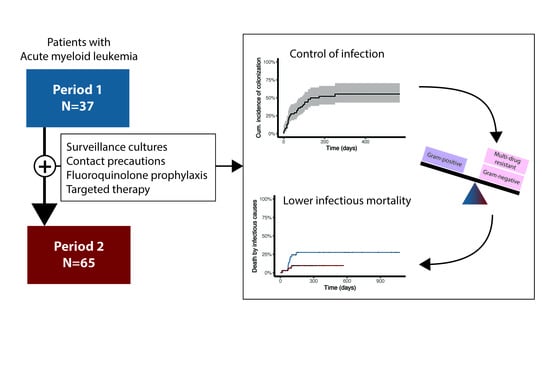
The Value of Adding Surveillance Cultures to Fluoroquinolone Prophylaxis in the Management of Multiresistant Gram Negative Bacterial Infections in Acute Myeloid Leukemia
Abstract
1. Introduction
2. Methods
2.1. Intervention
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Compliance with the Active Surveillance Program
3.2. Colonization
3.3. Infection
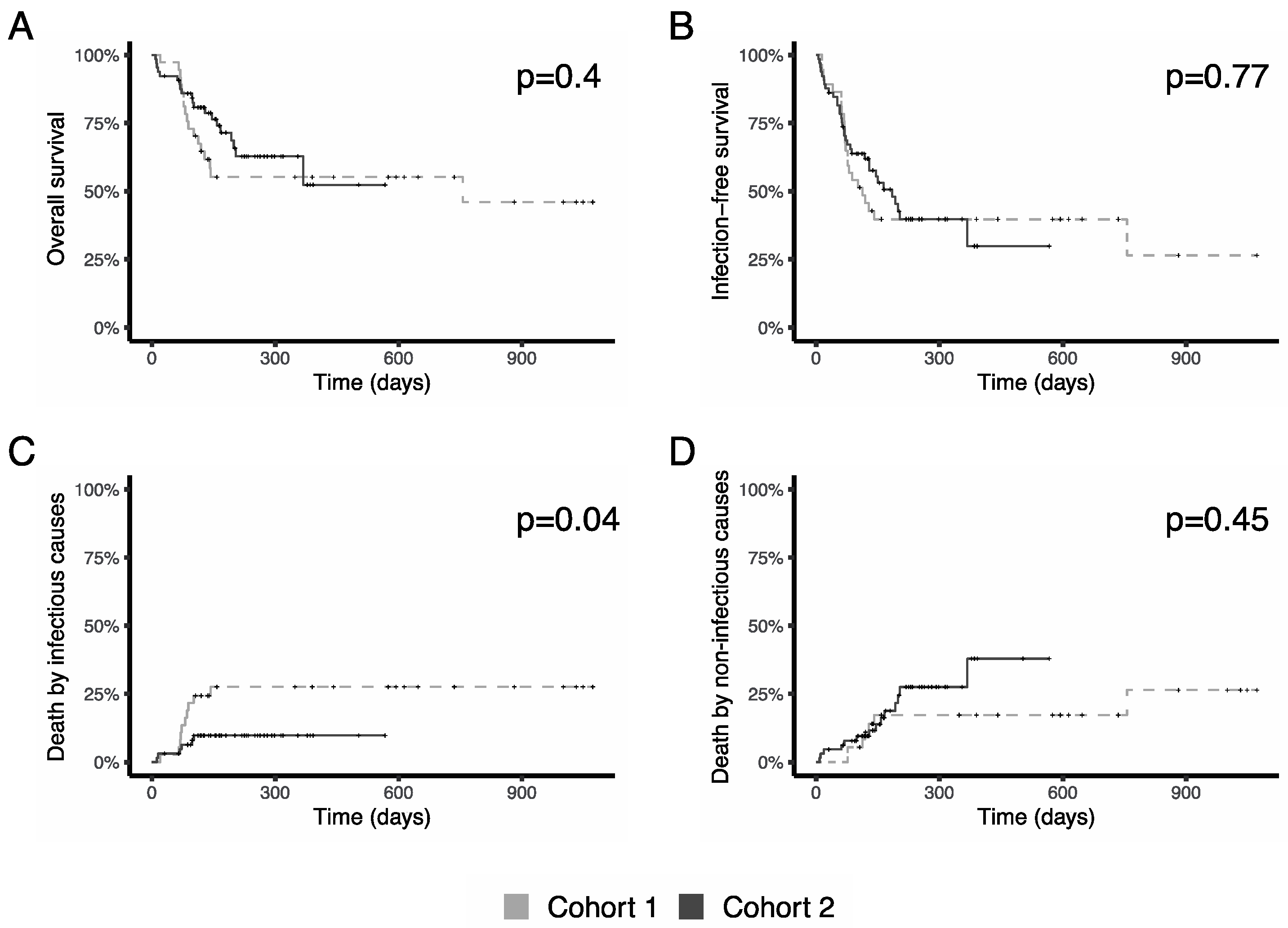
3.4. Survival
3.5. Targeted Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gudiol, C.; Aguilar-Guisado, M.; Azanza, J.R.; Candel, F.J.; Cantón, R.; Carratalà, J.; Garcia-Vidal, C.; Jarque, I.; Lizasoain, M.; Gil-Bermejo, J.M.; et al. Executive summary of the consensus document of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), the Spanish Network for Research in Infectious Diseases (REIPI) and the Spanish Society of Haematology and Haemotherapy (SEHH) on the management of febrile neutropenia in patients with hematological malignancies. Enferm. Infecc. Microbiol. Clin. 2019. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Micozzi, A.; Gentile, G.; Minotti, C.; Cartoni, C.; Capria, S.; Ballarò, D.; Santilli, S.; Pacetti, E.; Grammatico, S.; Bucaneve, G.; et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: Factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect. Dis. 2017, 17, 203. [Google Scholar] [CrossRef] [PubMed]
- Ballo, O.; Tarazzit, I.; Stratmann, J.; Reinheimer, C.; Hogardt, M.; Wichelhaus, T.A.; Kempf, V.; Serve, H.; Finkelmeier, F.; Brandts, C. Colonization with multidrug resistant organisms determines the clinical course of patients with acute myeloid leukemia undergoing intensive induction chemotherapy. PLoS ONE 2019, 14, e0210991. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Rossolini, G.M.; Piciocchi, A.; Bertaina, A.; Pisapia, G.; Pastore, D.; Sica, S.; Severino, A.; Cudillo, L.; Ciceri, F.; et al. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: A nationwide retrospective survey from Italy. Bone Marrow Transplant. 2015, 50, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Cometta, A.; Calandra, T.; Bille, J.; Glauser, M.P. Escherichia coli resistant to fluoroquinolones in patients with cancer and neutropenia. N. Engl. J. Med. 1994, 330, 1240–1241. [Google Scholar] [CrossRef]
- Karampatakis, T.; Tsergouli, K.; Iosifidis, E.; Antachopoulos, C.; Karapanagiotou, A.; Karyoti, A.; Gritsi-Gerogianni, N.; Tsakris, A.; Roilides, E. Impact of active surveillance and infection control measures on carbapenem-resistant Gram-negative bacterial colonization and infections in intensive care. J. Hosp. Infect. 2018, 99, 396–404. [Google Scholar] [CrossRef]
- Geladari, A.; Karampatakis, T.; Antachopoulos, C.; Iosifidis, E.; Tsiatsiou, O.; Politi, L.; Karyoti, A.; Papanikolaou, V.; Tsakris, A.; Roilides, E. Epidemiological surveillance of multidrug-resistant gram-negative bacteria in a solid organ transplantation department. Transpl. Infect. Dis. 2017, 19, e12686. [Google Scholar] [CrossRef]
- Mulanovich, V.; Kontoyiannis, D.P. Acute myeloid leukemia and the infectious diseases consultant. Leuk. Lymphoma 2018, 59, 1284–1291. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baño, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20 (Suppl. 1), 1–55. [Google Scholar] [CrossRef]
- Lorenzana, N.; Avila, L.F.; Alonso, S.; Colado, E.; Bernal, T. The impact of antimicrobial prophylaxis in morbidity and infections during azacitidine treatment. Ann. Hematol. 2017, 96, 1833–1840. [Google Scholar] [CrossRef]
- Bow, E.J. Fluoroquinolones, antimicrobial resistance and neutropenic cancer patients. Curr. Opin. Infect. Dis. 2011, 24, 545–553. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Sorror, M.L.; Sandmaier, B.M.; Storer, B.E.; Maris, M.B.; Baron, F.; Maloney, D.G.; Scott, B.L.; Deeg, H.J.; Appelbaum, F.R.; Storb, R. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2007, 25, 4246–4254. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.-A.H.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Desmet, S.; Verhaegen, J.; Glupzcynski, Y.; Van Eldere, J.; Melin, P.; Goossens, H.; Piérard, D.; Declercq, P.; Lagrou, K.; Boel, A.; et al. Development of a national EUCAST challenge panel for antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2016, 22, 704–710. [Google Scholar] [CrossRef]
- Fernández, J.; Poirel, L.; Rodicio, M.R.; Nordmann, P. Concomitant and multiclonal dissemination of OXA-48-producing Klebsiella pneumoniae in a Spanish hospital. J. Antimicrob. Chemother. 2016, 71, 1734–1736. [Google Scholar] [CrossRef]
- Fernández, J.; Montero, I.; Martínez, Ó.; Fleites, A.; Poirel, L.; Nordmann, P.; Rodicio, M.R. Dissemination of multiresistant Enterobacter cloacae isolates producing OXA-48 and CTX-M-15 in a Spanish hospital. Int. J. Antimicrob. Agents 2015, 46, 469–474. [Google Scholar] [CrossRef]
- Fernández, J.; Montero, I.; Fleites, A.; Rodicio, M.R. Cluster of Escherichia coli isolates producing a plasmid-mediated OXA-48 β-lactamase in a Spanish hospital in 2012. J. Clin. Microbiol. 2014, 52, 3414–3417. [Google Scholar] [CrossRef]
- Cattaneo, C.; Zappasodi, P.; Mancini, V.; Annaloro, C.; Pavesi, F.; Skert, C.; Ferrario, A.; Todisco, E.; Saccà, V.; Verga, L.; et al. Emerging resistant bacteria strains in bloodstream infections of acute leukaemia patients: Results of a prospective study by the Rete Ematologica Lombarda (Rel). Ann. Hematol. 2016, 95, 1955–1963. [Google Scholar] [CrossRef]
- Saini, L.; Rostein, C.; Atenafu, E.G.; Brandwein, J.M. Ambulatory consolidation chemotherapy for acute myeloid leukemia with antibacterial prophylaxis is associated with frequent bacteremia and the emergence of fluoroquinolone resistant E. Coli. BMC Infect. Dis. 2013, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Reuter, S.; Harris, S.R.; Glasner, C.; Feltwell, T.; Argimon, S.; Abudahab, K.; Goater, R.; Giani, T.; Errico, G.; et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 2019, 4, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Bertaina, A.; Piciocchi, A.; Perruccio, K.; Algarotti, A.; Busca, A.; Cattaneo, C.; Raiola, A.M.; Guidi, S.; Iori, A.P.; et al. Incidence, Risk Factors and Outcome of Pre-engraftment Gram-Negative Bacteremia After Allogeneic and Autologous Hematopoietic Stem Cell Transplantation: An Italian Prospective Multicenter Survey. Clin. Infect. Dis. 2017, 65, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Kern, W.V.; Sigge, A.; Döhner, H.; Marre, R.; Kern, P.; von Baum, H. Impact of fluoroquinolone prophylaxis on reduced infection-related mortality among patients with neutropenia and hematologic malignancies. Clin. Infect. Dis. 2005, 40, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.; Song, K.W.; Barnett, M.J.; Forrest, D.L.; Hogge, D.E.; Nantel, S.H.; Nevill, T.J.; Shepherd, J.D.; Smith, C.A.; Sutherland, H.J.; et al. Positive impact of selective outpatient management of high-risk acute myelogenous leukemia on the incidence of septicemia. Ann. Oncol. 2007, 18, 1246–1252. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Cardozo-Espinola, C.; Puerta-Alcalde, P.; Marco, F.; Tellez, A.; Agüero, D.; Romero-Santana, F.; Díaz-Beyá, M.; Giné, E.; Morata, L.; et al. Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PLoS ONE 2018, 13, e0199531. [Google Scholar] [CrossRef]
- Martinez-Nadal, G.; Puerta-Alcalde, P.; Gudiol, C.; Cardozo, C.; Albasanz-Puig, A.; Marco, F.; Laporte-Amargós, J.; Moreno-García, E.; Domingo-Doménech, E.; Chumbita, M.; et al. Inappropriate Empirical Antibiotic Treatment in High-risk Neutropenic Patients With Bacteremia in the Era of Multidrug Resistance. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef]



| Cohort 1 | Cohort 2 | p | |
|---|---|---|---|
| Number of patients | 37 | 65 | |
| Number of cycles | 87 | 146 | |
| Age, mean years (range) | 58 (44–64) | 58 (50–64) | 0.5 |
| Male sex N (%) | 16 (43) | 30 (46) | 0.9 |
| Prognostic group N (%) | |||
| Good | 15 (40) | 28 (43) | 0.8 |
| Intermediate | 11 (30) | 16 (25) | |
| Poor | 11 (30) | 21 (32) | |
| White Blood Cell Count × 109/L (range) | 10.07 (3.64–31.6) | 8.07 (2.43–21.9) | 0.2 |
| Comorbidity index (%) | |||
| 0–2 | 32 (86) | 53 (82) | 0.6 |
| >2 | 5 (14) | 12 (18) | |
| Phase of underlying disease (%) | |||
| Diagnosis | 36 (97) | 62 (95) | 0.1 |
| Relapse | 1 (3) | 3 (5) | |
| Type of chemotherapy (%) | |||
| Ida/arac (3/7) | 26 (70) | 51 (78.5) | |
| Fludarabine-based | |||
| Low dose Ara-C | 11 (30) | 12 (18.5) | 0.3 |
| Allogeneic stem cell transplant | 0 (0) | 2 (3) | |
| Quinolone prophylaxis (%) | 12/37 (32) | 20/65 (31) | 0.9 |
| Induction-1 | 12/37 (32) | 50/65 (77) | 0.001 |
| Induction-2 | 2/7 (29) | 9/11 (82) | 0.07 |
| Consolidation-1 | 5/26 (19) | 33/38 (87) | 0.001 |
| Consolidation-2 | 4/13 (31) | 18/20 (90) | 0.001 |
| Consolidation | 2/4 (50) | 12/12 (100) | 0.08 |
| Days of neutropenia (range) | |||
| Induction-1 | 21 (17–27) | 22 (19–28) | 0.4 |
| Induction-2 | 20 (16–33) | 18 (17–24) | 0.75 |
| Consolidation-1 | 15 (15–19) | 22 (13–27) | 0.27 |
| Consolidation-2 | 13 (11–18) | 16 (13–22) | 0.2 |
| Consolidation3 | 21 (17–27) | 15 (11–16) | 0.14 |
| Pathogen and Resistance Pattern N (% of Total Gram Negative Isolated) | N (%) |
|---|---|
| N of patients colonized/n of isolates | 32/35 |
| Klebisella pneumoniae, n isolates, % of isolates | 23 |
| • ESBL | 20 (85) |
| • ESBL-CPN | 3 (15) |
| Pseudomonas aeruginosa, n isolates, % of isolates | 5 |
| • XDR | 1/5 (20) |
| • Non XDR (carbapenem resistant) | 4/5 (80) |
| Escherichia coli ESBL, n isolates, % of isolates | 1 (100) |
| Enterobacter cloacae complex, n isolates, % of isolates | 4 |
| • ESBL | 1 (20) |
| • ESBL-CPN | 3 (75) |
| Citrobacter freundii complex CPN, n isolates, % of isolates | 1 (100) |
| Acinetobacter baumannii XDR, n isolates, % of isolates | 1 (100) |
| Cohort 1 | Cohort 2 | Overall | p | |
|---|---|---|---|---|
| No fever, no infection, n patients, number of cycles (%) | 9/87 (10) | 27/146 (18) | 36/233(15) | 0.9 |
| Fever of Unknown Origin, n febrile episodes, (%) | 21/89 (24) | 50/147 (34) | 71/236 (30) | 0.9 |
| Clinically documented, n febrile episodes, (%) | 12/89 (12) | 11/147 (7) | 23/236 (10) | 0.13 |
| Catheter | 0 (0) | 1 (9) | 1 (4) | 0.79 |
| Respiratory | 4 (33) | 5 (45) | 9 (39) | 0.67 |
| Soft tissues | 7 (58) | 3 (25) | 10 (44) | 0.03 |
| Gastrointestinal | 1 (8) | 2 (18) | 3 (13) | 0.8 |
| Microbiologically documented, n febrile episodes, (%) | 56/89 (63) | 87/147 (59) | 143/236 (61) | 0.6 |
| Gram negative | 33 (59) | 37 (43) | 70 (49) | 0.05 |
| Gram positive | 18 (32) | 43 (49) | 61 (43) | 0.12 |
| Viral disease | 1 (1) | 1 (1) | 2 (1) | 0.7 |
| Fungi | 2 (4) | 4 (5) | 6 (4) | 0.8 |
| Clostridium difficile | 2 (4) | 2 (2) | 4 (3) | 0.9 |
| MR-GNB and Resistance Pattern N/Total GNB Recovered (% Resistant) | Cohort 1 | Cohort 2 | p |
|---|---|---|---|
| Klebisella pneumoniae | |||
| • ESBL | 1/8 (12) | 11/18 (61) | 0.06 |
| • CPN | 0/8 (0) | 1/18 (6) | |
| • ESBL-CPN | 7/8 (88) | 6/18 (33) | |
| Pseudomonas aeruginosa | |||
| • XDR | 7/11 (36) | 6/9 (55) | 0.1 |
| • Other | 4/11 (36) | 3/9 (34) | |
| Escherichia coli | |||
| • ESBL | 0/9 | 6/13 (46) | 0.057 |
| • CPN | 1/9 (11) | 0/13 (0) | |
| • Other | 8/9 (89) | 7/13 (54) | |
| Enterobacter sp. ESBL | 0/3 (0) | 1/4 (25) | 0.8 |
| Stenotrophomonas maltophila | 1/1(100) | 1/1 (100) | 1 |
| Factor | Hazard Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Age | 1.005 | 0.98–1.03 | 0.68 |
| Comorbidity index | 2.025 | 0.88–4.63 | 0.09 |
| Genetic risk | 1.593 | 0.79–3.17 | 0.18 |
| Prophylactic fluoroquinolones with no active surveillance | 0.611 | 0.33–1.13 | 0.12 |
| Prophylactic fluoroquinolones with active surveillance | 0.555 | 0.38–0.79 | 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañón, C.; Fernández Moreno, A.; Fernández Verdugo, A.M.; Fernández, J.; Martínez Ortega, C.; Alaguero, M.; Nicolás, C.; Vilorio Marqués, L.; Bernal, T. The Value of Adding Surveillance Cultures to Fluoroquinolone Prophylaxis in the Management of Multiresistant Gram Negative Bacterial Infections in Acute Myeloid Leukemia. J. Clin. Med. 2019, 8, 1985. https://doi.org/10.3390/jcm8111985
Castañón C, Fernández Moreno A, Fernández Verdugo AM, Fernández J, Martínez Ortega C, Alaguero M, Nicolás C, Vilorio Marqués L, Bernal T. The Value of Adding Surveillance Cultures to Fluoroquinolone Prophylaxis in the Management of Multiresistant Gram Negative Bacterial Infections in Acute Myeloid Leukemia. Journal of Clinical Medicine. 2019; 8(11):1985. https://doi.org/10.3390/jcm8111985
Chicago/Turabian StyleCastañón, Christelle, Ahinoa Fernández Moreno, Ana María Fernández Verdugo, Javier Fernández, Carmen Martínez Ortega, Miguel Alaguero, Concepción Nicolás, Laura Vilorio Marqués, and Teresa Bernal. 2019. "The Value of Adding Surveillance Cultures to Fluoroquinolone Prophylaxis in the Management of Multiresistant Gram Negative Bacterial Infections in Acute Myeloid Leukemia" Journal of Clinical Medicine 8, no. 11: 1985. https://doi.org/10.3390/jcm8111985
APA StyleCastañón, C., Fernández Moreno, A., Fernández Verdugo, A. M., Fernández, J., Martínez Ortega, C., Alaguero, M., Nicolás, C., Vilorio Marqués, L., & Bernal, T. (2019). The Value of Adding Surveillance Cultures to Fluoroquinolone Prophylaxis in the Management of Multiresistant Gram Negative Bacterial Infections in Acute Myeloid Leukemia. Journal of Clinical Medicine, 8(11), 1985. https://doi.org/10.3390/jcm8111985