Seizures Do Not Affect Disability and Mortality Outcomes of Stroke: A Population-Based Study
Abstract
1. Introduction
2. Methods
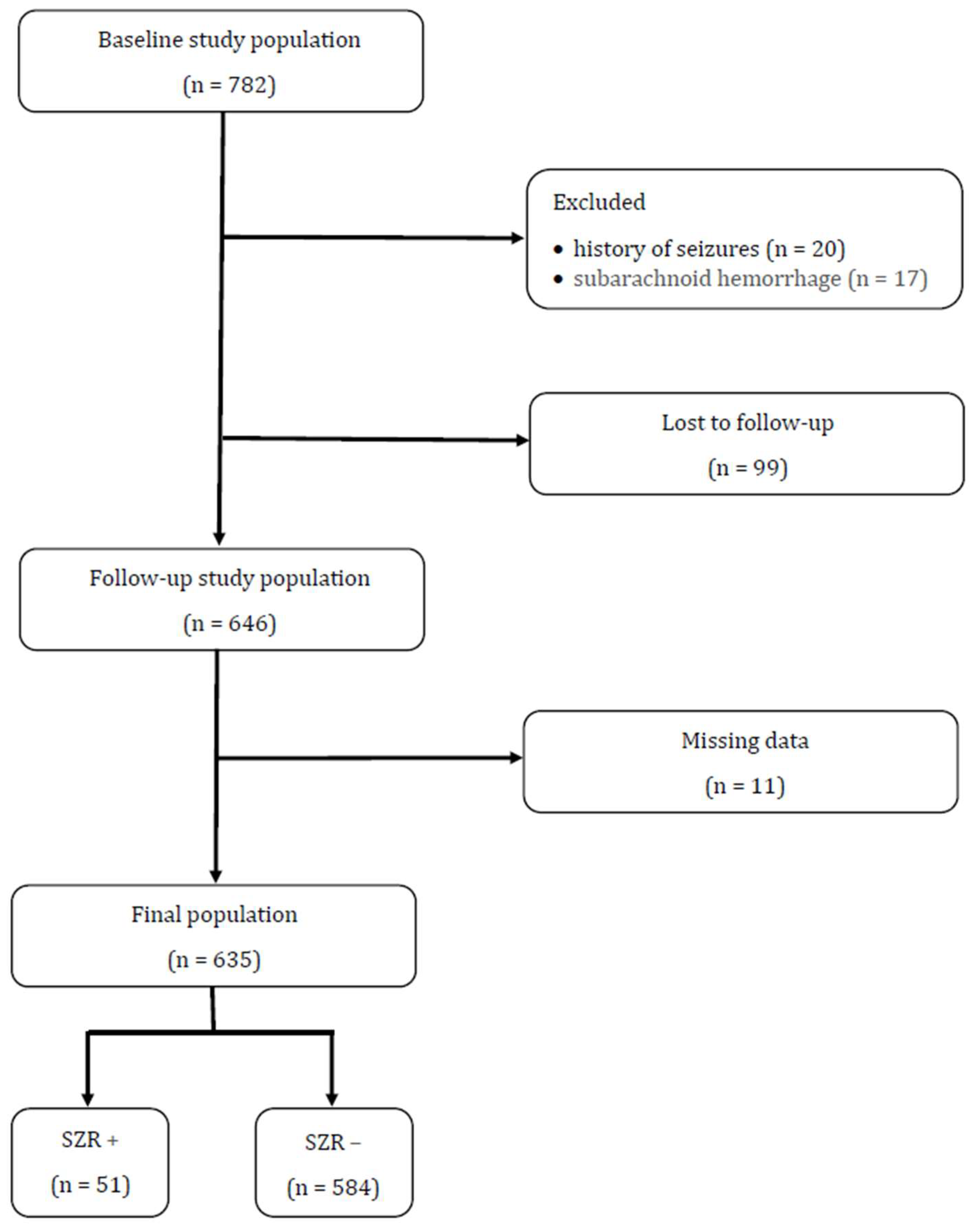
2.1. Study Population
2.2. Assessment of Post-Stroke Seizures
2.3. Data Collection
2.4. Follow-Up
2.5. Outcome Measures
- (1)
- Poor outcome at 24 months, as assessed by the mRS;
- (2)
- All-cause long-term mortality. Information on the permanence in life was systematically recorded thanks to the use of death certificates.
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Long-Term Functional Outcome
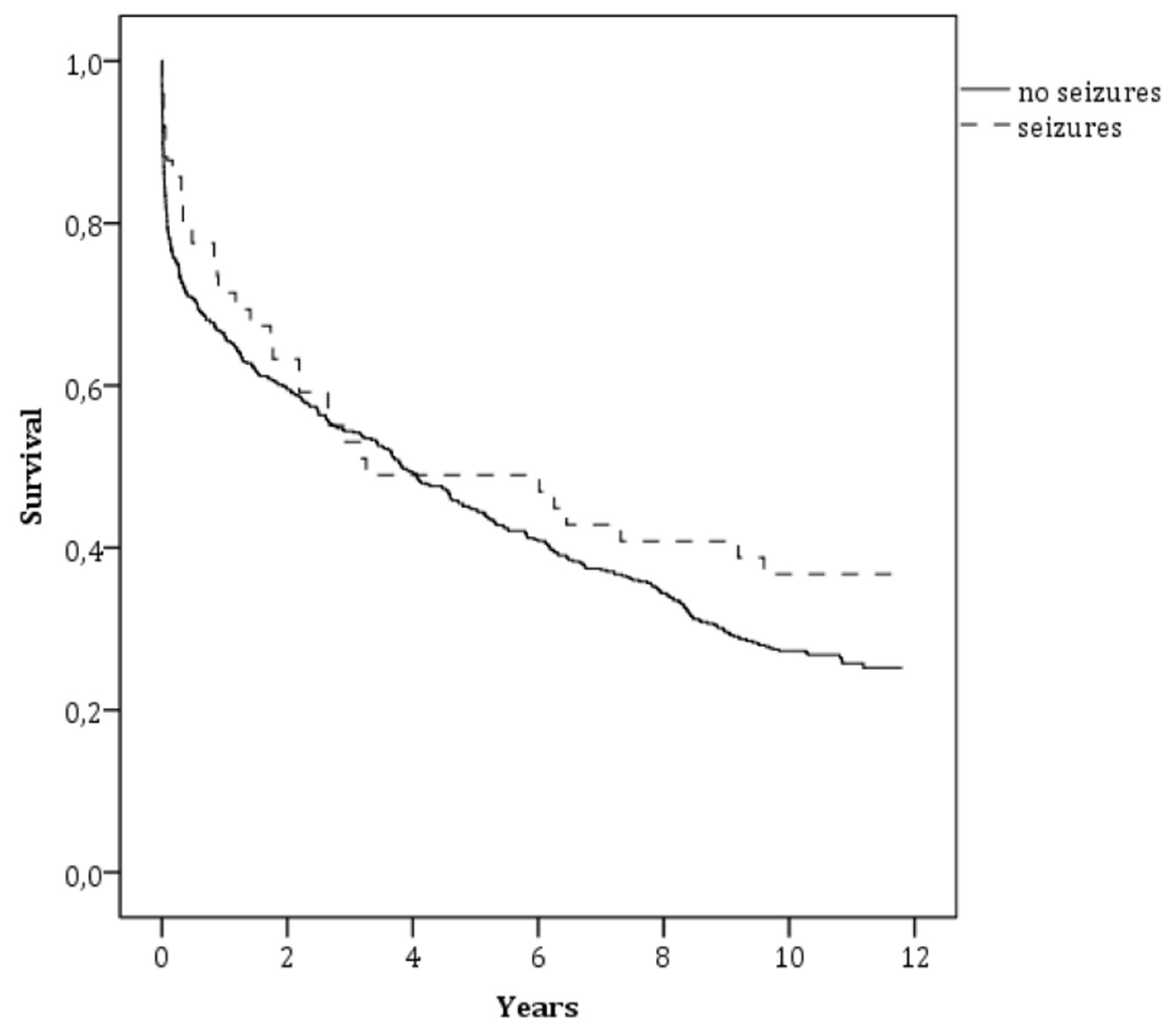
3.3. Long-Term Mortality
3.4. Seizure Type and Long-Term Outcome
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- So, E.L.; Annegers, J.F.; Hauser, W.A.; O’Brien, P.C.; Whisnant, J.P. Population-based study of seizure disorders after cerebral infarction. Neurology 1996, 46, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Labovitz, D.L.; Hauser, W.A.; Sacco, R.L. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology 2001, 57, 200–2006. [Google Scholar] [CrossRef]
- Beghi, E.; D’Alessandro, R.; Beretta, S.; Consoli, D.; Crespi, V.; Delaj, L.; Gandolfo, C.; Greco, G.; Neve, A.L.; Manfred, M.; et al. Incidence and predictors of acute symptomatic seizures after stroke. Neurology 2011, 77, 1785–1793. [Google Scholar] [CrossRef]
- Procaccianti, G.; Zaniboni, A.; Rondelli, F.; Crisci, M.; Sacquegna, T. Seizures in acute stroke: Incidence, risk factors and prognosis. Neuroepidemiology 2012, 39, 45–50. [Google Scholar] [CrossRef]
- Guo, J.; Guo, J.; Li, J.; Zhou, M.; Qin, F.; Zhang, S.; Wu, B.; He, L.; Zhou, D. Statin treatment reduces the risk of poststroke seizures. Neurology 2015, 85, 701–707. [Google Scholar] [CrossRef]
- Serafini, A.; Gigli, G.L.; Gregoraci, G.; Janes, F.; Cancelli, I.; Novello, S.; Valente, M. Are early seizures predictive of epilepsy after a stroke? Results of a population-based study. Neuroepidemiology 2015, 45, 50–58. [Google Scholar] [CrossRef]
- Roivainen, R.; Haapaniemi, E.; Putaala, J.; Kaste, M.; Tatlisumak, T. Young adult ischaemic stroke related acute symptomatic and late seizures: Risk factors. Eur. J. Neurol. 2013, 20, 1247–1255. [Google Scholar] [CrossRef]
- Haapaniemi, E.; Strbian, D.; Rossi, C.; Putaala, J.; Sipi, T.; Mustanoja, S.; Sairanen, T.; Curtze, S.; Satopää, J.; Roivainen, R.; et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke 2014, 45, 1971–1976. [Google Scholar] [CrossRef]
- Giroud, M.; Gras, P.; Fayolle, H.; André, N.; Soichot, P.; Dumas, R. Early seizures after acute stroke: A study of 1640 cases. Epilepsia 1994, 35, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Comes, E.; Massons, J.; Garcia, L.; Oliveres, M. Relevance of early seizures for in-hospital mortality in acute cerebrovascular disease. Neurology 1996, 47, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Dennis, M.; Bamford, J.; Sandercock, P.; Wade, D.; Warlow, C. Epileptic seizures after a first stroke: The Oxfordshire Community Stroke Project. BMJ 1997, 315, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Garcia-Eroles, L.; Massons, J.B.; Oliveres, M.; Comes, E. Predictive factors of early seizures after acute cerebrovascular disease. Stroke 1997, 28, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Reith, J.; Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Seizures in acute stroke: Predictors and prognostic significance: The Copenhagen Stroke Study. Stroke 1997, 28, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Bladin, C.F.; Alexandrov, A.V.; Bellavance, A.; Bornstein, N.; Chambers, B.; Coté, R.; Lebrun, L.; Pirisi, A.; Norris, J.W. Seizures after stroke: A prospective multicentre study. Arch. Neurol. 2000, 57, 1617–1622. [Google Scholar] [CrossRef]
- Bentes, C.; Pimentel, J.; Ferro, J. Epileptic seizures following subcortical infarcts. Cereb. Dis. 2001, 12, 331–334. [Google Scholar] [CrossRef]
- Pohlmann-Eden, B.; Fatar, M.; Hennerici, M. The preserved cortical island sigh is highly predictive of postischemic seizures. Cereb. Dis. 2001, 12, 282–283. [Google Scholar] [CrossRef]
- Lossius, M.I.; Ronning, O.M.; Mowinckel, P.; Gjerstad, L. Incidence and predictors for post-stroke epilepsy: A prospective controlled trial: The Akershus Stroke Study. Eur. J. Neurol. 2002, 9, 365–368. [Google Scholar] [CrossRef]
- Kilpatrick, C.J.; Davis, S.M.; Tress, B.M.; Rossiter, S.C.; Hopper, J.L.; Vandendriesen, M.L. Epileptic seizures in acute stroke. Arch. Neurol. 1990, 47, 157–160. [Google Scholar] [CrossRef]
- Burneo, J.G.; Fang, J.; Saposnik, G. Investigators of the Registry of the Canadian Stroke Network. Impact of seizures on morbidity and mortality after stroke: A Canadian multi-centre cohort study. Eur. J. Neurol. 2010, 17, 52–58. [Google Scholar] [CrossRef]
- Mullen, M.T.; Kasner, S.E.; Messé, S.R. Seizures do not increase in-hospital mortality after intracerebral hemorrhage in the nationwide inpatient sample. Neurocrit. Care 2013, 19, 19–24. [Google Scholar] [CrossRef]
- Hamidou, B.; Aboa-Eboulé, C.; Durier, J.; Jacquin, A.; Lemesle-Martin, M.; Giroud, M.; Béjot, Y. Prognostic value of early epileptic seizures on mortality and functional disability in acute stroke: The Dijon Stroke Registry (1985-2010). J. Neurol. 2013, 260, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Saposnik, G.; Fang, J.; Steven, D.A.; Burneo, J.G. Influence of seizures on stroke outcomes: A large multicenter study. Neurology 2014, 82, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Claessens, D.; Bekelaar, K.; Schreuder, F.H.B.M.; De Greef, B.T.; Vlooswijk, M.C.; Staals, J.; van Oostenbrugge, R.J.; Rouhl, R.P. Mortality after primary intracerebral hemorrhage in relation to post-stroke seizures. J. Neurol. 2017, 264, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Arntz, R.M.; Maaijwee, N.A.; Rutten-Jacobs, L.C.; Schoonderwaldt, H.C.; Dorresteijn, L.D.; van Dijk, E.J.; de Leeuw, F.E. Epilepsy after TIA or stroke in young patients impairs long-term functional outcome: The FUTURE Study. Neurology 2013, 81, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Arntz, R.M.; Rutten-Jacobs, L.C.; Maaijwee, N.A.; Schoonderwaldt, H.C.; Dorresteijn, L.D.; van Dijk, E.J.; de Leeuw, F.E. Poststroke Epilepsy Is Associated With a High Mortality After a Stroke at Young Age: Follow-Up of Transient Ischemic Attack and Stroke Patients and Unelucidated Risk Factor Evaluation Study. Stroke 2015, 46, 2309–2311. [Google Scholar] [CrossRef]
- Janes, F.; Gigli, G.L.; D’Anna, L.; Cancelli, I.; Perelli, A.; Canal, G.; Russo, V.; Zanchettin, B.; Valente, M. Stroke incidence and 30-day and six-month case fatality rates in Udine, Italy: A population-based prospective study. Int. J. Stroke 2013, 8, 100–105. [Google Scholar] [CrossRef]
- Cancelli, I.; Janes, F.; Gigli, G.L.; Perelli, A.; Zanchettin, B.; Canal, G.; D’Anna, L.; Russo, V.; Barbone, F.; Valente, M. Incidence of transient ischemic attack and early stroke risk: Validation of the ABCD2 score in an Italian population-based study. Stroke 2011, 42, 2751–2757. [Google Scholar] [CrossRef]
- Hatano, S. Experience from a multicenter stroke register: A preliminary report. Bull. World Health Organ. 1976, 54, 541–553. [Google Scholar]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, K.J.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position paper of the ILAE Commission for classification and terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bamford, J.; Sandercock, P.; Dennis, M.; Burn, J.; Warlow, C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991, 337, 1521–1526. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; Van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M.; Pinto, F. Poststroke epilepsy: Epidemiology, pathophysiology and management. Drugs Aging 2004, 21, 639–653. [Google Scholar] [CrossRef]
- Paolucci, S.; Silvestri, G.; Lubich, S.; Pratesi, L.; Traballesi, M.; Gigli, G.L. Postroke late seizures and their role in rehabilitation on inpatients. Epilepsia 1997, 38, 266–270. [Google Scholar] [CrossRef]
- Conrad, J.; Pawlowski, M.; Dogan, M.; Kovac, S.; Ritter, M.A.; Evers, S. Seizures after cerebrovascular events: Risk factors and clinical features. Seizure 2013, 22, 275–282. [Google Scholar] [CrossRef]
- Kammersgaard, L.P.; Olsen, T.S. Poststroke epilepsy in the Copenhagen stroke study: Incidence and predictors. J. Stroke Cereb. Dis. 2005, 14, 210–214. [Google Scholar] [CrossRef]
- Benbir, G.; Ince, B.; Bozluolcay, M. The epidemiology of post-stroke epilepsy according to stroke subtypes. Acta Neurol. Scand. 2006, 114, 8–12. [Google Scholar] [CrossRef]
- Alberti, A.; Paciaroni, M.; Caso, V.; Venti, M.; Palmerini, F.; Agnelli, G. Early seizures in patients with acute stroke: Frequency, predictive factors, and effect on clinical outcome. Vasc. Health Risk Manag. 2008, 4, 715–720. [Google Scholar]
- Sadrzadeh, S.M.H.; Eaton, J.W. Hemoglobin-mediated oxidant damage to the central nervous system requires endogenous ascorbate. J. Clin. Investig. 1988, 82, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.; Sastry, B. Effects of hemoglobin and its breakdown products on synaptic transmission in rat hippocampal CA1 neurons. Brain Res. 2000, 864, 1–12. [Google Scholar] [CrossRef]
- Ueda, Y.; Willmore, L.J.; Triggs, W.J. Amygdalar injection of FeCl3 causes spontaneous recurrent seizures. Exp. Neurol. 1998, 153, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Willmore, L.J.; Sypert, G.W.; Munson, J.B.; Hurd, R.W. Chronic focal epileptiform disharges induced by injection of iron into rat and cat cortex. Science 1978, 200, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.; Tsoi, T.H.; Au-Yeung, M.; Tang, A.S. Epileptic seizure after stroke in Chinese patients. J. Neurol. 2003, 250, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Galovic, M.; Döhler, N.; Erdélyi-Canavese, B.; Felbecker, A.; Siebel, P.; Conrad, J.; Evers, P.S.; Winklehner, M.; Oertzen, T.J.; Haring, H.P.; et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): A multivariable prediction model development and validation study. Lancet Neurol. 2018, 17, 143–152. [Google Scholar] [CrossRef]
| SZR + (n = 51) | SZR − (n = 584) | p | |
|---|---|---|---|
| Demographic Data and Baseline Clinical Characteristics | |||
| Age, years | 72.3 ± 14.8 | 77.5 ± 11.5 | 0.003 |
| Males, n (%) | 21 (41.2) | 276 (47.3) | 0.4 |
| Stroke subtypes | 0.001 | ||
| IS, n (%) | 35 (68.6) | 506 (86.6) | |
| ICH, n (%) | 16 (31.4) | 78 (13.4) | |
| IS subtypes based on TOAST classification | 0.08 | ||
| Large artery atherosclerosis, n (%) | 2 (5.7) | 58 (11.5) | |
| Cardioembolism, n (%) | 9 (25.7) | 98 (19.4) | |
| Small-vessel occlusion, n (%) | 1 (2.9) | 82 (16.2) | |
| Other determined etiology, n (%) | 2 (5.7) | 9 (1.8) | |
| Undetermined etiology, n (%) | 21 (60) | 259 (51.2) | |
| IS subtypes based on OCSP classification | 0.001 | ||
| TACI, n (%) | 9 (25.7) | 36 (7.1) | |
| PACI, n (%) | 22 (62.9) | 270 (53.4) | |
| POCI, n (%) | 1 (2.9) | 86 (17) | |
| LACI, n (%) | 3 (8.6) | 114 (22.5) | |
| ICH localization | 0.01 | ||
| Lobar, n (%) | 9 (56.3) | 18 (23.1) | |
| Deep, n (%) | 7 (43.8) | 60 (76.9) | |
| NIHSS score at admission, median (IQR) | 10 (4–17) | 5 (2–14) | 0.01 |
| pre-stroke mRS, median (IQR) | 0 (0-2) | 0 (0-2) | 0.2 |
| Vascular risk factors | |||
| Hypertension, n (%) | 37 (72.5) | 475 (81.3) | 0.9 |
| Atrial fibrillation, n (%) | 17 (33.3) | 195 (33.4) | 0.4 |
| Diabetes mellitus, n (%) | 11 (21.6) | 140 (24.0) | 0.7 |
| Hypercholesterolemia, n (%) | 13 (25.5) | 140 (24.0) | 0.8 |
| Smoking, n (%) | 9 (17.6) | 101 (17.3) | 0.9 |
| Laboratory findings | |||
| Sodium | 0.3 | ||
| Hyponatremia, n (%) | 7 (13.7) | 45 (7.7) | |
| Hypernatremia, n (%) | 1 (2.0) | 8 (1.4) | |
| Potassium | 0.4 | ||
| Hypokalemia, n (%) | 5 (9.8) | 98 (16.8) | |
| Hyperkalemia, n (%) | 4 (7.8) | 37 (6.3) | |
| Glucose | 0.2 | ||
| Hypoglycemia, n (%) | 0 (0) | 0 (0) | |
| Hyperglycemia, n (%) | 37 (72.5) | 370 (63.4) | |
| Unadjusted Analysis | Multivariate Analysis * | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.08 | 1.07–1.10 | 0.001 | 1.09 | 1.06–1.12 | 0.001 |
| Sex | ||||||
| Females | 1.00 | |||||
| Males | 0.43 | 0.30–0.57 | 0.001 | 0.96 | 0.60–1.54 | 0.9 |
| Post–stroke seizures | ||||||
| No | 1.00 | |||||
| Yes | 1.97 | 1.01–3.92 | 0.05 | 2.29 | 0.80–6–56 | 0.1 |
| Stroke subtypes | ||||||
| IS | 1.00 | |||||
| ICH | 1.96 | 1.17–3.82 | 0.01 | 3.01 | 1.50–6.07 | 0.002 |
| Small–vessel occlusion based on TOAST classification | ||||||
| No | 1.00 | |||||
| Yes | 0.17 | 0.10–0.29 | 0.001 | 0.58 | 0.21–1–60 | 0.3 |
| Undetermined etiology based on TOAST classification | ||||||
| No | 1.00 | |||||
| Yes | 3.28 | 2.27–4.74 | 0.001 | 1.99 | 0.98–4.01 | 0.06 |
| TACI based on OCSP classification | ||||||
| No | 1.00 | |||||
| Yes | 6.42 | 2.26–18–20 | 0.001 | 1.12 | 0.33–3.85 | 0.8 |
| LACI based on OCSP classification | ||||||
| No | 1.00 | |||||
| Yes | 0.42 | 0.28–0.64 | 0.001 | 1.34 | 0.68–2.65 | 0.4 |
| Hemorrhagic transformation | ||||||
| No | 1.00 | |||||
| Yes | 3.41 | 1.89–6.36 | 0.001 | 2.04 | 0.92–4.57 | 0.08 |
| Cortical stroke | ||||||
| No | 1.00 | |||||
| Yes | 1.84 | 1.28–2.65 | 0.001 | 1.33 | 0.78–2.27 | 0.3 |
| NIHSS at admission | 1.18 | 1.13–1.22 | 0.001 | 1.14 | 1.09–1.19 | 0.001 |
| Hypertension | ||||||
| No | 1.00 | |||||
| Yes | 1.76 | 1.18–2.63 | 0.005 | 1.15 | 0.63–2.12 | 0.6 |
| Atrial fibrillation | ||||||
| No | 1.00 | |||||
| Yes | 2.72 | 1.85–4.00 | 0.001 | 1.59 | 0.80–3.15 | 0.2 |
| Hypercholesterolemia | ||||||
| No | 1.00 | |||||
| Yes | 0.55 | 0.38–0.80 | 0.002 | 0.95 | 0.56–1.59 | 0.8 |
| Smoking | ||||||
| No | 1.00 | |||||
| Yes | 0.58 | 0.38–0.88 | 0.01 | 1.34 | 0.72–2.49 | 0.3 |
| Hyponatremia | ||||||
| No | 1.00 | |||||
| Yes | 2.02 | 1.02–4.02 | 0.04 | 1.74 | 0.72–4.24 | 0.2 |
| OR | 95% CI | p | |
|---|---|---|---|
| Age | 1.07 | 1.05–1.08 | 0.001 |
| Sex | |||
| Females | 1.00 | ||
| Males | 1.09 | 0.89–1.35 | 0.4 |
| Post-stroke seizures | |||
| No | 1.00 | ||
| Yes | 0.71 | 0.48–1.06 | 0.09 |
| Stroke subtypes | |||
| IS | 1.00 | ||
| ICH | 1.21 | 0.92–1.60 | 0.2 |
| NIHSS at admission | 1.07 | 1.05–1.08 | 0.001 |
| Pre-stroke mRS > 2 | |||
| No | 1.00 | ||
| Yes | 1.65 | 1.30–2.09 | 0.001 |
| SZR FA + (n = 15) | SZR − (n = 584) | p | |
| Disability, n (%) | 9 (60) | 379 (64.9) | 0.7 |
| Mortality, n (%) | 8 (53.3) | 436 (74.7) | 0.06 |
| SZR GTC + (n = 15) | SZR − (n = 584) | p | |
| Disability, n (%) | 13 (86.7) | 379 (64.9) | 0.08 |
| Mortality, n (%) | 14 (93.3) | 436 (74.7) | 0.08 |
| SZR FBTC + (n = 11) | SZR − (n = 584) | p | |
| Disability, n (%) | 10 (90.9) | 379 (64.9) | 0.06 |
| Mortality, n (%) | 8 (72.7) | 436 (74.7) | 0.5 |
| SZR FIA + (n = 10) | SZR − (n = 584) | p | |
| Disability, n (%) | 8 (80) | 379 (64.9) | 0.3 |
| Mortality, n (%) | 6 (60) | 436 (74.7) | 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlino, G.; Gigli, G.L.; Bax, F.; Serafini, A.; Corazza, E.; Valente, M. Seizures Do Not Affect Disability and Mortality Outcomes of Stroke: A Population-Based Study. J. Clin. Med. 2019, 8, 2006. https://doi.org/10.3390/jcm8112006
Merlino G, Gigli GL, Bax F, Serafini A, Corazza E, Valente M. Seizures Do Not Affect Disability and Mortality Outcomes of Stroke: A Population-Based Study. Journal of Clinical Medicine. 2019; 8(11):2006. https://doi.org/10.3390/jcm8112006
Chicago/Turabian StyleMerlino, Giovanni, Gian Luigi Gigli, Francesco Bax, Anna Serafini, Elisa Corazza, and Mariarosaria Valente. 2019. "Seizures Do Not Affect Disability and Mortality Outcomes of Stroke: A Population-Based Study" Journal of Clinical Medicine 8, no. 11: 2006. https://doi.org/10.3390/jcm8112006
APA StyleMerlino, G., Gigli, G. L., Bax, F., Serafini, A., Corazza, E., & Valente, M. (2019). Seizures Do Not Affect Disability and Mortality Outcomes of Stroke: A Population-Based Study. Journal of Clinical Medicine, 8(11), 2006. https://doi.org/10.3390/jcm8112006





