Neurocircuitry of Reward and Addiction: Potential Impact of Dopamine–Glutamate Co-release as Future Target in Substance Use Disorder
Abstract
:1. Dopamine and Substance Use Disorder
2. DA-Glutamate Co-Release in the Mesolimbic System
2.1. DA Neurons in the VTA
2.2. Vesicular Glutamate Transporters and the Concept of DA-Glutamate Co-Release
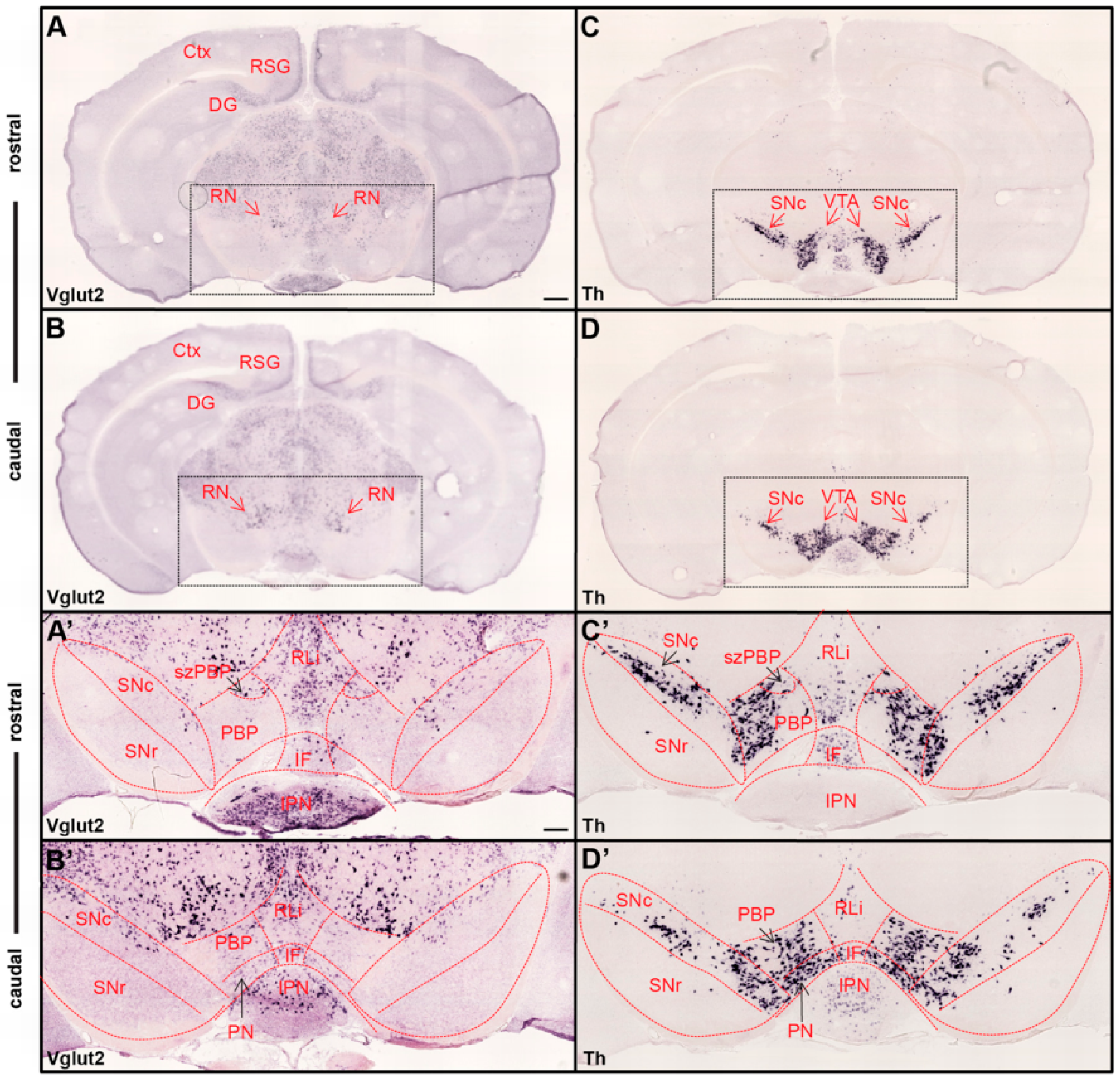
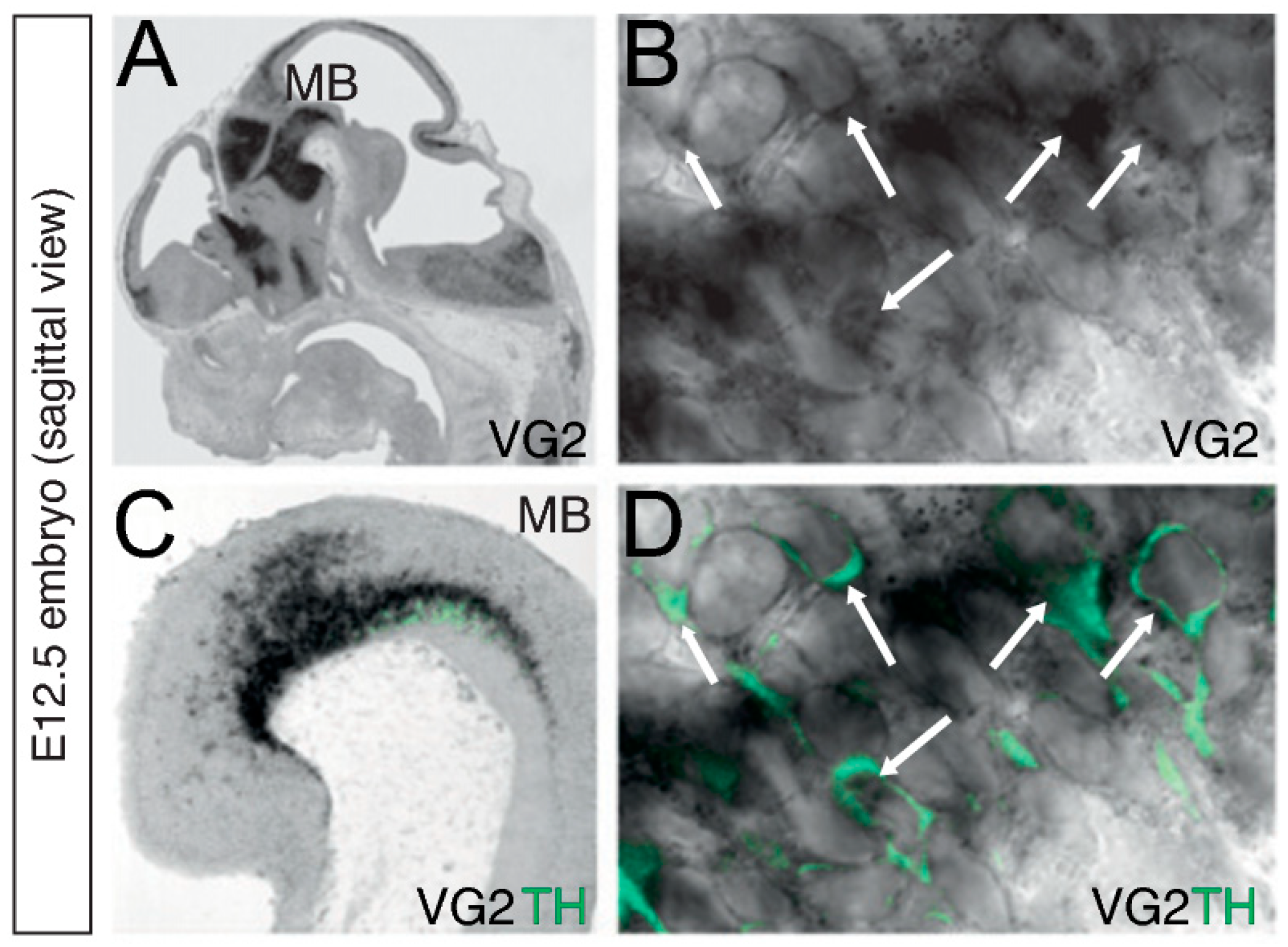
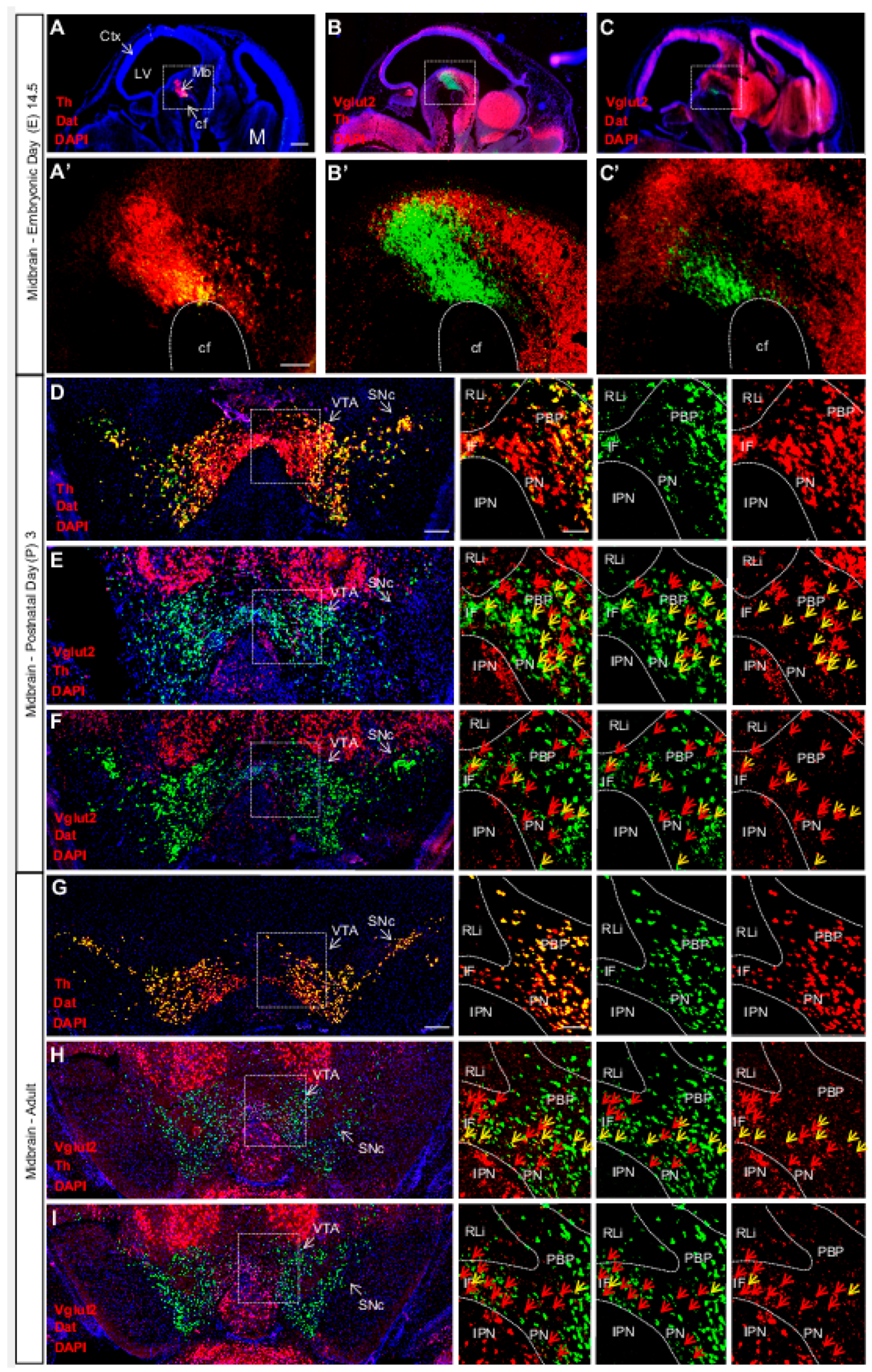
2.3. Expression Patterns of VGLUT2 in the Midbrain DA System and Validation of Glutamate Co-Release
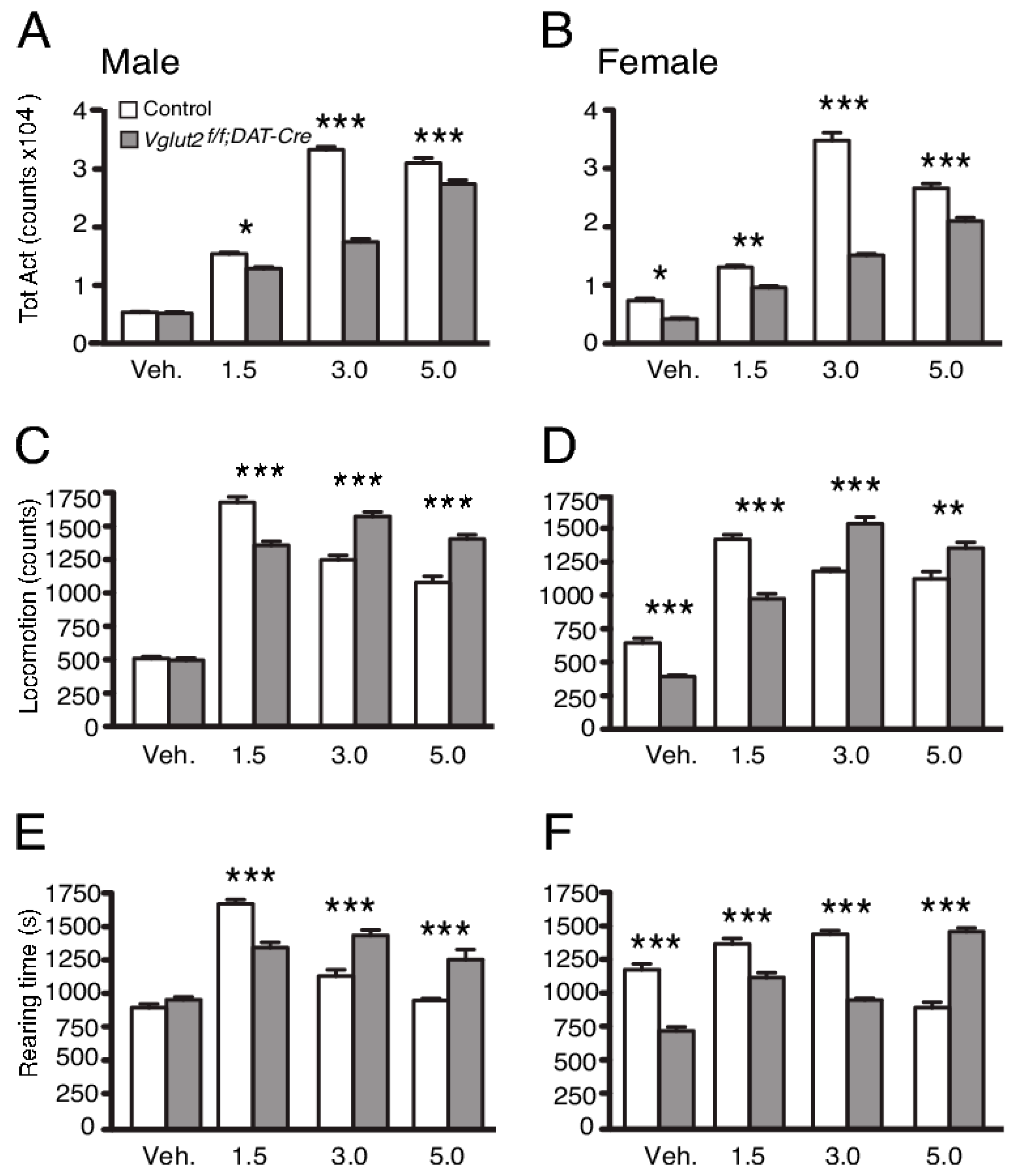
2.4. Behavioral Consequences upon Disrupted Dopamine–Glutamate Co-Release in Transgenic Mice
2.5. DAT-Cre-Mediated Gene Targeting of VGLUT2 in Studies Aiming to Unravel the Importance of Glutamate Co-Release in Neurocircuitry of Reward and Addiction
2.6. DAT-Cre-Mediated Gene Targeting of Phosphate-Activated Glutaminase (GLS1)
2.7. Some Caveats in the Implementation of Transgenics to Address Neuronal Function
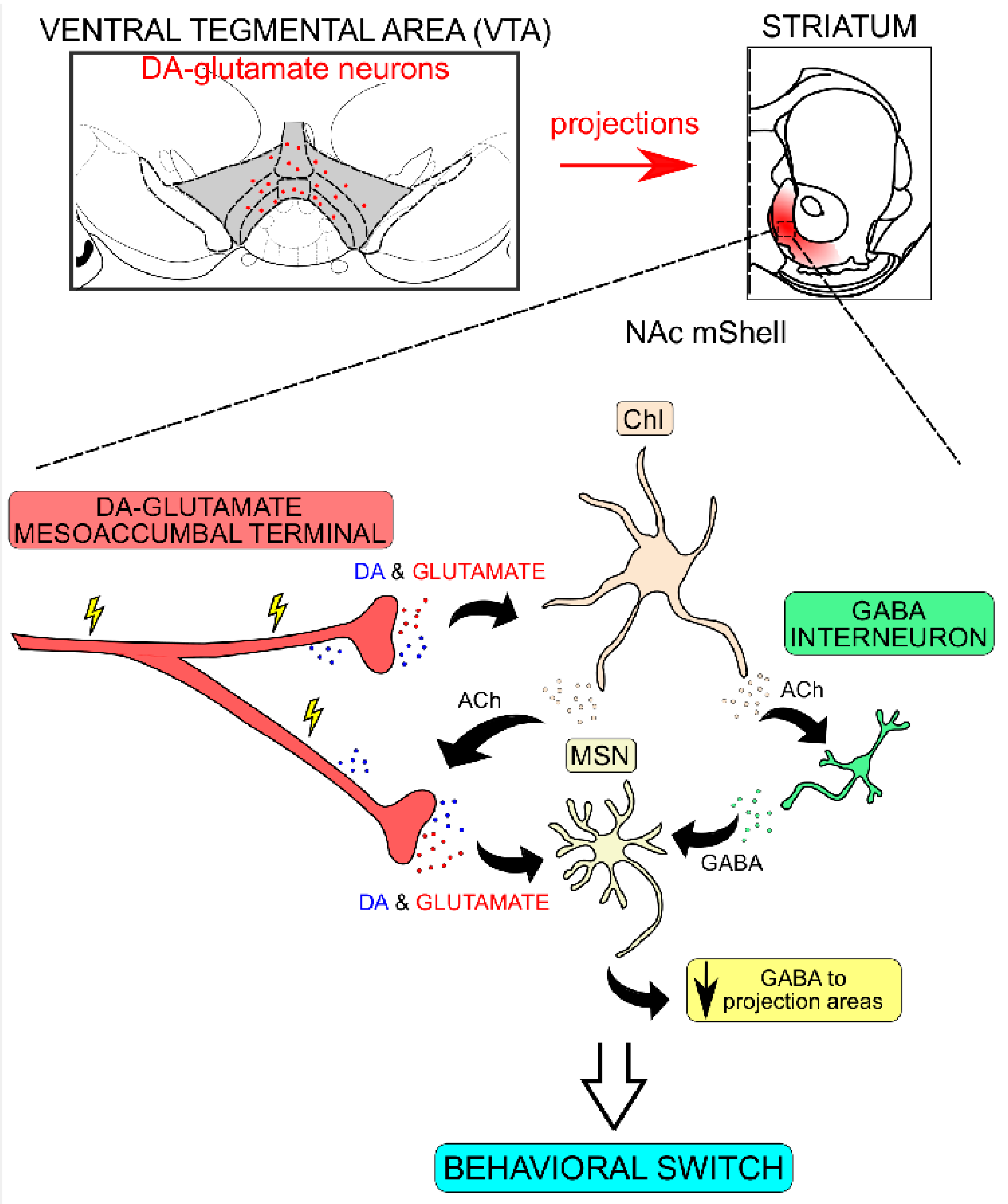
2.8. DA–Glutamate Co-Release in Neuronal Plasticity within the Ventral Striatum
2.9. Dopamine–Glutamate Co-Release: Implications for Reward Processing
2.10. Whole-Brain Analysis and Improved Selectivity in Animal Models Should Enhance Current Knowledge of DA-Glutamate Co-Releasing Neurons
2.11. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lüscher, C. The Emergence of a Circuit Model for Addiction. Annu. Rev. Neurosci. 2016, 39, 257–276. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [Green Version]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Kourrich, S.; Calu, D.J.; Bonci, A. Intrinsic plasticity: An emerging player in addiction. Nat. Rev. Neurosci. 2015, 16, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Van Huijstee, A.N.; Mansvelder, H.D. Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction. Front. Cell. Neurosci. 2015, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Logan, J.; Childress, A.R.; Jayne, M.; Ma, Y.; Wong, C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J. Neurosci. 2006, 26, 6583–6588. [Google Scholar] [CrossRef] [PubMed]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in Motivational Control: Rewarding, Aversive, and Alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, K.C.; Robinson, T.E. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998, 28, 309–369. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef]
- Ikemoto, S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 2007, 56, 27–78. [Google Scholar] [CrossRef]
- Threlfell, S.; Cragg, S.J. Dopamine signaling in dorsal versus ventral striatum: The dynamic role of cholinergic interneurons. Front. Syst. Neurosci. 2011, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Wang, G. The Addicted Human Brain: Insights from Imaging Studies Find the Latest Version: The Addicted Human Brain: Insights from Imaging Studies. J. Clin. Investig. 2003, 111, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef] [PubMed]
- Pontieri, F.E.; Tanda, G.; Di Chiara, G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc. Natl. Acad. Sci. USA 1995, 92, 12304–12308. [Google Scholar] [CrossRef]
- Ungless, M.A.; Whistler, J.L.; Malenka, R.C.; Bonci, A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 2001, 411, 583–587. [Google Scholar] [CrossRef]
- Chen, B.T.; Bowers, M.S.; Martin, M.; Hopf, F.W.; Guillory, A.M.; Carelli, R.M.; Chou, J.K.; Bonci, A. Cocaine but Not Natural Reward Self-Administration nor Passive Cocaine Infusion Produces Persistent LTP in the VTA. Neuron 2008, 59, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Kourrich, S.; Rothwell, P.E.; Klug, J.R.; Thomas, M.J. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 2007, 27, 7921–7928. [Google Scholar] [CrossRef]
- Pascoli, V.; Turiault, M.; Lüscher, C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature 2012, 481, 71–76. [Google Scholar] [CrossRef]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef]
- Swanson, L.W. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982, 9, 321–353. [Google Scholar] [CrossRef]
- Fu, Y.H.; Yuan, Y.; Halliday, G.; Rusznák, Z.; Watson, C.; Paxinos, G. A cytoarchitectonic and chemoarchitectonic analysis of the dopamine cell groups in the substantia nigra, ventral tegmental area, and retrorubral field in the mouse. Brain Struct. Funct. 2012, 217, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Pupe, S.; Wallén-Mackenzie, Å. Cre-driven optogenetics in the heterogeneous genetic panorama of the VTA. Trends Neurosci. 2015, 38, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Viereckel, T.; Dumas, S.; Smith-Anttila, C.J.A.; Vlcek, B.; Bimpisidis, Z.; Lagerström, M.C.; Konradsson-Geuken, Å.; Wallén-Mackenzie, Å. Midbrain Gene Screening Identifies a New Mesoaccumbal Glutamatergic Pathway and a Marker for Dopamine Cells Neuroprotected in Parkinson’s Disease. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Papathanou, M.; Creed, M.; Dorst, M.C.; Bimpisidis, Z.; Dumas, S.; Pettersson, H.; Bellone, C.; Silberberg, G.; Lüscher, C.; Wallén-Mackenzie, Å. Targeting VGLUT2 in Mature Dopamine Neurons Decreases Mesoaccumbal Glutamatergic Transmission and Identifies a Role for Glutamate Co-release in Synaptic Plasticity by Increasing Baseline AMPA/NMDA Ratio. Front. Neural Circuits 2018, 12, 1–20. [Google Scholar] [CrossRef]
- Falck, B.; Hillarp, N.-Å.; Thieme, G.; Torp, A. Fluorescence of catechol amines and related compounds condensed with formaldehyde. J. Histochem. Cytochem. 1962, 10, 348–354. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I.Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. Suppl. 1964, 1–55. [Google Scholar]
- Carlsson, A.; Falck, B.; Hillarp, N.A. Cellular localization of brain monoamines. Acta Physiol. Scand. Suppl. 1962, 56, 1–28. [Google Scholar]
- Bai, L.; Xu, H.; Collins, J.F.; Ghishan, F.K. Molecular and Functional Analysis of a Novel Neuronal Vesicular Glutamate Transporter. J. Biol. Chem. 2001, 276, 36764–36769. [Google Scholar] [CrossRef] [Green Version]
- Bellocchio, E.E.; Reimer, R.J.; Fremeau, J.; Edwards, R.H. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 2000, 289, 957–960. [Google Scholar] [CrossRef]
- Herzog, E.; Bellenchi, G.C.; Gras, C.; Bernard, V.; Ravassard, P.; Bedet, C.; Gasnier, B.; Giros, B.; El Mestikawy, S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J. Neurosci. 2001, 21, 2–7. [Google Scholar] [CrossRef]
- Takamori, S.; Rhee, J.S.; Rosenmund, C.; Jahn, R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2). J. Neurosci. 2001, 21, 1–6. [Google Scholar] [CrossRef]
- Varoqui, H.; Schäfer, M.K.H.; Zhu, H.; Weihe, E.; Erickson, J.D. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J. Neurosci. 2002, 22, 142–155. [Google Scholar] [CrossRef] [PubMed]
- El Mestikawy, S.; Wallén-Mackenzie, Å.; Fortin, G.M.; Descarries, L.; Trudeau, L.E. From glutamate co-release to vesicular synergy: Vesicular glutamate transporters. Nat. Rev. Neurosci. 2011, 12, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, L.E.; Hnasko, T.S.; Wallén-Mackenzie, Å.; Morales, M.; Rayport, S.; Sulzer, D. The multilingual nature of dopamine neurons. Prog. Brain Res. 2014, 211, 141–164. [Google Scholar] [Green Version]
- Sulzer, D.; Joyce, M.P.; Lin, L.; Geldwert, D.; Haber, S.N.; Hattori, T.; Rayport, S. Dopamine neurons make glutamatergic synapses in vitro. J. Neurosci. 1998, 18, 4588–4602. [Google Scholar] [CrossRef]
- Bourque, M.J.; Trudeau, L.E. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur. J. Neurosci. 2000, 12, 3172–3180. [Google Scholar] [CrossRef]
- Joyce, M.P.; Rayport, S. Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience 2000, 99, 445–456. [Google Scholar] [CrossRef]
- Chuhma, N.; Zhang, H.; Masson, J.; Zhuang, X.; Sulzer, D.; Hen, R.; Rayport, S. Dopamine Neurons Mediate a Fast Excitatory Signal via Their Glutamatergic Synapses. J. Neurosci. 2004, 24, 972–981. [Google Scholar] [CrossRef] [Green Version]
- Lavin, A.; Nogueira, L.; Lapish, C.C.; Wightman, R.M.; Phillips, P.E.M.; Seamans, J.K. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J. Neurosci. 2005, 25, 5013–5023. [Google Scholar] [CrossRef]
- Dal Bo, G.; St.-Gelais, F.; Danik, M.; Williams, S.; Cotton, M.; Trudeau, L.E. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J. Neurochem. 2004, 88, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J.; Root, D.H.; Zhang, S.; Morales, M. Multiplexed neurochemical signaling by neurons of the ventral tegmental area. J. Chem. Neuroanat. 2016, 73, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Qi, J.; Li, X.; Wang, H.L.; Britt, J.P.; Hoffman, A.F.; Bonci, A.; Lupica, C.R.; Morales, M. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat. Neurosci. 2015, 18, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, L.-E.; El Mestikawy, S. Glutamate Cotransmission in Cholinergic, GABAergic and Monoamine Systems: Contrasts and Commonalities. Front. Neural Circuits 2018, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Mingote, S.; Amsellem, A.; Kempf, A.; Rayport, S.; Chuhma, N. Dopamine-Glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching. Neurochem. Int. 2019, 129, 104482. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, T.S.; Edwards, R.H. Neurotransmitter Corelease: Mechanism and Physiological Role. Annu. Rev. Physiol. 2012, 74, 225–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, M.; Kawasaki, A.; Sakata-Haga, H.; Fukui, Y.; Kawano, H.; Nogami, H.; Hisano, S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J. Comp. Neurol. 2006, 498, 581–592. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Wang, H.-L.; Li, X.; Ng, T.H.; Morales, M. Mesocorticolimbic Glutamatergic Pathway. J. Neurosci. 2011, 31, 8476–8490. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Qi, J.; Wang, H.L.; Zhang, S.; Morales, M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur. J. Neurosci. 2015, 41, 760–772. [Google Scholar] [CrossRef] [Green Version]
- Root, D.H.; Wang, H.L.; Liu, B.; Barker, D.J.; Mód, L.; Szocsics, P.; Silva, A.C.; Maglóczky, Z.; Morales, M. Glutamate neurons are intermixed with midbrain dopamine neurons in nonhuman primates and humans. Sci. Rep. 2016, 6, 30615. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Wang, H.L.; Morales, M. Glutamate neurons in the substantia nigra compacta and retrorubral field. Eur. J. Neurosci. 2013, 38, 3602–3610. [Google Scholar] [CrossRef]
- Morales, M.; Root, D.H. Glutamate neurons within the midbrain dopamine regions. Neuroscience 2014, 282, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, T.; Sheen, W.; Morales, M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur. J. Neurosci. 2007, 25, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez, J.A.; Bourque, M.J.; Dal Bo, G.; Bourdeau, M.L.; Danik, M.; Williams, S.; Lacaille, J.C.; Trudeau, L.E. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J. Neurosci. 2008, 28, 6309–6318. [Google Scholar] [CrossRef] [PubMed]
- Birgner, C.; Nordenankar, K.; Lundblad, M.; Mendez, J.A.; Smith, C.; le Greves, M.; Galter, D.; Olson, L.; Fredriksson, A.; Trudeau, L.-E.; et al. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc. Natl. Acad. Sci. USA 2010, 107, 389–394. [Google Scholar] [CrossRef]
- Steinkellner, T.; Zell, V.; Farino, Z.J.; Sonders, M.S.; Villeneuve, M.; Freyberg, R.J.; Przedborski, S.; Lu, W.; Freyberg, Z.; Hnasko, T.S. Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons. J. Clin. Investig. 2018, 128, 774–788. [Google Scholar] [CrossRef] [Green Version]
- Tecuapetla, F.; Patel, J.C.; Xenias, H.; English, D.; Tadros, I.; Shah, F.; Berlin, J.; Deisseroth, K.; Rice, M.E.; Tepper, J.M.; et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J. Neurosci. 2010, 30, 7105–7110. [Google Scholar] [CrossRef]
- Stuber, G.D.; Hnasko, T.S.; Britt, J.P.; Edwards, R.H.; Bonci, A. Dopaminergic Terminals in the Nucleus Accumbens But Not the Dorsal Striatum Corelease Glutamate. J. Neurosci. 2010, 30, 8229–8233. [Google Scholar] [CrossRef]
- Chuhma, N.; Mingote, S.; Moore, H.; Rayport, S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron 2014, 81, 901–912. [Google Scholar] [CrossRef]
- Chuhma, N.; Mingote, S.; Yetnikoff, L.; Kalmbach, A.; Ma, T.; Ztaou, S.; Sienna, A.C.; Tepler, S.; Poulin, J.F.; Ansorge, M.; et al. Dopamine neuron glutamate cotransmission evokes a delayed excitation in lateral dorsal striatal cholinergic interneurons. eLife 2018, 7, 1–29. [Google Scholar] [CrossRef]
- Cai, Y.; Ford, C.P. Dopamine Cells Differentially Regulate Striatal Cholinergic Transmission across Regions through Corelease of Dopamine and Glutamate. Cell Rep. 2018, 25, 3148.e3–3157.e3. [Google Scholar] [CrossRef]
- Alsiö, J.; Nordenankar, K.; Arvidsson, E.; Birgner, C.; Mahmoudi, S.; Halbout, B.; Smith, C.; Fortin, G.M.; Olson, L.; Descarries, L.; et al. Enhanced Sucrose and Cocaine Self-Administration and Cue-Induced Drug Seeking after Loss of VGLUT2 in Midbrain Dopamine Neurons in Mice. J. Neurosci. 2011, 31, 12593–12603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortin, G.M.; Bourque, M.-J.; Mendez, J.A.; Leo, D.; Nordenankar, K.; Birgner, C.; Arvidsson, E.; Rymar, V.V.; Berube-Carriere, N.; Claveau, A.-M.; et al. Glutamate Corelease Promotes Growth and Survival of Midbrain Dopamine Neurons. J. Neurosci. 2012, 32, 17477–17491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hnasko, T.S.; Chuhma, N.; Zhang, H.; Goh, G.Y.; Sulzer, D.; Palmiter, R.D.; Rayport, S.; Edwards, R.H. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 2010, 65, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Mingote, S.; Chuhma, N.; Kalmbach, A.; Thomsen, G.M.; Wang, Y.; Mihali, A.; Sferrazza, C.; Zucker-Scharff, I.; Siena, A.C.; Welch, M.G.; et al. Dopamine neuron dependent behaviours mediated by glutamate cotransmission. eLife 2017, 6, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.V.; Viereckel, T.; Zell, V.; Konradsson-Geuken, Å.; Broker, C.J.; Talishinsky, A.; Yoo, J.H.; Galinato, M.H.; Arvidsson, E.; Kesner, A.J.; et al. Disrupting Glutamate Co-transmission Does Not Affect Acquisition of Conditioned Behavior Reinforced by Dopamine Neuron Activation. Cell Rep. 2017, 18, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Wallén-Mackenzie, Å.; Gezelius, H.; Thoby-Brisson, M.; Nygard, A.; Enjin, A.; Fujiyama, F.; Fortin, G.; Kullander, K. Vesicular Glutamate Transporter 2 Is Required for Central Respiratory Rhythm Generation But Not for Locomotor Central Pattern Generation. J. Neurosci. 2006, 26, 12294–12307. [Google Scholar] [CrossRef]
- Wallén-Mackenzie, Å.; Nordenankar, K.; Fejgin, K.; Lagerström, M.C.; Emilsson, L.; Fredriksson, R.; Wass, C.; Andersson, D.; Egecioglu, E.; Andersson, M.; et al. Restricted cortical and amygdaloid removal of vesicular glutamate transporter 2 in preadolescent mice impacts dopaminergic activity and neuronal circuitry of higher brain function. J. Neurosci. 2009, 29, 2238–2251. [Google Scholar] [CrossRef]
- Stornetta, R.L.; Sevigny, C.P.; Schreihofer, A.M.; Rosin, D.L.; Guyenet, P.G. Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J. Comp. Neurol. 2002, 444, 207–220. [Google Scholar] [CrossRef]
- Stornetta, R.L.; Sevigny, C.P.; Guyenet, P.G. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J. Comp. Neurol. 2002, 444, 191–206. [Google Scholar] [CrossRef]
- Nordenankar, K.; Smith-Anttila, C.J.A.; Schweizer, N.; Viereckel, T.; Birgner, C.; Mejia-Toiber, J.; Morales, M.; Leao, R.N.; Wallén-Mackenzie, Å. Increased hippocampal excitability and impaired spatial memory function in mice lacking VGLUT2 selectively in neurons defined by tyrosine hydroxylase promoter activity. Brain Struct. Funct. 2015, 220, 2171–2190. [Google Scholar] [CrossRef]
- Campos-Melo, D.; Galleguillos, D.; Sánchez, N.; Gysling, K.; Andrés, M.E. Nur transcription factors in stress and addiction. Front. Mol. Neurosci. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.I.; Dunn, M.; Mingote, S.; Karam, C.S.; Farino, Z.J.; Sonders, M.S.; Choi, S.J.; Grygoruk, A.; Zhang, Y.; Cela, C.; et al. Neuronal Depolarization Drives Increased Dopamine Synaptic Vesicle Loading via VGLUT. Neuron 2017, 95, 1074.e7–1088.e7. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-C.; Zhang, F.; Adamantidis, A.R.; Stuber, G.D.; Bonci, A.; de Lecea, L.; Deisseroth, K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 2009, 324, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Ilango, A.; Kesner, A.J.; Broker, C.J.; Wang, D.V.; Ikemoto, S. Phasic excitation of ventral tegmental dopamine neurons potentiates the initiation of conditioned approach behavior: Parametric and reinforcement-schedule analyses. Front. Behav. Neurosci. 2014, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Baratta, M.V.; Yang, A.; Lee, D.; Boyden, E.S.; Fiorillo, C.D. Optogenetic mimicry of the transient activation of dopamine neurons by natural reward is sufficient for operant reinforcement. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Pascoli, V.; Terrier, J.; Hiver, A.; Lüscher, C. Sufficiency of Mesolimbic Dopamine Neuron Stimulation for the Progression to Addiction. Neuron 2015, 88, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Viereckel, T.; Konradsson-Geuken, Å.; Wallén-Mackenzie, Å. Validated multi-step approach for in vivo recording and analysis of optogenetically evoked glutamate in the mouse globus pallidus. J. Neurochem. 2018, 145, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Gaisler-Salomon, I.; Miller, G.M.; Chuhma, N.; Lee, S.; Zhang, H.; Ghoddoussi, F.; Lewandowski, N.; Fairhurst, S.; Wang, Y.; Conjard-Duplany, A.; et al. Glutaminase-deficient mice display hippocampal hypoactivity, insensitivity to pro-psychotic drugs and potentiated latent inhibition: Relevance to schizophrenia. Neuropsychopharmacology 2009, 34, 2305–2322. [Google Scholar] [CrossRef]
- Ang, S.L. Transcriptional control of midbrain dopaminergic neuron development. Development 2006, 133, 3499–3506. [Google Scholar] [CrossRef] [Green Version]
- Lammel, S.; Steinberg, E.E.; Földy, C.; Wall, N.R.; Beier, K.; Luo, L.; Malenka, R.C. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron 2015, 85, 429–438. [Google Scholar] [CrossRef]
- Stuber, G.D.; Stamatakis, A.M.; Kantak, P.A. Considerations when using cre-driver rodent lines for studying ventral tegmental area circuitry. Neuron 2015, 85, 439–445. [Google Scholar] [CrossRef]
- Papathanou, M.; Dumas, S.; Pettersson, H.; Olson, L.; Wallén-Mackenzie, Å. Off-target effects in transgenic mice: Characterization of Dopamine transporter (DAT)-Cre transgenic mouse lines exposes multiple non-dopaminergic neuronal clusters available for selective targeting within limbic neurocircuitry. Eneuro 2019. [Google Scholar] [CrossRef]
- Engblom, D.; Bilbao, A.; Sanchis-Segura, C.; Dahan, L.; Perreau-Lenz, S.; Balland, B.; Parkitna, J.R.; Luján, R.; Halbout, B.; Mameli, M.; et al. Glutamate Receptors on Dopamine Neurons Control the Persistence of Cocaine Seeking. Neuron 2008, 59, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Adrover, M.F.; Shin, J.H.; Alvarez, V.A. Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. J. Neurosci. 2014, 34, 3183–3192. [Google Scholar] [CrossRef]
- Ishikawa, M.; Otaka, M.; Neumann, P.A.; Wang, Z.; Cook, J.M.; Schlüter, O.M.; Dong, Y.; Huang, Y.H. Exposure to cocaine regulates inhibitory synaptic transmission from the ventral tegmental area to the nucleus accumbens. J. Physiol. 2013, 591, 4827–4841. [Google Scholar] [CrossRef] [PubMed]
- Mingote, S.; Chuhma, N.; Kusnoor, S.V.; Field, B.; Deutch, A.Y.; Rayport, S. Functional Connectome Analysis of Dopamine Neuron Glutamatergic Connections in Forebrain Regions. J. Neurosci. 2015, 35, 16259–16271. [Google Scholar] [CrossRef] [Green Version]
- Cachope, R.; Mateo, Y.; Mathur, B.N.; Irving, J.; Wang, H.L.; Morales, M.; Lovinger, D.M.; Cheer, J.F. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: Setting the tone for reward processing. Cell Rep. 2012, 2, 33–41. [Google Scholar] [CrossRef]
- Descarries, L.; Gisiger, V.; Steriade, M. Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol. 1997, 53, 603–625. [Google Scholar] [CrossRef]
- Threlfell, S.; Lalic, T.; Platt, N.J.; Jennings, K.A.; Deisseroth, K.; Cragg, S.J. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 2012, 75, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Arkadir, D.; Nevet, A.; Vaadia, E.; Bergman, H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 2004, 43, 133–143. [Google Scholar] [CrossRef]
- Ikemoto, S.; Bonci, A. Neurocircuitry of drug reward. Neuropharmacology 2014, 76, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Poulin, J.-F.; Caronia, G.; Hofer, C.; Cui, Q.; Helm, B.; Ramakrishnan, C.; Chan, C.S.; Dombeck, D.A.; Deisseroth, K.; Awatramani, R. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 2018, 21, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.C.; Sahr, R.N.; Sari, Y.; Behbahani, K. Glutamate and dopamine synaptic terminals in extended amygdala after 14-week chronic alcohol drinking in inbred alcohol-preferring rats. Alcohol 2006, 39, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Comasco, E.; Hallman, J.; Wallén-Mackenzie, Å. Haplotype-tag single nucleotide polymorphism analysis of the Vesicular Glutamate Transporter (VGLUT) genes in severely alcoholic women. Psychiatry Res. 2014, 219, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, T.S.; Hjelmstad, G.O.; Fields, H.L.; Edwards, R.H. Ventral Tegmental Area Glutamate Neurons: Electrophysiological Properties and Projections. J. Neurosci. 2012, 32, 15076–15085. [Google Scholar] [CrossRef] [Green Version]
- Gorelova, N.; Mulholland, P.J.; Chandler, L.J.; Seamans, J.K. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cereb. Cortex 2012, 22, 327–336. [Google Scholar] [CrossRef]
- Pérez-López, J.L.; Contreras-López, R.; Ramírez-Jarquín, J.O.; Tecuapetla, F. Direct Glutamatergic Signaling From Midbrain Dopaminergic Neurons Onto Pyramidal Prefrontal Cortex Neurons. Front. Neural Circuits 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Lammel, S.; Ion, D.I.; Roeper, J.; Malenka, R.C. Projection-Specific Modulation of Dopamine Neuron Synapses by Aversive and Rewarding Stimuli. Neuron 2011, 70, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Vander Weele, C.M.; Siciliano, C.A.; Matthews, G.A.; Namburi, P.; Izadmehr, E.M.; Espinel, I.C.; Nieh, E.H.; Schut, E.H.S.; Padilla-Coreano, N.; Burgos-Robles, A.; et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 2018, 563, 397–401. [Google Scholar] [CrossRef]
- Bimpisidis, Z.; De Luca, M.A.; Pisanu, A.; Di Chiara, G. Lesion of medial prefrontal dopamine terminals abolishes habituation of accumbens shell dopamine responsiveness to taste stimuli. Eur. J. Neurosci. 2013, 37, 613–622. [Google Scholar] [CrossRef]
- Beyer, C.E.; Steketee, J.D. Dopamine depletion in the medial prefrontal cortex induces sensitized- like behavioral and neurochemical responses to cocaine. Brain Res. 1999, 833, 133–141. [Google Scholar] [CrossRef]
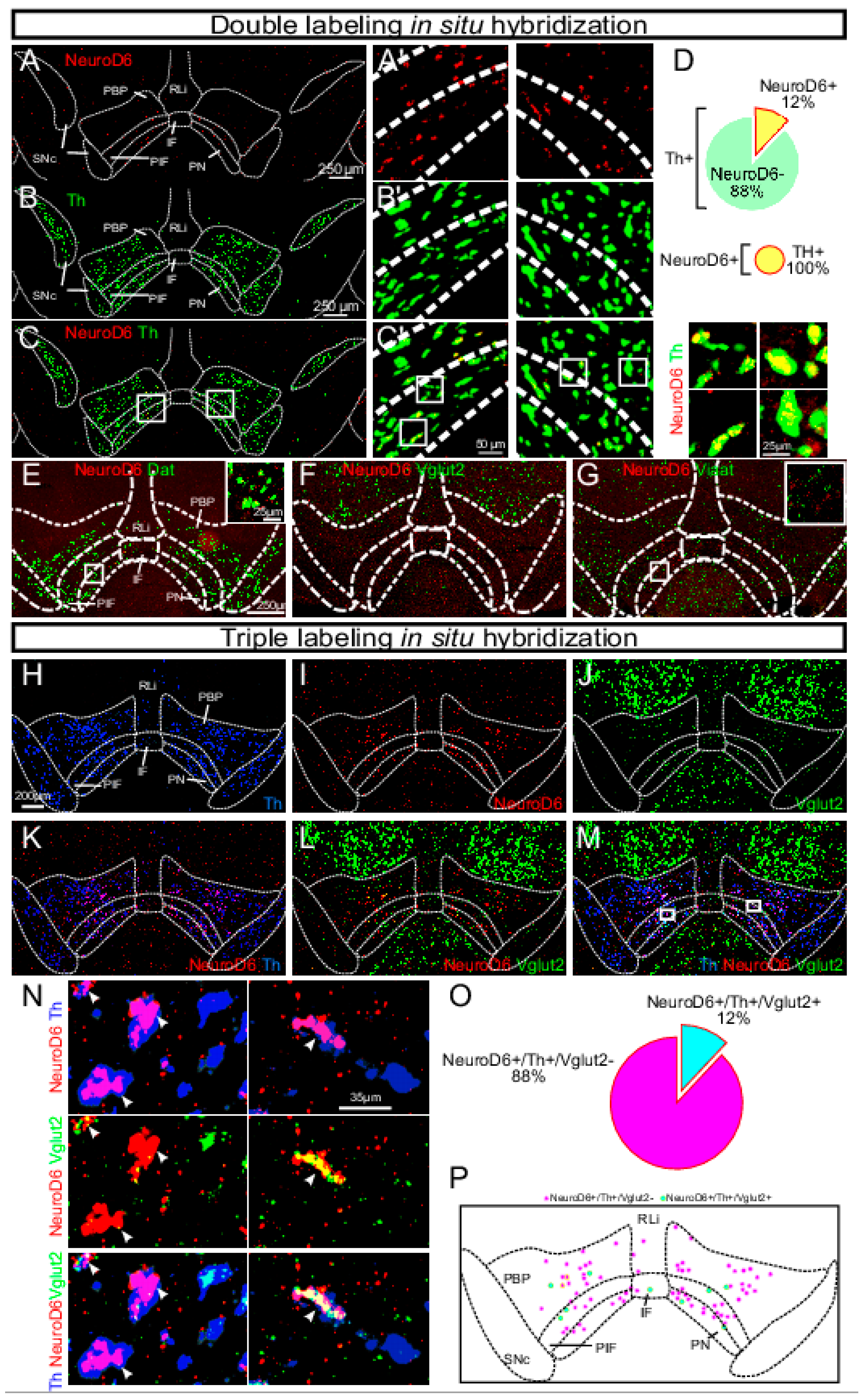
- Bimpisidis, Z.; König, N.; Stagkourakis, S.; Zell, V.; Vlcek, B.; Dumas, S.; Giros, B.; Broberger, C.; Hnasko, T.S.; Wallén-Mackenzie, Å. The NeuroD6 subtype of VTA neurons contributes to psychostimulant sensitization and behavioral reinforcement. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Seo, H.; Sonntag, K.C.; Brooks, A.; Lin, L.; Isacson, O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum. Mol. Genet. 2005, 14, 1709–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, J.G.; Dingledine, R.; Greenamyre, J.T. Gene expression profiling of rat midbrain dopamine neurons: Implications for selective vulnerability in parkinsonism. Neurobiol. Dis. 2005, 18, 19–31. [Google Scholar] [CrossRef]
- La Manno, G.; Gyllborg, D.; Codeluppi, S.; Nishimura, K.; Salto, C.; Zeisel, A.; Borm, L.E.; Stott, S.R.W.; Toledo, E.M.; Villaescusa, J.C.; et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell 2016, 167, 566.e19–580.e19. [Google Scholar] [CrossRef]
- Poulin, J.F.; Zou, J.; Drouin-Ouellet, J.; Kim, K.Y.A.; Cicchetti, F.; Awatramani, R.B. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 2014, 9, 930–943. [Google Scholar] [CrossRef]
- Kramer, D.J.; Risso, D.; Kosillo, P.; Ngai, J.; Bateup, H.S. Combinatorial Expression of Grp and Neurod6 Defines Dopamine Neuron Populations with Distinct Projection Patterns and Disease Vulnerability Combinatorial expression of Grp and Neurod6 defines dopamine neuron populations with distinct projection patterns and disease vulnerability. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Khan, S.; Stott, S.R.W.; Chabrat, A.; Truckenbrodt, A.M.; Spencer-Dene, B.; Nave, K.-A.; Guillemot, F.; Levesque, M.; Ang, S.-L. Survival of a Novel Subset of Midbrain Dopaminergic Neurons Projecting to the Lateral Septum Is Dependent on NeuroD Proteins. J. Neurosci. 2017, 37, 2305–2316. [Google Scholar] [CrossRef] [Green Version]

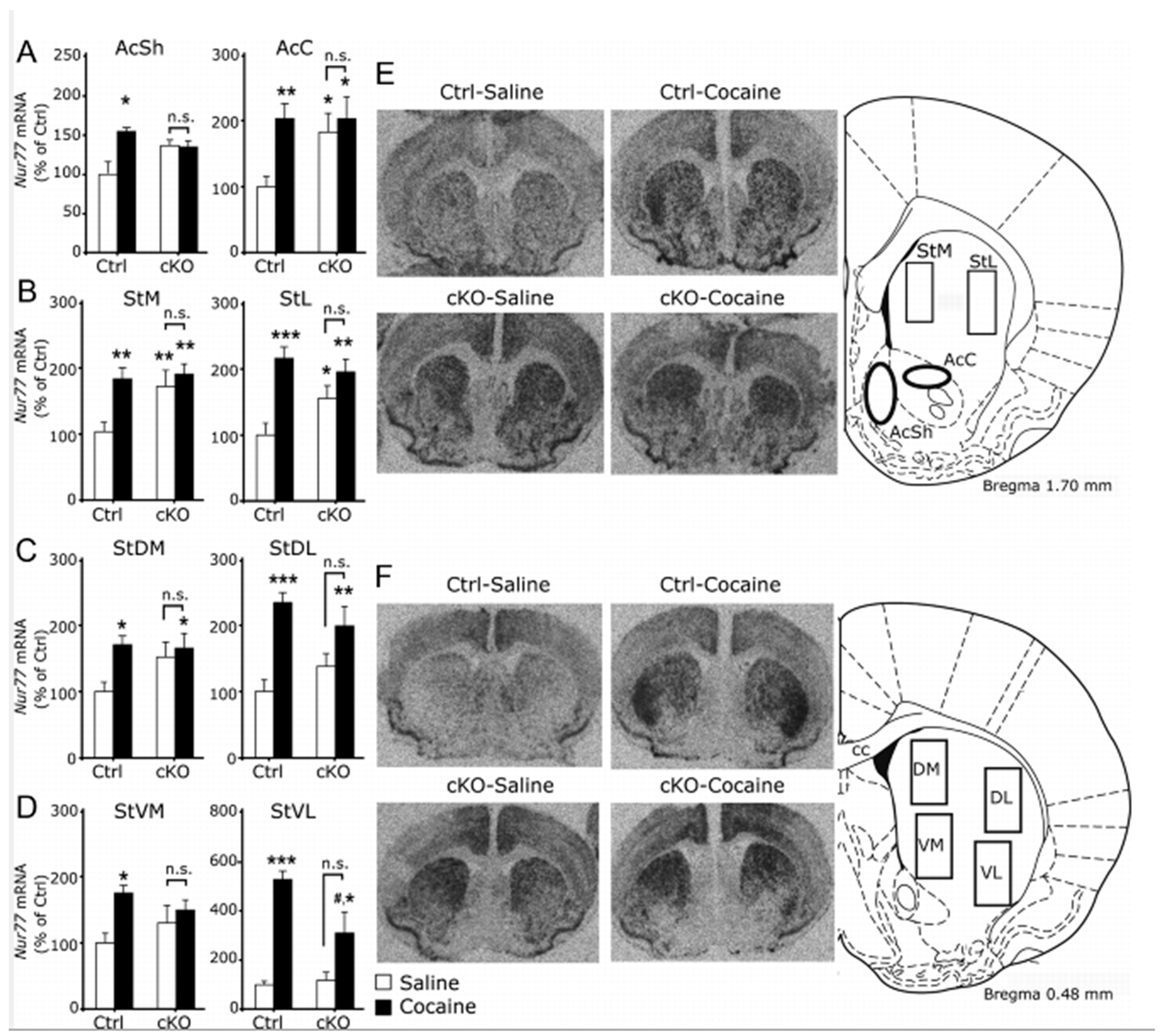
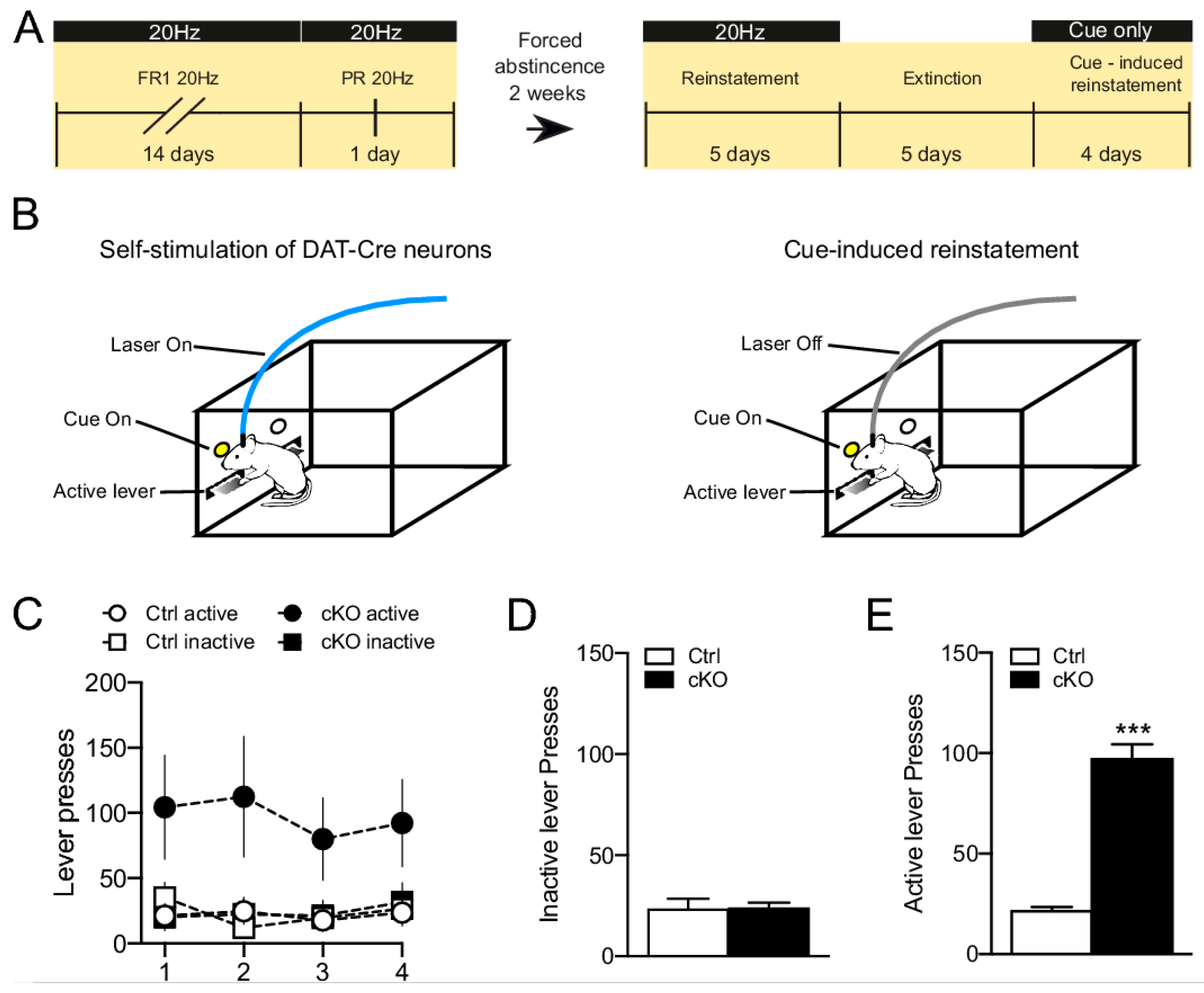
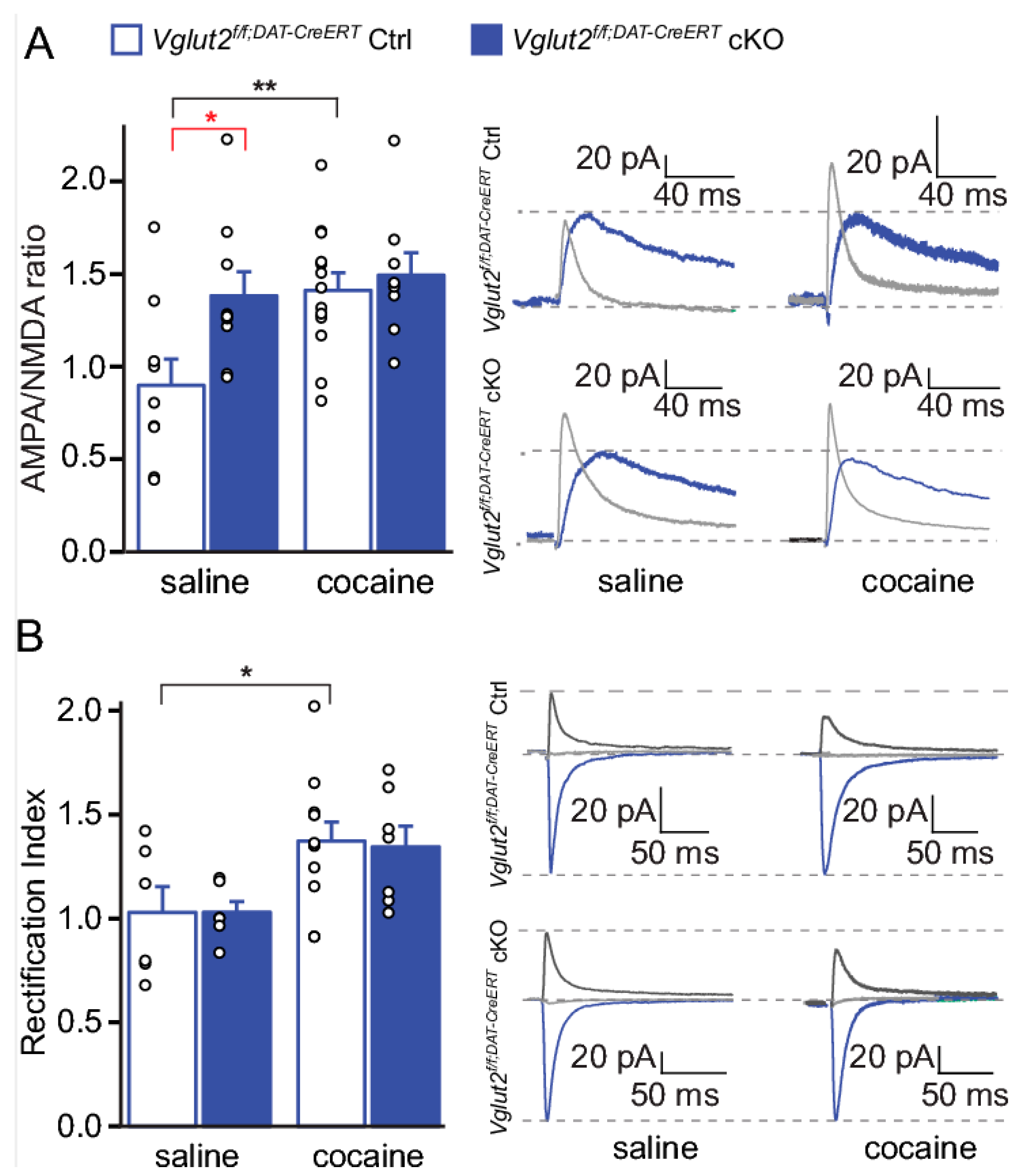
| Study (Listed in Alphabetical Order) | Strategy to Target DA-Glutamate Co-Release | Test | Effect |
|---|---|---|---|
| Alsiö et al., 2011 [61] | Vglut2f/f;DAT-Cre | Sucrose preference | lower threshold |
| Sucrose self-administration | ↑ responding under food restriction | ||
| ↑ consumption when fed ad libitum | |||
| Cocaine self-administration | ↑ for lower doses | ||
| ↑ responding for cocaine-associated cues | |||
| Birgner et al., 2010 [54] | Vglut2f/f;DAT-Cre | Rotarod (crude motor coordination) | − |
| Beam walking (fine motor coordination) | − | ||
| Elevated plus maze (anxiety) | ↑ latency to move | ||
| Multi-variate concentric square field (anxiety and risk analysis) | ↑ risk-taking behavior | ||
| Forced swim test (depression) | − | ||
| Radial maze | − | ||
| Acute amphetamine | dose-dependent alterations | ||
| Fortin et al., 2012 [62] | Vglut2f/f;DAT-Cre | Rotarod (crude motor coordination) | ↓ motor performance |
| Forced swim test (depression) | ↓ latency to immobility | ||
| Spontaneous activity in novel environment | ↓ horizontal activity | ||
| Acute amphetamine | ↓ locomotor responses | ||
| Acute cocaine | ↓ locomotor responses | ||
| Hnasko et al., 2010 [63] | Vglut2f/f;DAT-Cre | Spontaneous locomotion | − |
| Rotarod (crude motor coordination) | − | ||
| Acute cocaine | ↓ locomotor responses | ||
| Cocaine sensitization | − | ||
| Cocaine conditioned place preference (CPP) | − | ||
| Mingote et al., 2017 [64] | DATIREScre/+::GLS1lox/+ | Rotarod (crude motor coordination) | − |
| Novelty induced locomotion | − | ||
| Elevated plus maze (anxiety) | − | ||
| Fear conditioning | − | ||
| Acute amphetamine | − | ||
| Amphetamine sensitization | ↓ | ||
| Latent inhibition | ↑ | ||
| Papathanou et al., 2018 [24] | Vglut2f/f;DAT-CreERT2 | Amphetamine and cocaine sensitization | − (baseline AMPA/NMDA ratio altered) |
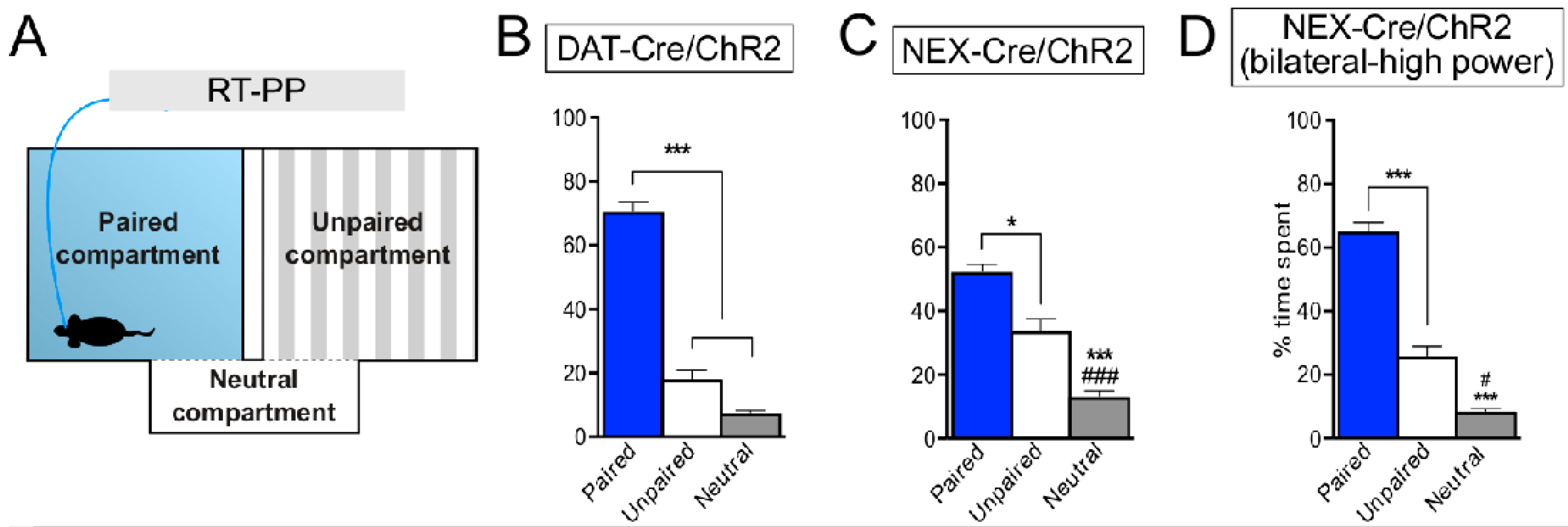
| Wang et al., 2017 [65] | Vglut2f/f;DAT-Cre | RT-PP (optogenetics in VTA) | − |
| Self-stimulation (optogenetics in VTA) | no effects on acquisition ↓ responses in higher laser power stimulation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bimpisidis, Z.; Wallén-Mackenzie, Å. Neurocircuitry of Reward and Addiction: Potential Impact of Dopamine–Glutamate Co-release as Future Target in Substance Use Disorder. J. Clin. Med. 2019, 8, 1887. https://doi.org/10.3390/jcm8111887
Bimpisidis Z, Wallén-Mackenzie Å. Neurocircuitry of Reward and Addiction: Potential Impact of Dopamine–Glutamate Co-release as Future Target in Substance Use Disorder. Journal of Clinical Medicine. 2019; 8(11):1887. https://doi.org/10.3390/jcm8111887
Chicago/Turabian StyleBimpisidis, Zisis, and Åsa Wallén-Mackenzie. 2019. "Neurocircuitry of Reward and Addiction: Potential Impact of Dopamine–Glutamate Co-release as Future Target in Substance Use Disorder" Journal of Clinical Medicine 8, no. 11: 1887. https://doi.org/10.3390/jcm8111887
APA StyleBimpisidis, Z., & Wallén-Mackenzie, Å. (2019). Neurocircuitry of Reward and Addiction: Potential Impact of Dopamine–Glutamate Co-release as Future Target in Substance Use Disorder. Journal of Clinical Medicine, 8(11), 1887. https://doi.org/10.3390/jcm8111887




