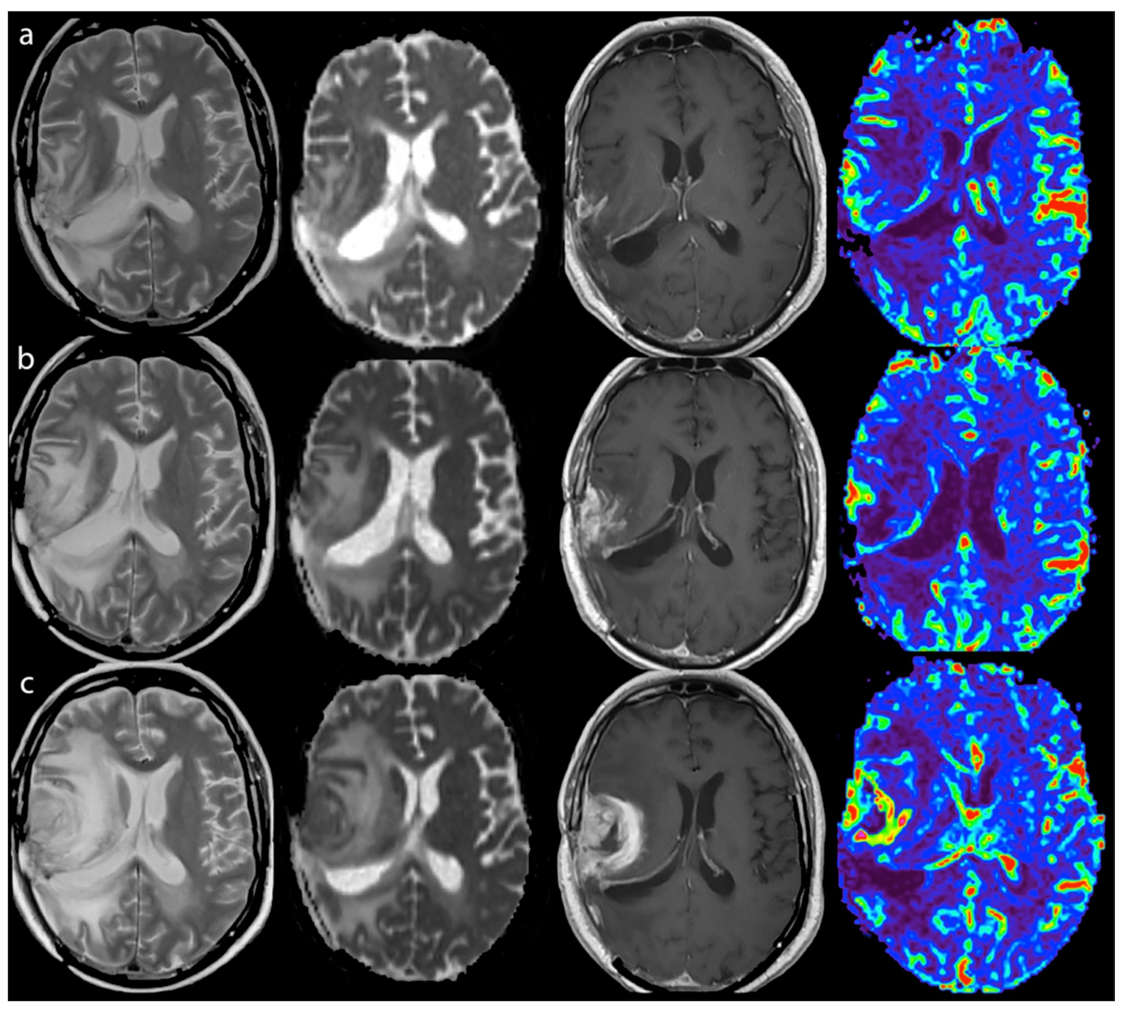
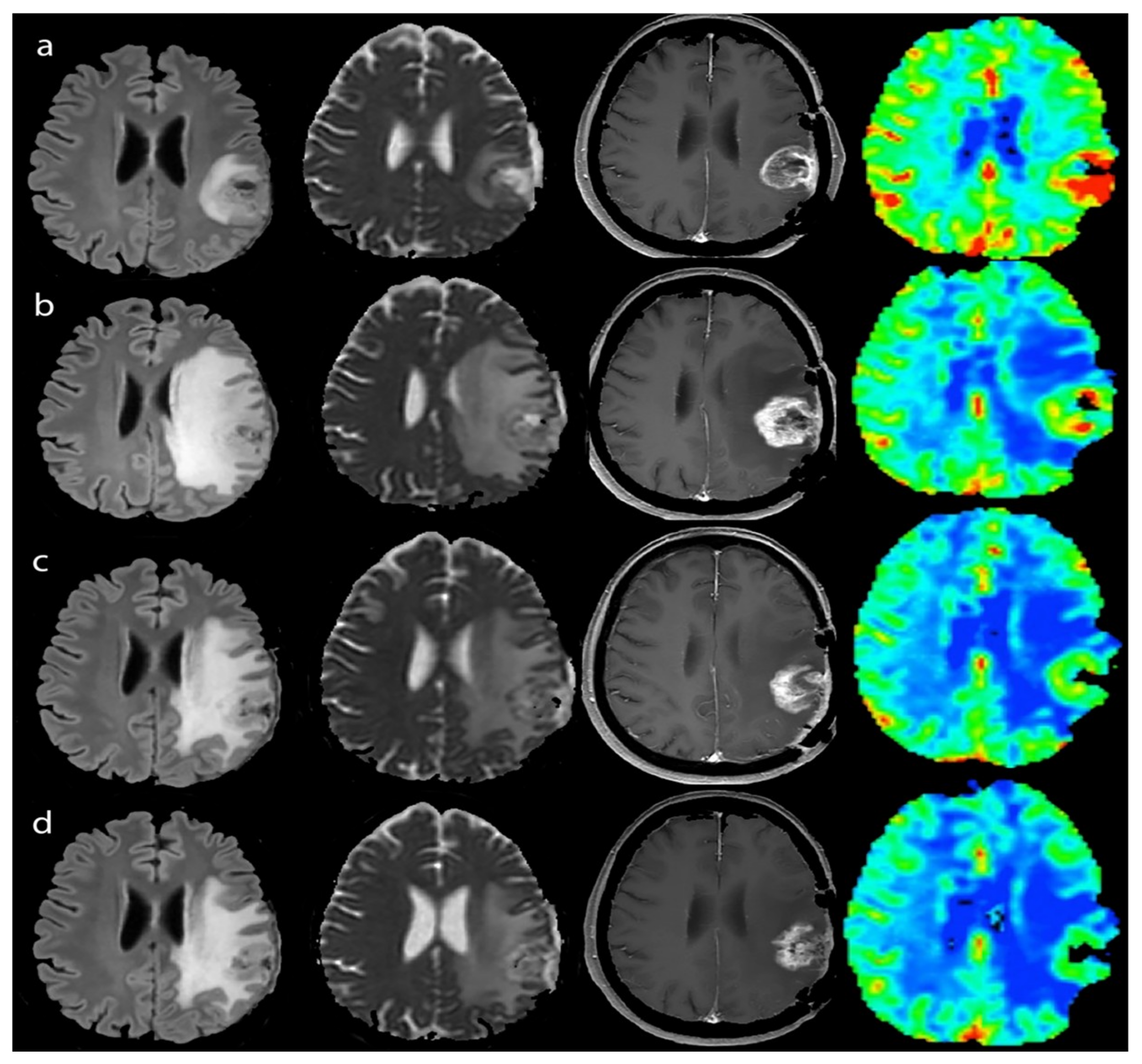
3.2.1. Response Assessment
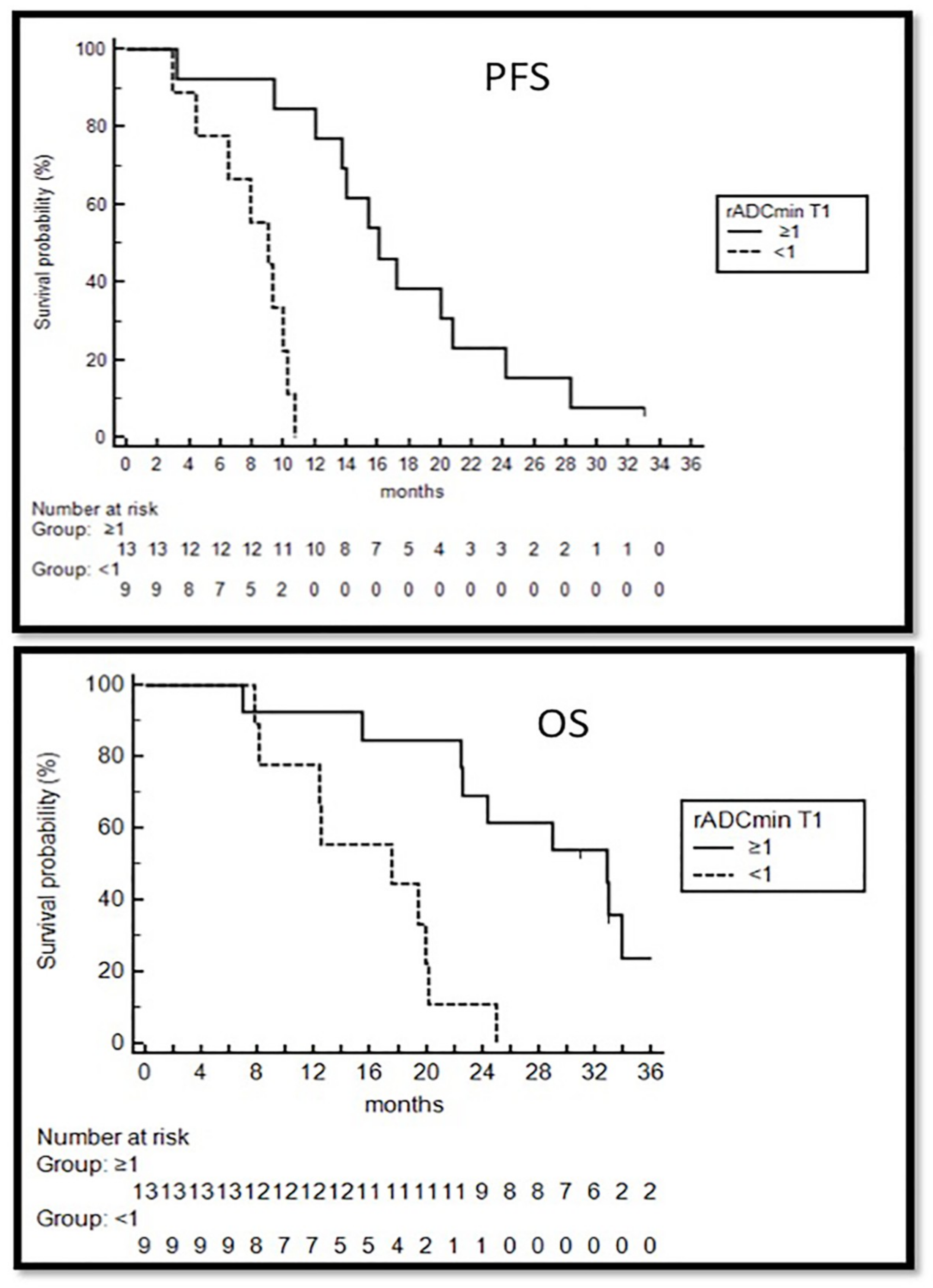
3.2.2. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, L.W.; Chow, K.K.H.; Lim, M.; Li, G. Current vaccine trials in glioblastoma: A review. J. Immunol. Res. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Weller, M.; Chiocca, E.A. Current state of immune-based therapies for glioblastoma. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e132–e139. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbe, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, X.; Stroiney, A.; Qu, J.; Helgager, J.; Reardon, D.A.; Young, G.S. Advanced MRI assessment to predict benefit of anti-programmed cell death 1 protein immunotherapy response in patients with recurrent glioblastoma. Neuroradiology 2017, 59, 135–145. [Google Scholar] [CrossRef]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef]
- Pellegatta, S.; Eoli, M.; Cuccarini, V.; Anghileri, E.; Pollo, B.; Pessina, S.; Frigerio, S.; Servida, M.; Cuppini, L.; Antozzi, C.; et al. Survival gain in glioblastoma patients treated with dendritic cell immunotherapy is associated with increased NK but not CD8 + T cell activation in the presence of adjuvant temozolomide. Oncoimmunology 2018, 7, e1412901. [Google Scholar] [CrossRef]
- Lucas, M.; Schachterle, W.; Oberle, K.; Aichele, P.; Diefenbach, A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 2007, 26, 503–517. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant Temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Lee, J.; Choi, S.H.; Kim, J.-H.; Sohn, C.-H.; Lee, S.; Jeong, J. Glioma grading using apparent diffusion coefficient map: Application of histogram analysis based on automatic segmentation. NMR Biomed. 2014, 27, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Dossena, M.; Pogliani, S.; Pellegatta, S.; Antozzi, C.; Baggi, F.; Gellera, C.; Pollo, B.; Parati, E.A.; Finocchiaro, G.; et al. An optimized method for manufacturing a clinical scale dendritic cell-based vaccine for the treatment of glioblastoma. PLoS ONE 2012, 7, e52301. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J. Neurooncol. 2017, 134, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017, 14, 307–320. [Google Scholar] [CrossRef]
- Huang, R.Y.; Neagu, M.R.; Reardon, D.A.; Wen, P.Y. Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and immuno/targeted therapy - detecting illusive disease, defining response. Front. Neurol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Roth, P.; Valavanis, A.; Weller, M. Long-term control and partial remission after initial pseudoprogression of glioblastoma by anti–PD-1 treatment with nivolumab. Neuro Oncol. 2017, 19, 454–456. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; Van Den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Sugahara, T.; Korogi, Y.; Tomiguchi, S.; Shigematsu, Y.; Ikushima, I.; Kira, T.; Liang, L.; Ushio, Y.; Takahashi, M. Posttherapeutic intraaxial brain tumor: The value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR. Am. J. Neuroradiol. 2000, 21, 901–909. [Google Scholar]
- Barajas, R.F.; Chang, J.S.; Segal, M.R.; Parsa, A.T.; McDermott, M.W.; Berger, M.S.; Cha, S. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced Perfusion MR Imaging. Radiology 2009, 253, 486–496. [Google Scholar] [CrossRef]
- Gasparetto, E.L.; Pawlak, M.A.; Patel, S.H.; Huse, J.; Woo, J.H.; Krejza, J.; Rosenfeld, M.R.; O’Rourke, D.M.; Lustig, R.; Melhem, E.R.; et al. Posttreatment recurrence of malignant brain neoplasm: Accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology 2009, 250, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Mangla, R.; Singh, G.; Ziegelitz, D.; Milano, M.T.; Korones, D.N.; Zhong, J.; Ekholm, S.E. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology 2010, 256, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Tsien, C.; Galbán, C.J.; Chenevert, T.L.; Johnson, T.D.; Hamstra, D.A.; Sundgren, P.C.; Junck, L.; Meyer, C.R.; Rehemtulla, A.; Lawrence, T.; et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J. Clin. Oncol. 2010, 28, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Baradaran, H.; Delgado, D.; Askin, G.; Christos, P.; John Tsiouris, A.; Gupta, A. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: A systematic review and meta-analysis. Neuro Oncol. 2017, 19, 118–127. [Google Scholar] [CrossRef]
- Boxerman, J.L.; Ellingson, B.M.; Jeyapalan, S.; Elinzano, H.; Harris, R.J.; Rogg, J.M.; Pope, W.B.; Safran, H. Longitudinal DSC-MRI for distinguishing tumor recurrence from pseudoprogression in patients with a high-grade glioma. Am. J. Clin. Oncol. 2017, 40, 228–234. [Google Scholar] [CrossRef]
- Di Chiro, G.; Oldfield, E.; Wright, D.; De Michele, D.; Katz, D.; Patronas, N.; Doppman, J.; Larson, S.; Ito, M.; Kufta, C. Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. Am. J. Roentgenol. 1988, 150, 189–197. [Google Scholar] [CrossRef]
- Hein, P.A.; Eskey, C.J.; Dunn, J.F.; Hug, E.B. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: Tumor recurrence versus radiation injury. AJNR Am. J. Neuroradiol. 2004, 25, 201–209. [Google Scholar]
- Asao, C.; Korogi, Y.; Kitajima, M.; Hirai, T.; Baba, Y.; Makino, K.; Kochi, M.; Morishita, S.; Yamashita, Y. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR. Am. J. Neuroradiol. 2005, 26, 1455–1460. [Google Scholar]
- Lee, W.; Choi, S.H.; Park, C.K.; Yi, K.S.; Kim, T.M.; Lee, S.H.; Kim, J.H.; Sohn, C.H.; Park, S.H.; Kim, I.H. Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with Temozolomide in patients with newly diagnosed high-grade gliomas. Acad. Radiol. 2012, 19, 1353–1361. [Google Scholar] [CrossRef]
- Vrabec, M.; Van Cauter, S.; Himmelreich, U.; Van Gool, S.W.; Sunaert, S.; De Vleeschouwer, S.; Šuput, D.; Demaerel, P. MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: A pilot study. Neuroradiology 2011, 53, 721–731. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Harris, R.J.; Woodworth, D.C.; Leu, K.; Zaw, O.; Mason, W.P.; Sahebjam, S.; Abrey, L.E.; Aftab, D.T.; Schwab, G.M.; et al. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: Evidence from single and multicenter trials. Neuro Oncol. 2017, 19, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Malkin, M.G.; Rand, S.D.; Connelly, J.M.; Quinsey, C.; LaViolette, P.S.; Bedekar, D.P.; Schmainda, K.M. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J. Magn. Reson. Imaging 2010, 31, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Schuessler, A.; Walker, D.G.; Khanna, R. Cellular immunotherapy directed against human cytomegalovirus as a novel approach for glioblastoma treatment. Oncoimmunology 2014, 3, e29381. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.A.; Cepeda, L.; Owens, B.; Johnson, S.D.; Stears, J.; Lillehei, K.O. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol. Immunother. 1997, 45, 77–87. [Google Scholar] [CrossRef]
- Agur, Z.; Halevi-Tobias, K.; Kogan, Y.; Shlagman, O. Employing dynamical computational models for personalizing cancer immunotherapy. Expert Opin. Biol. Ther. 2016, 16, 1373–1385. [Google Scholar] [CrossRef]
| HighNK | LowNK | |
|---|---|---|
| N° | 13 | 9 |
| F/M | 3/10 | 3/6 |
| Age | 54.7 (36–70) | 54.7 (45–62) |
| KPS | 90 | 90 |
| MGMT Hypermethylation | 6/13 | 1/9 |
| Post-Surgery TV | 0.77 (0–2.1) | 1.74 (0.2–3.83) |
| (a) | |||||
| TTP | |||||
| MRIpreTTP | MRITTP | Δ (MRITTP-MRIpreTTP) | |||
| Vol (cm3) | 5.66 (0.24–25.83) | 12.34 (1.26–38.31) | 6.02 (0.5–29.5) | ||
| rCBVmax | 3.98 (0.87–9.28) a | 5.87 (2.81–15.18) b | 2.32 (−0.81–8.29) | ||
| rADCmin | 1 (0.81–1.59) c | 0.93 (0.67–1.32) d | −0.14 (−0.62–0.26) | ||
| ADCmean (10−3 mm2/s) | 1.4 (1–1.7) | 1.4 (0.8–1.7) | 0.03 (−0.5–0.3) | ||
| ADCmode (10−3 mm2/s) | 1.2 (0.2–2) | 1.1 (0.06–2.5) | −0.07 (−0.96–0.3) | ||
| ADCskewness | 1.43 (0.93–3.77) | 1.68 (1.05–10.9) | 0.03 (−0.1–1.52) | ||
| (b) | |||||
| PsP | |||||
| MRIprePsP | MRIPsP | MRIpostPsP | Δ (MRIPsP-MRIprePsP) | Δ (MRIpostPsP-MRIPsP) | |
| Vol (cm3) | 5.44 (0.45–9.41) | 7.70 (0.60–20.72) | 5.41 (0.4–13.7) | 2.88 (0.25–14.15) | −0.89 (−6.96–0.72) |
| rCBVmax | 5.6 (1.85–14.14) | 3.91 (0.75–10.04) | 4.31 (2.97–7.63) | −2.64 (−5.35–3.68) | 0.4 (−1.91–6.88) |
| rADCmin | 1.1 (0.81–1.43) | 1.01 (0.82–1.34) | 1.1 (0.88–1.01) | −0.09 (−0.27–0.2) | −0.01 (−0.22–1.18) |
| ADCmean (10−3 mm2/s) | 1.42 (1–1.7) | 1.49 (0.9–1.66) | 1.52 (1–1.6) | 0.07 (−0.2–0.3) | −0.03 (−0.07–0.1) |
| ADCmode (10−3 mm2/s) | 1.22 (0.3–1.5) | 1.28 (0.3–1.8) e | 1.14 (0.2–1.6) f | 0.6 (−0.3–0.5) | −0.2 (−0.5–−0.1) |
| ADCskewness | 1.77 (1.15–2.75) | 1.47 (1.18–2.09) | 1.56 (1.1–3.3) | −0.33 (−1.5–0.15) | −0.09 (−0.36–2) |
| High/LowNK | aMRI-T0 | aMRI-T2 | aMRI-T4 | aMRI-T12 | |
|---|---|---|---|---|---|
| Median TV T1 CE (cm3) | HighNK | 3.2 (0–11.2) | 2.71 (0–38.34) | 3.3 (0–13.57) a | 0.94 (0–8.3) |
| LowNK | 5.7 (0.86–25.22) | 6.6 (1.1–27) | 7.5 (0.71–20.72) b | - | |
| rCBVmax | HighNK | 5.29 (0.56–10.55) | 3.29 (0.92–14.14) | 3.79 (0.76–10,04) | 2.6 (0.71–6.89) |
| LowNK | 5.25 (2.27–9,69) | 3.91 (1.81–7.52) | 4.85 (1.08–6.82) | - | |
| rADCmin | HighNK | 1.15 (0.82–1.47) c | 1.01 (0.76–1.51) d | 0.97 (0.67–1.34) e | 1.02 (0.85–1.32) |
| LowNK | 0.97 (0.69–1.61) | 1 (0.82–1.47) | 0.99 (0.7–1.11) | - | |
| ADCskewness | HighNK | 1.38 (0.99–3.15) | 1.62 (0.84–2.75) | 1.59 (0.71–2.1) | 1.79 (1.07–4.47) |
| LowNK | 1.95 (0.85–2.44) | 1.98 (1.4–2.3) | 1.73 (1.4–2.35) | - | |
| ADCmode (10−3 mm2/s) | HighNK | 1.22 (0.3–2) f | 1.24 (0.06–1.6) | 0.8 (0.2–1.5) | 1.1 (0.9–1.2) |
| LowNK | 0.8 (0.4–1.1) g | 1.24 (0.9–1.8) h | 1.3 (0.2–1) | - | |
| ADCmean (10−3 mm2/s) | HighNK | 1.4 (1–1.7) | 1.4 (0.8–1.7) | 1.4 (0.9–1.8) | 1.2 (0.9–1.9) |
| LowNK | 1.3 (1.1–1.6) i | 1.4 (1.3–1.6) l | 1.5 (1.2–1.7) m | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuccarini, V.; Aquino, D.; Gioppo, A.; Anghileri, E.; Pellegatta, S.; Schettino, C.; Mazzi, F.; Finocchiaro, G.; Bruzzone, M.G.; Eoli, M. Advanced MRI Assessment during Dendritic Cell Immunotherapy Added to Standard Treatment Against Glioblastoma. J. Clin. Med. 2019, 8, 2007. https://doi.org/10.3390/jcm8112007
Cuccarini V, Aquino D, Gioppo A, Anghileri E, Pellegatta S, Schettino C, Mazzi F, Finocchiaro G, Bruzzone MG, Eoli M. Advanced MRI Assessment during Dendritic Cell Immunotherapy Added to Standard Treatment Against Glioblastoma. Journal of Clinical Medicine. 2019; 8(11):2007. https://doi.org/10.3390/jcm8112007
Chicago/Turabian StyleCuccarini, Valeria, Domenico Aquino, Andrea Gioppo, Elena Anghileri, Serena Pellegatta, Carla Schettino, Federica Mazzi, Gaetano Finocchiaro, Maria Grazia Bruzzone, and Marica Eoli. 2019. "Advanced MRI Assessment during Dendritic Cell Immunotherapy Added to Standard Treatment Against Glioblastoma" Journal of Clinical Medicine 8, no. 11: 2007. https://doi.org/10.3390/jcm8112007
APA StyleCuccarini, V., Aquino, D., Gioppo, A., Anghileri, E., Pellegatta, S., Schettino, C., Mazzi, F., Finocchiaro, G., Bruzzone, M. G., & Eoli, M. (2019). Advanced MRI Assessment during Dendritic Cell Immunotherapy Added to Standard Treatment Against Glioblastoma. Journal of Clinical Medicine, 8(11), 2007. https://doi.org/10.3390/jcm8112007






