Clinical Relevance of Immunobiology in Umbilical Cord Blood Transplantation
Abstract
1. Introduction
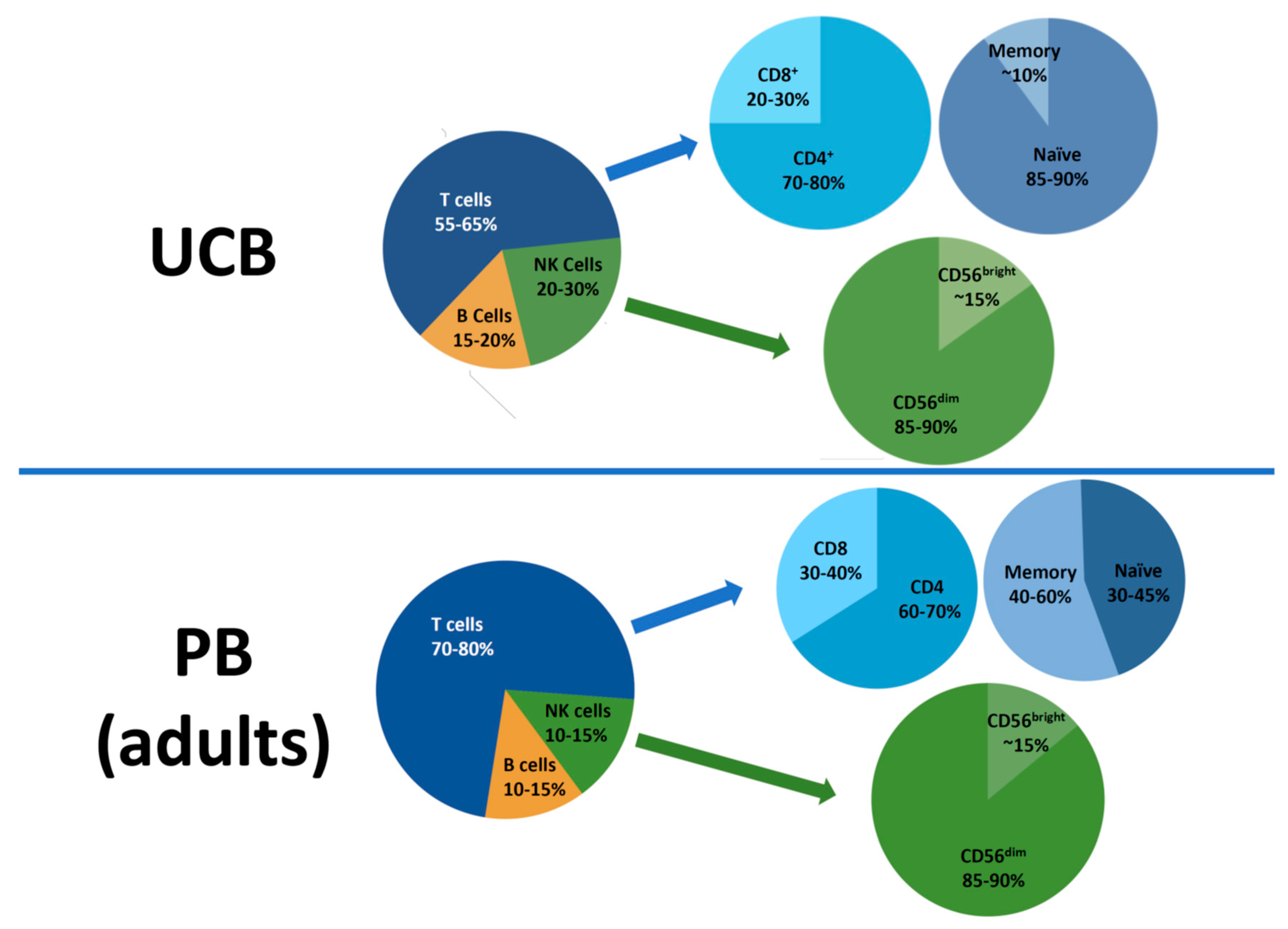
2. Lymphocyte Subsets in the UCB Graft
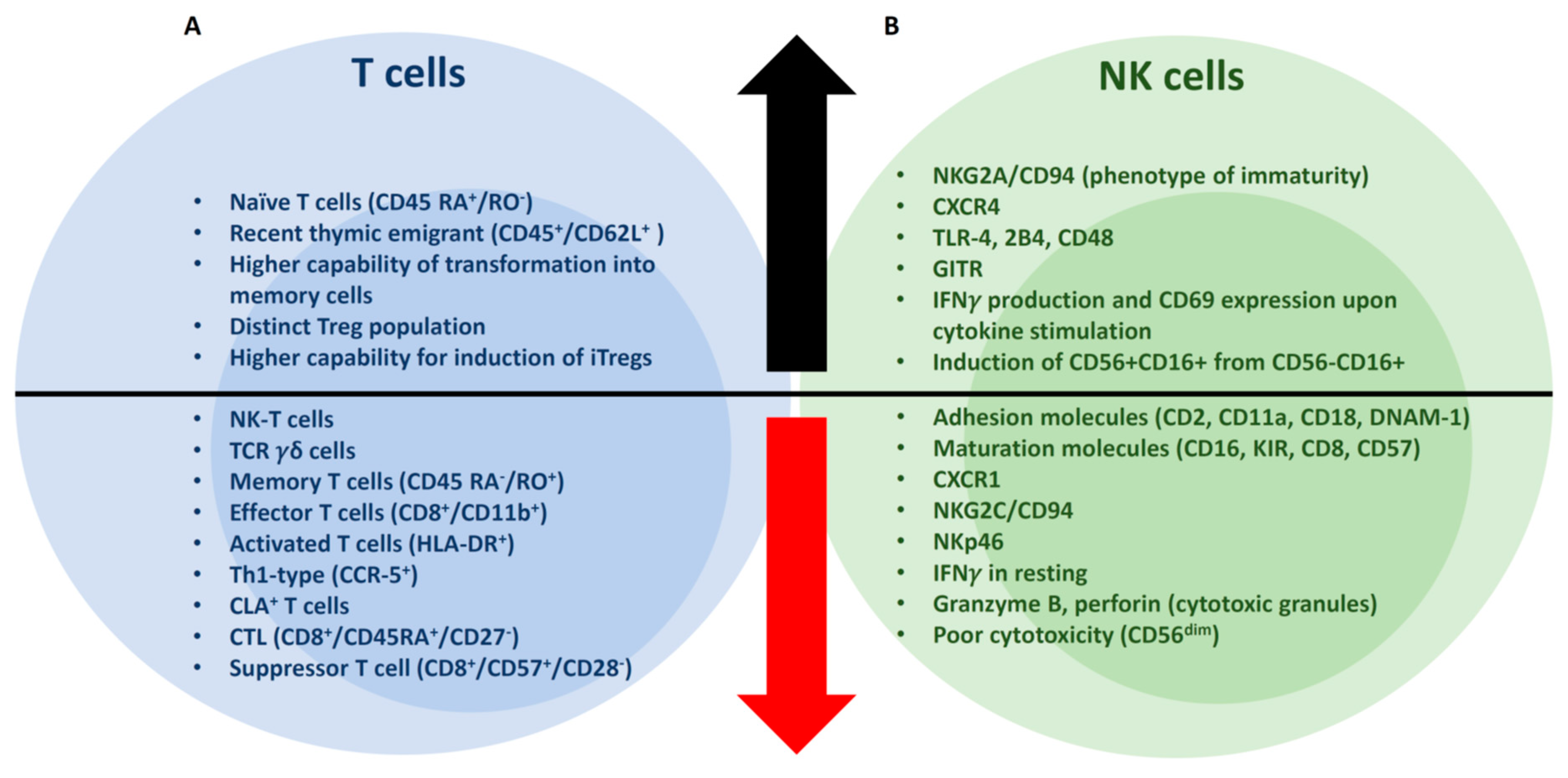
2.1. CD3+ T cells
2.2. Tregs
2.3. NK Cells
3. Immune Reconstitution in UCBT
3.1. T cells
3.2. NK Cells
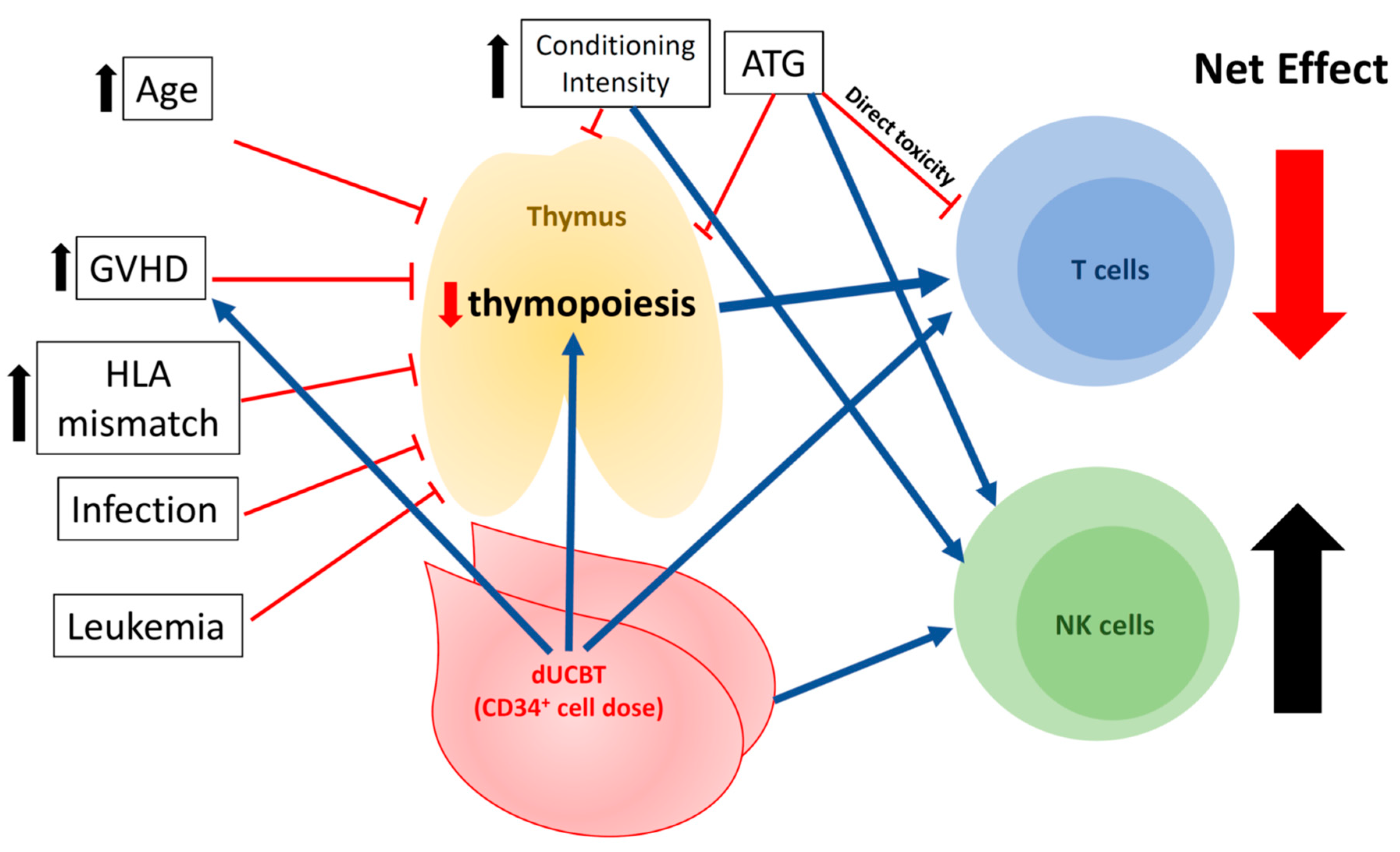
4. Clinical Factors Associated with Immune Reconstitution in UCBT
4.1. Viral Infections
4.2. GVHD
4.3. Conditioning Regimen
4.4. Age
4.5. Cell Dose: CD34+ Progenitor Counts in Grafts and Single vs. Double Unit(s) of UCBT
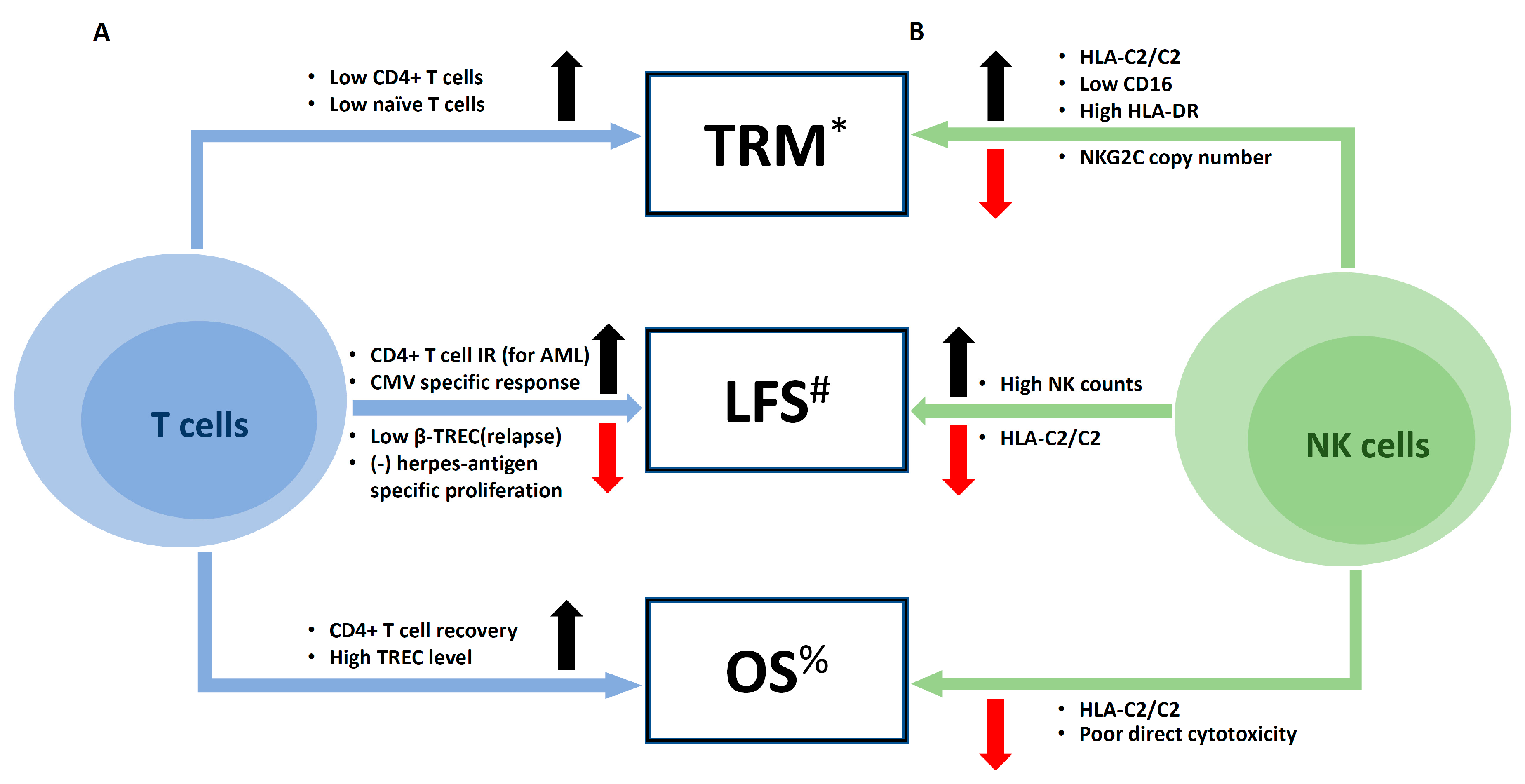
5. Clinical Impacts of Immune Reconstitution on Outcomes of UCBT
5.1. Infections
5.2. Major Clinical Outcomes: Relapse, Mortality, and Survival
6. Closing Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szabolcs, P.; Park, K.D.; Reese, M.; Marti, L.; Broadwater, G.; Kurtzberg, J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp. Hematol. 2003, 31, 708–714. [Google Scholar] [CrossRef]
- Lopez, M.C.; Palmer, B.E.; Lawrence, D.A. Phenotypic differences between cord blood and adult peripheral blood. Cytom. Part B Clin. Cytom. 2009, 76, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, I.M.; Janossy, G.; Contreras, M.; Navarrete, C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood 1998, 92, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.N.; Wagner, J.E. Umbilical-cord blood transplantation for the treatment of cancer. Nat. Rev. Cancer 2003, 3, 526–532. [Google Scholar] [CrossRef]
- Early, E.; Reen, D.J. Rapid conversion of naive to effector T cell function counteracts diminished primary human newborn T cell responses. Clin. Exp. Immunol. 1999, 116, 527–533. [Google Scholar] [CrossRef]
- Kaminski, B.A.; Kadereit, S.; Miller, R.E.; Leahy, P.; Stein, K.R.; Topa, D.A.; Radivoyevitch, T.; Veigl, M.L.; Laughlin, M.J. Reduced expression of NFAT-associated genes in UCB versus adult CD4+ T lymphocytes during primary stimulation. Blood 2003, 102, 4608–4617. [Google Scholar] [CrossRef]
- Hiwarkar, P.; Qasim, W.; Ricciardelli, I.; Gilmour, K.; Quezada, S.; Saudemont, A.; Amrolia, P.; Veys, P. Cord blood T cells mediate enhanced antitumor effects compared with adult peripheral blood T cells. Blood 2015, 126, 2882–2891. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Godfrey, W.R.; Spoden, D.J.; Ge, Y.G.; Baker, S.R.; Liu, B.; Levine, B.L.; June, C.H.; Blazar, B.R.; Porter, S.B. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood 2005, 105, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Lindgren, S.; Kollberg, G.; Lundgren, A.; Harris, R.A.; Rudin, A.; Lundin, S.; Suri-Payer, E. CD4 T cell activation by myelin oligodendrocyte glycoprotein is suppressed by adult but not cord blood CD25+ T cells. Eur. J. Immunol. 2003, 33, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Satwani, P.; Oberfield, N.; Vlad, G.; Simpson, L.L.; Cairo, M.S. Increased induction of allogeneic-specific cord blood CD4+CD25+ regulatory T (Treg) cells: A comparative study of naive and antigenic-specific cord blood Treg cells. Exp. Hematol. 2005, 33, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Torelli, G.F.; Maggio, R.; Peragine, N.; Chiaretti, S.; De Propris, M.S.; Lucarelli, B.; Screnci, M.; Mascolo, M.G.; Milano, F.; Iori, A.P.; et al. Functional analysis and gene expression profile of umbilical cord blood regulatory T cells. Ann. Hematol. 2012, 91, 155–161. [Google Scholar] [CrossRef]
- Brunstein, C.G.; Miller, J.S.; McKenna, D.H.; Hippen, K.L.; DeFor, T.E.; Sumstad, D.; Curtsinger, J.; Verneris, M.R.; MacMillan, M.L.; Levine, B.L.; et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: Kinetics, toxicity profile, and clinical effect. Blood 2016, 127, 1044–1051. [Google Scholar] [CrossRef]
- Brunstein, C.G.; Miller, J.S.; Cao, Q.; McKenna, D.H.; Hippen, K.L.; Curtsinger, J.; Defor, T.; Levine, B.L.; June, C.H.; Rubinstein, P.; et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood 2011, 117, 1061–1070. [Google Scholar] [CrossRef]
- Sarvaria, A.; Jawdat, D.; Madrigal, J.A.; Saudemont, A. Umbilical Cord Blood Natural Killer Cells, Their Characteristics, and Potential Clinical Applications. Front. Immunol. 2017, 8, 329. [Google Scholar] [CrossRef]
- Verneris, M.R.; Miller, J.S. The phenotypic and functional characteristics of umbilical cord blood and peripheral blood natural killer cells. Br. J. Haematol. 2009, 147, 185–191. [Google Scholar] [CrossRef]
- Kotylo, P.K.; Baenzinger, J.C.; Yoder, M.C.; Engle, W.A.; Bolinger, C.D. Rapid analysis of lymphocyte subsets in cord blood. Am. J. Clin. Pathol. 1990, 93, 263–266. [Google Scholar] [CrossRef]
- Dalle, J.H.; Menezes, J.; Wagner, E.; Blagdon, M.; Champagne, J.; Champagne, M.A.; Duval, M. Characterization of cord blood natural killer cells: Implications for transplantation and neonatal infections. Pediatric Res. 2005, 57, 649–655. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell. Mol. Immunol. 2007, 4, 377–382. [Google Scholar] [PubMed]
- Tanaka, H.; Kai, S.; Yamaguchi, M.; Misawa, M.; Fujimori, Y.; Yamamoto, M.; Hara, H. Analysis of natural killer (NK) cell activity and adhesion molecules on NK cells from umbilical cord blood. Eur. J. Haematol. 2003, 71, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Luevano, M.; Daryouzeh, M.; Alnabhan, R.; Querol, S.; Khakoo, S.; Madrigal, A.; Saudemont, A. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum. Immunol. 2012, 73, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Milano, F.; Gooley, T.; Wood, B.; Woolfrey, A.; Flowers, M.E.; Doney, K.; Witherspoon, R.; Mielcarek, M.; Deeg, J.H.; Sorror, M.; et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N. Engl. J. Med. 2016, 375, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Della Chiesa, M.; Falco, M.; Podesta, M.; Locatelli, F.; Moretta, L.; Frassoni, F.; Moretta, A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: A role for human cytomegalovirus? Blood 2012, 119, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.; Broxmeyer, H.E. Cord blood CD16+56- cells with low lytic activity are possible precursors of mature natural killer cells. Cell. Immunol. 1997, 180, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Takada, H.; Jin, C.H.; Tanaka, T.; Ohga, S.; Hara, T. Functional analyses of cord blood natural killer cells and T cells: A distinctive interleukin-18 response. Exp. Hematol. 2001, 29, 1169–1176. [Google Scholar] [CrossRef]
- Mehta, R.S.; Shpall, E.J.; Rezvani, K. Cord Blood as a Source of Natural Killer Cells. Front. Med. 2015, 2, 93. [Google Scholar] [CrossRef]
- Ogonek, J.; Kralj Juric, M.; Ghimire, S.; Varanasi, P.R.; Holler, E.; Greinix, H.; Weissinger, E. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2016, 7, 507. [Google Scholar] [CrossRef]
- Renard, C.; Barlogis, V.; Mialou, V.; Galambrun, C.; Bernoux, D.; Goutagny, M.P.; Glasman, L.; Loundou, A.D.; Poitevin-Later, F.; Dignat-George, F.; et al. Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. Br. J. Haematol. 2011, 152, 322–330. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Turki, A.T.; McDonough, S.M.; Stevenson, K.E.; Kim, H.T.; Kao, G.; Herrera, M.I.; Reynolds, C.G.; Alyea, E.P.; Ho, V.T.; et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: Comparison with unrelated peripheral blood stem cell transplantation. Biol. Blood Marrow Transplant. 2012, 18, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.S.; Velardi, E.; Malard, F.; van den Brink, M.R. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation: Time To T Up the Thymus. J. Immunol. 2017, 198, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Servais, S.; Hannon, M.; Peffault de Latour, R.; Socie, G.; Beguin, Y. Reconstitution of adaptive immunity after umbilical cord blood transplantation: Impact on infectious complications. Stem Cell Investig. 2017, 4, 40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jameson, S.C. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2002, 2, 547–556. [Google Scholar] [CrossRef]
- Thiant, S.; Yakoub-Agha, I.; Magro, L.; Trauet, J.; Coiteux, V.; Jouet, J.P.; Dessaint, J.P.; Labalette, M. Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse. Bone Marrow Transpl. 2010, 45, 1546–1552. [Google Scholar] [CrossRef]
- Williams, K.M.; Hakim, F.T.; Gress, R.E. T cell immune reconstitution following lymphodepletion. Semin. Immunol. 2007, 19, 318–330. [Google Scholar] [CrossRef]
- Rufer, N.; Brummendorf, T.H.; Chapuis, B.; Helg, C.; Lansdorp, P.M.; Roosnek, E. Accelerated telomere shortening in hematological lineages is limited to the first year following stem cell transplantation. Blood 2001, 97, 575–577. [Google Scholar] [CrossRef]
- Tanchot, C.; Lemonnier, F.A.; Perarnau, B.; Freitas, A.A.; Rocha, B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science 1997, 276, 2057–2062. [Google Scholar] [CrossRef]
- Suessmuth, Y.; Mukherjee, R.; Watkins, B.; Koura, D.T.; Finstermeier, K.; Desmarais, C.; Stempora, L.; Horan, J.T.; Langston, A.; Qayed, M.; et al. CMV reactivation drives posttransplant T-cell reconstitution and results in defects in the underlying TCRbeta repertoire. Blood 2015, 125, 3835–3850. [Google Scholar] [CrossRef]
- Klein, A.K.; Patel, D.D.; Gooding, M.E.; Sempowski, G.D.; Chen, B.J.; Liu, C.; Kurtzberg, J.; Haynes, B.F.; Chao, N.J. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol. Blood Marrow Transplant. 2001, 7, 454–466. [Google Scholar] [CrossRef]
- Komanduri, K.V.; St John, L.S.; de Lima, M.; McMannis, J.; Rosinski, S.; McNiece, I.; Bryan, S.G.; Kaur, I.; Martin, S.; Wieder, E.D.; et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood 2007, 110, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, A.; Peffault de Latour, R.; Carmagnat, M.; Clave, E.; Douay, C.; Larghero, J.; Cayuela, J.M.; Traineau, R.; Robin, M.; Madureira, A.; et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl. Infect. Dis. 2011, 13, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Kyewski, B.; Allen, P.M.; Hogquist, K.A. Positive and negative selection of the T cell repertoire: What thymocytes see (and don’t see). Nat. Rev. Immunol. 2014, 14, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, K.; Blazar, B.R.; Wagner, J.E.; Agura, E.; Hill, B.J.; Smogorzewska, M.; Koup, R.A.; Betts, M.R.; Collins, R.H.; Douek, D.C. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood 2001, 97, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Politikos, I.; Boussiotis, V.A. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood 2014, 124, 3201–3211. [Google Scholar] [CrossRef]
- Douek, D.C.; Vescio, R.A.; Betts, M.R.; Brenchley, J.M.; Hill, B.J.; Zhang, L.; Berenson, J.R.; Collins, R.H.; Koup, R.A. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 2000, 355, 1875–1881. [Google Scholar] [CrossRef]
- Clave, E.; Busson, M.; Douay, C.; Peffault de Latour, R.; Berrou, J.; Rabian, C.; Carmagnat, M.; Rocha, V.; Charron, D.; Socie, G.; et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood 2009, 113, 6477–6484. [Google Scholar] [CrossRef]
- Talvensaari, K.; Clave, E.; Douay, C.; Rabian, C.; Garderet, L.; Busson, M.; Garnier, F.; Douek, D.; Gluckman, E.; Charron, D.; et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood 2002, 99, 1458–1464. [Google Scholar] [CrossRef]
- Nguyen, S.; Achour, A.; Souchet, L.; Vigouroux, S.; Chevallier, P.; Furst, S.; Sirvent, A.; Bay, J.O.; Socie, G.; Ceballos, P.; et al. Clinical impact of NK-cell reconstitution after reduced intensity conditioned unrelated cord blood transplantation in patients with acute myeloid leukemia: Analysis of a prospective phase II multicenter trial on behalf of the Societe Francaise de Greffe de Moelle Osseuse et Therapie Cellulaire and Eurocord. Bone Marrow Transpl. 2017, 52, 1428–1435. [Google Scholar] [CrossRef]
- Beziat, V.; Nguyen, S.; Lapusan, S.; Hervier, B.; Dhedin, N.; Bories, D.; Uzunov, M.; Boudifa, A.; Trebeden-Negre, H.; Norol, F.; et al. Fully functional NK cells after unrelated cord blood transplantation. Leukemia 2009, 23, 721–728. [Google Scholar] [CrossRef]
- Oshrine, B.R.; Li, Y.; Teachey, D.T.; Heimall, J.; Barrett, D.M.; Bunin, N. Immunologic recovery in children after alternative donor allogeneic transplantation for hematologic malignancies: Comparison of recipients of partially T cell-depleted peripheral blood stem cells and umbilical cord blood. Biol. Blood Marrow Transplant. 2013, 19, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Foley, B.; Felices, M.; Cichocki, F.; Cooley, S.; Verneris, M.R.; Miller, J.S. The biology of NK cells and their receptors affects clinical outcomes after hematopoietic cell transplantation (HCT). Immunol. Rev. 2014, 258, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.C.; Ottinger, H.; Ferencik, S.; Sribar, M.; Punzel, M.; Beelen, D.W.; Schwan, M.A.; Grosse-Wilde, H.; Wernet, P.; Uhrberg, M. Relevance of C1 and C2 epitopes for hemopoietic stem cell transplantation: Role for sequential acquisition of HLA-C-specific inhibitory killer Ig-like receptor. J. Immunol. 2007, 178, 3918–3923. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, P.; Hebia-Fellah, I.; Planche, L.; Guillaume, T.; Bressolette-Bodin, C.; Coste-Burel, M.; Rialland, F.; Mohty, M.; Imbert-Marcille, B.M. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: A comparison with matched unrelated donors as stem cell source. Bone Marrow Transpl. 2010, 45, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Sashihara, J.; Tanaka-Taya, K.; Tanaka, S.; Amo, K.; Miyagawa, H.; Hosoi, G.; Taniguchi, T.; Fukui, T.; Kasuga, N.; Aono, T.; et al. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood 2002, 100, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.L.; Pritchett, J.C.; Leifer, C.; Zerr, D.M.; Koelle, D.M.; Di Luca, D.; Lusso, P. HHV-6B infection, T-cell reconstitution, and graft-vs-host disease after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2018, 53, 1508–1517. [Google Scholar] [CrossRef]
- De Koning, C.; Admiraal, R.; Nierkens, S.; Boelens, J.J. Human herpesvirus 6 viremia affects T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2018, 2, 428–432. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Said, B.; Cao, Q.; Miller, J.S.; Weisdorf, D.J. Weisdorf Early HHV-6 Reactivation in CMV-Seronegative Cord Blood Transplant Recipients is Associated with Inferior Relapse-Free and Overall Survival. Biol. Blood Marrow Transplant. 2018, 24, S368–S369. [Google Scholar] [CrossRef]
- Van Heijst, J.W.; Ceberio, I.; Lipuma, L.B.; Samilo, D.W.; Wasilewski, G.D.; Gonzales, A.M.; Nieves, J.L.; van den Brink, M.R.; Perales, M.A.; Pamer, E.G. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat. Med. 2013, 19, 372–377. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, M.; Song, R.; Ma, J.; Shi, Y.; Qin, W.; Jin, Y. A glucocorticoid amplifies IL-2-induced selective expansion of CD4(+)CD25(+)FOXP3(+) regulatory T cells in vivo and suppresses graft-versus-host disease after allogeneic lymphocyte transplantation. Acta Biochim. Biophys. Sin. 2009, 41, 781–791. [Google Scholar] [CrossRef]
- Chen, X.; Oppenheim, J.J.; Winkler-Pickett, R.T.; Ortaldo, J.R.; Howard, O.M. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 2006, 36, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Meng, C.; Ivashkiv, L.B. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc. Natl. Acad. Sci. USA 2000, 97, 9573–9578. [Google Scholar] [CrossRef] [PubMed]
- Theiss-Suennemann, J.; Jorss, K.; Messmann, J.J.; Reichardt, S.D.; Montes-Cobos, E.; Luhder, F.; Tuckermann, J.P.; AWolff, H.; Dressel, R.; Grone, H.J.; et al. Glucocorticoids attenuate acute graft-versus-host disease by suppressing the cytotoxic capacity of CD8(+) T cells. J. Pathol. 2015, 235, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Vanderheyde, N.; Verhasselt, V.; Goldman, M.; Willems, F. Inhibition of human dendritic cell functions by methylprednisolone. Transplantation 1999, 67, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Franchimont, D. Overview of the actions of glucocorticoids on the immune response: A good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. N. Y. Acad. Sci. 2004, 1024, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Krenger, W.; Rossi, S.; Hollander, G.A. Apoptosis of thymocytes during acute graft-versus-host disease is independent of glucocorticoids. Transplantation 2000, 69, 2190–2193. [Google Scholar] [CrossRef] [PubMed]
- Krenger, W.; Hollander, G.A. The immunopathology of thymic GVHD. Semin. Immunopathol. 2008, 30, 439–456. [Google Scholar] [CrossRef]
- Admiraal, R.; Lindemans, C.A.; van Kesteren, C.; Bierings, M.B.; Versluijs, A.B.; Nierkens, S.; Boelens, J.J. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood 2016, 128, 2734–2741. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Chiesa, R.; Amrolia, P.J.; Rao, K.; Nikolajeva, O.; de Wildt, A.; Gerhardt, C.E.; Gilmour, K.; Bierings, M.; Veys, P.; et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood 2014, 123, 126–132. [Google Scholar] [CrossRef]
- Zheng, C.; Luan, Z.; Fang, J.; Sun, X.; Chen, J.; Li, C.K.; Hu, S.; Zhu, Y.; Sun, Z. Comparison of conditioning regimens with or without antithymocyte globulin for unrelated cord blood transplantation in children with high-risk or advanced hematological malignancies. Biol. Blood Marrow Transplant. 2015, 21, 707–712. [Google Scholar] [CrossRef]
- Tong, J.; Xuan, L.; Sun, Y.; Huang, D.; Liu, H.; Zheng, C.; Zhu, X.; Tang, B.; Song, K.; Zhang, X.; et al. Umbilical Cord Blood Transplantation without Antithymocyte Globulin Results in Similar Survival but Better Quality of Life Compared with Unrelated Peripheral Blood Stem Cell Transplantation for the Treatment of Acute Leukemia-A Retrospective Study in China. Biol. Blood Marrow Transplant. 2017, 23, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.B.; Jacobson, J.S.; Freedman, J.; George, D.; Moore, V.; van de Ven, C.; Satwani, P.; Bhatia, M.; Garvin, J.H.; Bradley, M.B.; et al. A comparison of immune reconstitution and graft-versus-host disease following myeloablative conditioning versus reduced toxicity conditioning and umbilical cord blood transplantation in paediatric recipients. Br. J. Haematol. 2011, 155, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Chao, N.J.; Liu, C.X.; Rooney, B.; Chen, B.J.; Long, G.D.; Vredenburgh, J.J.; Morris, A.; Gasparetto, C.; Rizzieri, D.A. Nonmyeloablative regimen preserves “niches” allowing for peripheral expansion of donor T-cells. Biol. Blood Marrow Transplant. 2002, 8, 249–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moretta, A.; Maccario, R.; Fagioli, F.; Giraldi, E.; Busca, A.; Montagna, D.; Miniero, R.; Comoli, P.; Giorgiani, G.; Zecca, M.; et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp. Hematol. 2001, 29, 371–379. [Google Scholar] [CrossRef]
- Clave, E.; Lisini, D.; Douay, C.; Giorgiani, G.; Busson, M.; Zecca, M.; Moretta, F.; Acquafredda, G.; Brescia, L.P.; Locatelli, F.; et al. Thymic function recovery after unrelated donor cord blood or T-cell depleted HLA-haploidentical stem cell transplantation correlates with leukemia relapse. Front. Immunol. 2013, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, M.; Wollny, G.; Tzaribaschev, N.; Dietz, K.; Brugger, D.; Bader, P.; Lang, P.; Schilbach, K.; Winkler, B.; Niethammer, D.; et al. Onset of thymic recovery and plateau of thymic output are differentially regulated after stem cell transplantation in children. Biol. Blood Marrow Transplant. 2005, 11, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.E., Jr.; Eapen, M.; Carter, S.; Wang, Y.; Schultz, K.R.; Wall, D.A.; Bunin, N.; Delaney, C.; Haut, P.; Margolis, D.; et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N. Engl. J. Med. 2014, 371, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; Galambrun, C.; Sirvent, A.; Pochon, C.; Bruno, B.; Jubert, C.; Loundou, A.; Yakoub-Agha, I.; Milpied, N.; Lutz, P.; et al. Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood 2016, 127, 3450–3457. [Google Scholar] [CrossRef]
- Brown, J.A.; Stevenson, K.; Kim, H.T.; Cutler, C.; Ballen, K.; McDonough, S.; Reynolds, C.; Herrera, M.; Liney, D.; Ho, V.; et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood 2010, 115, 4111–4119. [Google Scholar] [CrossRef]
- Verneris, M.R.; Brunstein, C.G.; Barker, J.; MacMillan, M.L.; DeFor, T.; McKenna, D.H.; Burke, M.J.; Blazar, B.R.; Miller, J.S.; McGlave, P.B.; et al. Relapse risk after umbilical cord blood transplantation: Enhanced graft-versus-leukemia effect in recipients of 2 units. Blood 2009, 114, 4293–4299. [Google Scholar] [CrossRef]
- Sauter, C.; Abboud, M.; Jia, X.; Heller, G.; Gonzales, A.M.; Lubin, M.; Hawke, R.; Perales, M.A.; van den Brink, M.R.; Giralt, S.; et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol. Blood Marrow Transplant. 2011, 17, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Szabolcs, P.; Niedzwiecki, D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy 2007, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Admiraal, R.; de Koning, C.C.H.; Lindemans, C.A.; Bierings, M.B.; Wensing, A.M.J.; Versluys, A.B.; Wolfs, T.F.W.; Nierkens, S.; Boelens, J.J. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J. Allergy Clin. Immunol. 2017, 140, 1643–1650.e1496. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.C.; Wagner, J.E.; DeFor, T.E.; Brunstein, C.G.; Schleiss, M.R.; Young, J.A.; Weisdorf, D.H.; Cooley, S.; Miller, J.S.; Verneris, M.R. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol. Blood Marrow Transplant. 2010, 16, 215–222. [Google Scholar] [CrossRef] [PubMed]
- McGoldrick, S.M.; Bleakley, M.E.; Guerrero, A.; Turtle, C.J.; Yamamoto, T.N.; Pereira, S.E.; Delaney, C.S.; Riddell, S.R. Cytomegalovirus-specific T cells are primed early after cord blood transplant but fail to control virus in vivo. Blood 2013, 121, 2796–2803. [Google Scholar] [CrossRef]
- Cao, K.; Marin, D.; Sekine, T.; Rondon, G.; Zhao, W.; Smith, N.T.; Daher, M.; Wang, Q.; Li, L.; Saliba, R.M.; et al. Donor NKG2C Copy Number: An Independent Predictor for CMV Reactivation After Double Cord Blood Transplantation. Front. Immunol. 2018, 9, 2444. [Google Scholar] [CrossRef]
- Bejanyan, N.; Brunstein, C.G.; Cao, Q.; Lazaryan, A.; Luo, X.; Curtsinger, J.; Mehta, R.S.; Warlick, E.; Cooley, S.A.; Blazar, B.R.; et al. Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv. 2018, 2, 909–922. [Google Scholar] [CrossRef]
- Admiraal, R.; Chiesa, R.; Lindemans, C.A.; Nierkens, S.; Bierings, M.B.; Versluijs, A.B.; Hiwarkar, P.; Furtado Silva, J.M.; Veys, P.; Boelens, J.J. Leukemia-free survival in myeloid leukemia, but not in lymphoid leukemia, is predicted by early CD4+ reconstitution following unrelated cord blood transplantation in children: A multicenter retrospective cohort analysis. Bone Marrow Transpl. 2016, 51, 1376–1378. [Google Scholar] [CrossRef]
- Parkman, R.; Cohen, G.; Carter, S.L.; Weinberg, K.I.; Masinsin, B.; Guinan, E.; Kurtzberg, J.; Wagner, J.E.; Kernan, N.A. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol. Blood Marrow Transplant. 2006, 12, 919–927. [Google Scholar] [CrossRef][Green Version]
- Brunstein, C.G.; Gutman, J.A.; Weisdorf, D.J.; Woolfrey, A.E.; Defor, T.E.; Gooley, T.A.; Verneris, M.R.; Appelbaum, F.R.; Wagner, J.E.; Delaney, C. Allogeneic hematopoietic cell transplantation for hematologic malignancy: Relative risks and benefits of double umbilical cord blood. Blood 2010, 116, 4693–4699. [Google Scholar] [CrossRef]
- Barker, J.N.; Weisdorf, D.J.; DeFor, T.E.; Blazar, B.R.; Miller, J.S.; Wagner, J.E. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood 2003, 102, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.E., Jr.; Brunstein, C.G.; Boitano, A.E.; DeFor, T.E.; McKenna, D.; Sumstad, D.; Blazar, B.R.; Tolar, J.; Le, C.; Jones, J.; et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell 2016, 18, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.E.; Chao, N.J.; Rizzieri, D.A.; Long, G.D.; Sullivan, K.M.; Gasparetto, C.; Chute, J.P.; Morris, A.; McDonald, C.; Waters-Pick, B.; et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J. Clin. Investig. 2014, 124, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.D.; Varma, A.; Hussain, M.J.; Nathan, S.; Brunstein, C. Clinical Relevance of Immunobiology in Umbilical Cord Blood Transplantation. J. Clin. Med. 2019, 8, 1968. https://doi.org/10.3390/jcm8111968
Yun HD, Varma A, Hussain MJ, Nathan S, Brunstein C. Clinical Relevance of Immunobiology in Umbilical Cord Blood Transplantation. Journal of Clinical Medicine. 2019; 8(11):1968. https://doi.org/10.3390/jcm8111968
Chicago/Turabian StyleYun, Hyun Don, Ankur Varma, Mohammad J. Hussain, Sunita Nathan, and Claudio Brunstein. 2019. "Clinical Relevance of Immunobiology in Umbilical Cord Blood Transplantation" Journal of Clinical Medicine 8, no. 11: 1968. https://doi.org/10.3390/jcm8111968
APA StyleYun, H. D., Varma, A., Hussain, M. J., Nathan, S., & Brunstein, C. (2019). Clinical Relevance of Immunobiology in Umbilical Cord Blood Transplantation. Journal of Clinical Medicine, 8(11), 1968. https://doi.org/10.3390/jcm8111968



