Risk of Cancers in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: Results from the Korea National Health Insurance Claims Database 2010–2018
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of Patient and the Source of Data
2.2. Cancer Case Ascertainment and Charlson Comorbidity Index
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients with AAV
3.2. Comparison of Variables between AAV Patients with and without Cancer
3.3. Estimation of Cancer Risk in AAV Patients According to Sex
3.4. Risk of Cancer Based on AAV Subtypes
3.5. Risk of Cancer Based on Immunosuppressive Agent Usage
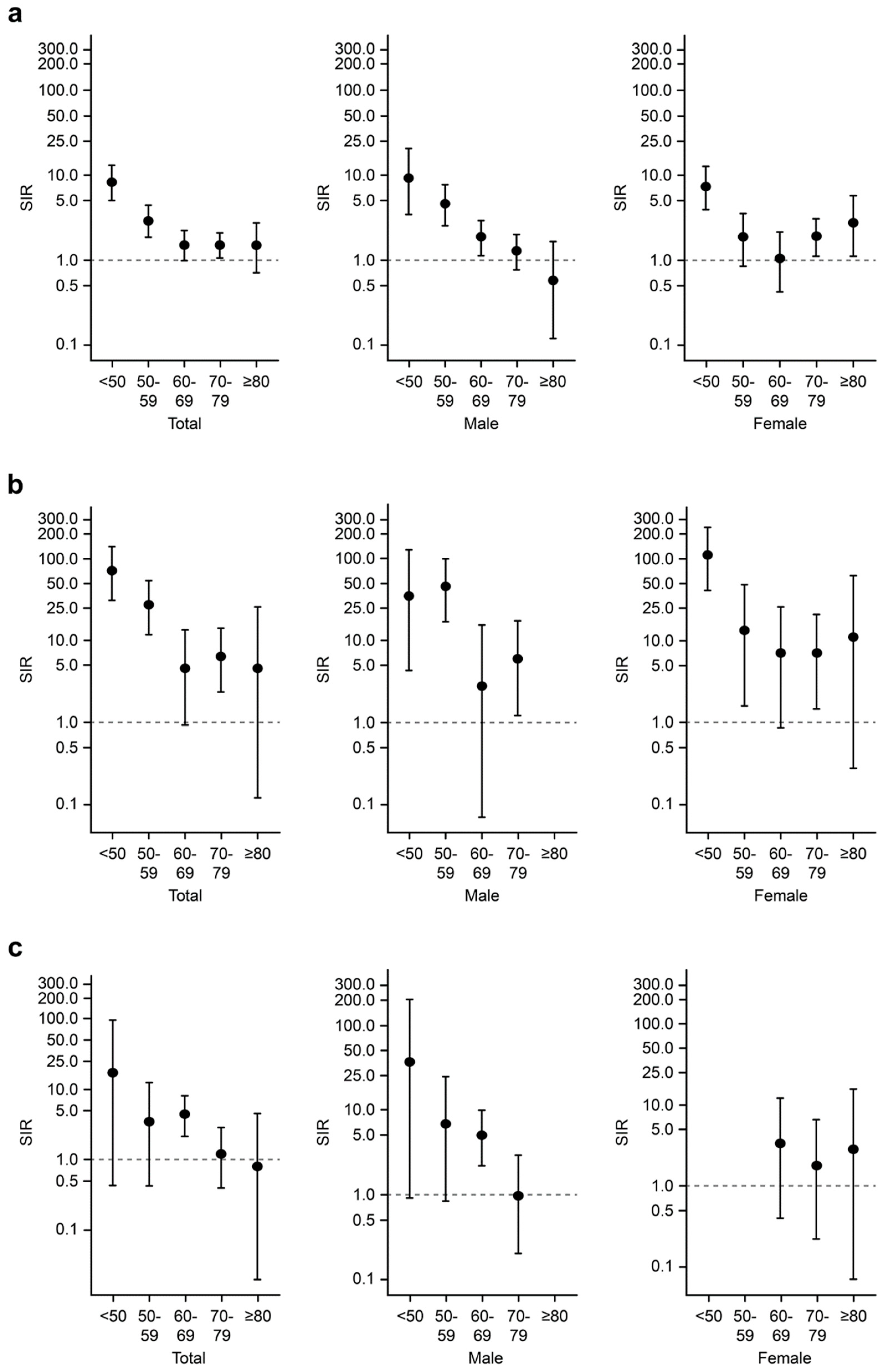
3.6. Overall, Hematological, and Lung Cancer Risks in Patients with AAV According to Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kallenberg, C.G. Pathophysiology of ANCA-associated small vessel vasculitis. Curr. Rheumatol. Rep. 2010, 12, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kallenberg, C.G. Key advances in the clinical approach to ANCA-associated vasculitis. Nat. Rev. Rheumatol. 2014, 10, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Ntatsaki, E.; Watts, R.A.; Scott, D.G. Epidemiology of ANCA-associated vasculitis. Rheum. Dis. Clin. N. Am. 2010, 36, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Nachman, P.H. ANCA Glomerulonephritis and Vasculitis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1680–1691. [Google Scholar] [CrossRef]
- Watts, R.A.; Mahr, A.; Mohammad, A.J.; Gatenby, P.; Basu, N.; Flores-Suarez, L.F. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol. Dial. Transp. 2015, 30 (Suppl. 1), i14–i22. [Google Scholar] [CrossRef] [PubMed]
- Wasko, M.C. Comorbid conditions in patients with rheumatic diseases: An update. Curr. Opin. Rheumatol. 2004, 16, 109–113. [Google Scholar] [CrossRef]
- Turesson, C.; Matteson, E.L. Malignancy as a comorbidity in rheumatic diseases. Rheumatology 2013, 52, 5–14. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Michaud, K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res. Ther. 2009, 11, 229. [Google Scholar] [CrossRef]
- Park, J.K.; Yang, J.A.; Ahn, E.Y.; Chang, S.H.; Song, Y.W.; Curtis, J.R.; Lee, E.B. Survival rates of cancer patients with and without rheumatic disease: A retrospective cohort analysis. BMC Cancer 2016, 16, 381. [Google Scholar] [CrossRef]
- Giat, E.; Ehrenfeld, M.; Shoenfeld, Y. Cancer and autoimmune diseases. Autoimmun. Rev. 2017, 16, 1049–1057. [Google Scholar] [CrossRef]
- Egiziano, G.; Bernatsky, S.; Shah, A.A. Cancer and autoimmunity: Harnessing longitudinal cohorts to probe the link. Best Prac. Res. Clin. Rheumatol. 2016, 30, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Lin, M.S.; Su, Y.J.; Cheng, T.T.; Lin, Y.S.; Chen, Y.C.; Chiu, W.C.; Chen, T.H. Cumulative immunosuppressant exposure is associated with diversified cancer risk among 14 832 patients with systemic lupus erythematosus: A nested case-control study. Rheumatology 2017, 56, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Vial, T.; Descotes, J. Immunosuppressive drugs and cancer. Toxicology 2003, 185, 229–240. [Google Scholar] [CrossRef]
- Trejo, M.A.C.W.; Bajema, I.M.; van Daalen, E.E. Antineutrophil cytoplasmic antibody-associated vasculitis and malignancy. Curr. Opin. Rheumatol. 2018, 30, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Ning, Y.; Xu, X.; Li, M.; Guo, S.; Han, M.; Zeng, R.; Ge, S.; Xu, G. Incidence of Cancer in ANCA-Associated Vasculitis: A Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0126016. [Google Scholar] [CrossRef]
- Mahr, A.; Heijl, C.; Le Guenno, G.; Faurschou, M. ANCA-associated vasculitis and malignancy: Current evidence for cause and consequence relationships. Best Pract. Res. Clin. Rheumatol. 2013, 27, 45–56. [Google Scholar] [CrossRef]
- Westman, K.W.; Bygren, P.G.; Olsson, H.; Ranstam, J.; Wieslander, J. Relapse rate, renal survival, and cancer morbidity in patients with Wegener’s granulomatosis or microscopic polyangiitis with renal involvement. J. Am. Soc. Nephrol. 1998, 9, 842–852. [Google Scholar]
- Heijl, C.; Harper, L.; Flossmann, O.; Stucker, I.; Scott, D.G.; Watts, R.A.; Hoglund, P.; Westman, K.; Mahr, A. European Vasculitis Study Group. Incidence of malignancy in patients treated for antineutrophil cytoplasm antibody-associated vasculitis: Follow-up data from European Vasculitis Study Group clinical trials. Ann. Rheum. Dis. 2011, 70, 1415–1421. [Google Scholar] [CrossRef]
- Tatsis, E.; Reinhold-Keller, E.; Steindorf, K.; Feller, A.C.; Gross, W.L. Wegener’s granulomatosis associated with renal cell carcinoma. Arthritis Rheum. 1999, 42, 751–756. [Google Scholar] [CrossRef]
- Li, J.; Cui, Z.; Long, J.Y.; Huang, W.; Wang, J.W.; Wang, H.; Zhang, L.; Chen, M.; Zhao, M.H. The frequency of ANCA-associated vasculitis in a national database of hospitalized patients in China. Arthritis Res. Ther. 2018, 20, 226. [Google Scholar] [CrossRef]
- Pearce, F.A.; Lanyon, P.C.; Grainge, M.J.; Shaunak, R.; Mahr, A.; Hubbard, R.B.; Watts, R.A. Incidence of ANCA-associated vasculitis in a UK mixed ethnicity population. Rheumatology 2016, 55, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Zahm, S.H.; Fraumeni, J.F., Jr. Racial, ethnic, and gender variations in cancer risk: Considerations for future epidemiologic research. Environ. Health Perspect. 1995, 103 (Suppl. 8), 283–286. [Google Scholar] [CrossRef] [PubMed]
- Doll, R. The geographical distribution of cancer. Br. J. Cancer 1969, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Yoon, S.; Kim, L.Y.; Kim, D.S. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J. Korean Med. Sci. 2017, 32, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Oh, C.M.; Kong, H.J.; Lee, D.H.; Lee, K.H. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2014. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2017, 49, 292–305. [Google Scholar] [CrossRef]
- Seo, H.J.; Oh, I.H.; Yoon, S.J. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac. J. Cancer Prev. 2012, 13, 6163–6168. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Firestein, G.S.; Budd, R.C.; Gabriel, S.E.; McInnes, I.B.; O’Dell, J.R. Kelley and Firestein’s Textbook of Rheumatology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2011, 2, 98. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nishikawa, H. Roles of regulatory T cells in cancer immunity. Int. Immunol. 2016, 28, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M. Foxp3(+) T Regulatory Cells: Still Many Unanswered Questions-A Perspective After 20 Years of Study. Front. Immunol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.J.; Mortensen, K.H.; Babar, J.; Smith, R.; Jones, R.B.; Nakagomi, D.; Sivasothy, P.; Jayne, D.R.W. Pulmonary Involvement in Antineutrophil Cytoplasmic Antibodies (ANCA)-associated Vasculitis: The Influence of ANCA Subtype. J. Rheumatol. 2017, 44, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Thickett, D.R.; Richter, A.G.; Nathani, N.; Perkins, G.D.; Harper, L. Pulmonary manifestations of anti-neutrophil cytoplasmic antibody (ANCA)-positive vasculitis. Rheumatology 2006, 45, 261–268. [Google Scholar] [CrossRef]
- Martin, D.N.; Mikhail, I.S.; Landgren, O. Autoimmunity and hematologic malignancies: Associations and mechanisms. Leuk. Lymphoma 2009, 50, 541–550. [Google Scholar] [CrossRef]
- Gutierrez-Dalmau, A.; Campistol, J.M. Immunosuppressive therapy and malignancy in organ transplant recipients: A systematic review. Drugs 2007, 67, 1167–1198. [Google Scholar] [CrossRef]
- Grulich, A.E.; van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef]
- Bugelski, P.J.; Volk, A.; Walker, M.R.; Krayer, J.H.; Martin, P.; Descotes, J. Critical review of preclinical approaches to evaluate the potential of immunosuppressive drugs to influence human neoplasia. Int. J. Toxicol. 2010, 29, 435–466. [Google Scholar] [CrossRef]
- Weaver, J.L. Establishing the carcinogenic risk of immunomodulatory drugs. Toxicol. Pathol. 2012, 40, 267–271. [Google Scholar] [CrossRef]
- Kang, E.H.; Lee, S.J.; Ascherman, D.P.; Lee, Y.J.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Temporal relationship between cancer and myositis identifies two distinctive subgroups of cancers: Impact on cancer risk and survival in patients with myositis. Rheumatology 2016, 55, 1631–1641. [Google Scholar] [CrossRef]
- Yang, H.; Peng, Q.; Yin, L.; Li, S.; Shi, J.; Zhang, Y.; Lu, X.; Shu, X.; Zhang, S.; Wang, G. Identification of multiple cancer-associated myositis-specific autoantibodies in idiopathic inflammatory myopathies: A large longitudinal cohort study. Arthritis Res. Ther. 2017, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- van Daalen, E.E.; Rahmattulla, C.; Wolterbeek, R.; Bruijn, J.A.; Bajema, I.M. Incidence of Malignancy Prior to Antineutrophil Cytoplasmic Antibody-associated Vasculitis Compared to the General Population. J. Rheumatol. 2017, 44, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, R.; Barber, M.; Heuzenroeder, L.; Wluka, A.E.; Giles, G.; Hall, S.; Harkness, A.; Lewis, D.; Littlejohn, G.; Miller, M.H.; et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 2008, 59, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, B.; Svanstrom, H.; Schmiegelow, K.; Jess, T.; Hviid, A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am. J. Epidemiol. 2013, 177, 1296–1305. [Google Scholar] [CrossRef]
- van Daalen, E.E.; Rizzo, R.; Kronbichler, A.; Wolterbeek, R.; Bruijn, J.A.; Jayne, D.R.; Bajema, I.M.; Rahmattulla, C. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann. Rheum. Dis. 2017, 76, 1064–1069. [Google Scholar] [CrossRef]
- Vlaovic, P.; Jewett, M.A. Cyclophosphamide-induced bladder cancer. Can. J. Urol. 1999, 6, 745–748. [Google Scholar]
- Travis, L.B.; Curtis, R.E.; Glimelius, B.; Holowaty, E.J.; Van Leeuwen, F.E.; Lynch, C.F.; Hagenbeek, A.; Stovall, M.; Banks, P.M.; Adami, J.; et al. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J. Natl. Cancer Inst. 1995, 87, 524–530. [Google Scholar] [CrossRef]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46, S7–S15. [Google Scholar] [CrossRef]
- Sada, K.-E.; Yamamura, M.; Harigai, M.; Fujii, T.; Dobashi, H.; Takasaki, Y.; Ito, S.; Yamada, H.; Wada, T.; Hirahashi, J.; et al. Classification and characteristics of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis in a nationwide, prospective, inception cohort study. Arthritis Res. Ther. 2014, 16, R101. [Google Scholar] [CrossRef]
- Sato, S.; Yashiro, M.; Matsuoka, N.; Asano, T.; Kobayashi, H.; Watanabe, H.; Migita, K. Clinical features and outcomes in patients with elderly-onset anti-neutrophil cytoplasmic antibody-associated vasculitis. Geriatr. Gerontol. Int. 2018, 18, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Kim, H.J.; Ahn, S.S.; Jung, S.M.; Song, J.J.; Park, Y.-B.; Lee, S.-W. Clinical and prognostic features of Korean patients with MPO-ANCA, PR3-ANCA and ANCA-negative vasculitis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 103), 111–118. [Google Scholar]
- Sauer, A.G.; Siegel, R.L.; Jemal, A.; Fedewa, S.A. Updated Review of Prevalence of Major Risk Factors and Use of Screening Tests for Cancer in the United States. Cancer Epidemiol. Prev. Biomark. 2017, 26, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
| Total n = 2097 | AAV Patients with Cancer n = 114 | AAV Patients without Cancer n = 1983 | p-Value | |
|---|---|---|---|---|
| Age at diagnosis | 59.8 ± 15.6 | 61.7 ± 13.9 | 59.7 ± 15.7 | 0.1723 |
| Sex, n (%) | ||||
| Female | 1168 (55.7) | 53 (46.5) | 1115 (56.2) | 0.0526 |
| Male | 929 (44.3) | 61 (53.5) | 868 (43.8) | |
| Diagnosis, n (%) | ||||
| MPA | 947 (45.2) | 38 (33.3) | 909 (45.8) | 0.0004 |
| GPA | 568 (27.1) | 49 (43.0) | 519 (26.2) | |
| EGPA | 582 (27.7) | 27 (23.7) | 555 (28.0) | |
| Type of insurance, n (%) | ||||
| National Health Insurance | 2004 (95.6) | 109 (95.6) | 1895 (95.6) | 1.0000 |
| Medical Aid | 93 (4.4) | 5 (4.4) | 88 (4.4) | |
| CCI subcategories, n (%) a | ||||
| Cardiovascular disorder | ||||
| Myocardial infarction | 53 (2.5) | 2 (1.8) | 51 (2.6) | 1.0000 |
| Congestive heart failure | 208 (9.9) | 16 (14.0) | 192 (9.7) | 0.1767 |
| Peripheral vascular disease | 389 (18.6) | 19 (16.7) | 370 (18.7) | 0.6831 |
| Cerebrovascular disease | 303 (14.5) | 11 (9.7) | 292 (14.7) | 0.1732 |
| Diabetes | 702 (33.5) | 36 (31.6) | 666 (33.6) | 0.7343 |
| Diabetes with chronic complication | 240 (11.4) | 11 (9.7) | 229 (11.6) | 0.6397 |
| Gastrointestinal disorder | ||||
| Mild liver disease | 769 (36.7) | 36 (31.6) | 733 (37.0) | 0.2890 |
| Moderate or severe liver disease | 12 (0.6) | 1 (0.9) | 11 (0.6) | 0.4896 |
| Peptic ulcer disease | 840 (40.1) | 41 (36.0) | 799 (40.3) | 0.4130 |
| Pulmonary disorder | ||||
| Chronic pulmonary disease | 1420 (67.7) | 64 (56.1) | 1356 (68.4) | 0.0089 |
| Rheumatologic disorder | ||||
| Rheumatologic disease | 330 (15.7) | 15 (13.2) | 315 (15.9) | 0.5187 |
| Kidney disorder | ||||
| Renal disease except unspecified kidney failure | 373 (17.8) | 14 (12.3) | 359 (18.1) | 0.1456 |
| Others | ||||
| Dementia | 86 (4.1) | 2 (1.8) | 84 (4.2) | 0.3247 |
| Hemiplegia or paraplegia | 48 (2.3) | 1 (0.9) | 47 (2.4) | 0.5159 |
| Malignancy (nonmetastatic cancer) | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a |
| Metastatic solid tumor | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a |
| Acquired immune deficiency syndrome | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a |
| Medication usage, n (%) | ||||
| Glucocorticoid steroid usage ≥ 1 year | 1037 (49.5) | 67 (58.8) | 970 (48.9) | 0.0511 |
| Cyclophosphamide | 1022 (48.7) | 63 (55.3) | 959 (48.4) | 0.1811 |
| Rituximab | 250 (11.9) | 8 (7.0) | 242 (12.2) | 0.1303 |
| Azathioprine/mizoribine | 835 (39.8) | 48 (42.1) | 787 (39.7) | 0.6785 |
| Methotrexate | 293 (14.0) | 25 (21.9) | 268 (13.5) | 0.0173 |
| Cancer (ICD-10) | Total | |||
|---|---|---|---|---|
| Expected | Observed | SIR | 95% CI | |
| Overall cancer (C00–C96) | 60.06 | 114 | 1.90 | (1.57–2.28) |
| Stomach (C16) | 9.12 | 9 | 0.99 | (0.45–1.87) |
| Colon and rectum (C18–C20) | 8.41 | 9 | 1.07 | (0.49–2.03) |
| Liver (C22) | 4.83 | 4 | 0.83 | (0.23–2.12) |
| Gallbladder, etc. (C23–C24) | 1.97 | 5 | 2.54 | (0.82–5.93) |
| Pancreas (C25) | 2.03 | 3 | 1.48 | (0.30–4.32) |
| Lung (C33–C34) | 8.51 | 19 | 2.23 | (1.34–3.48) |
| Breast (C50) | 3.28 | 3 | 0.91 | (0.19–2.67) |
| Ovary (C56) | 0.50 | 2 | 3.98 | (0.48–14.39) |
| Prostate (C61) | 3.84 | 1 | 0.26 | (0.01–1.45) |
| Kidney (C64) | 1.18 | 4 | 3.40 | (0.93–8.70) |
| Bladder (C67) | 1.36 | 4 | 2.94 | (0.80–7.54) |
| Thyroid (C73) | 4.65 | 2 | 0.43 | (0.05–1.55) |
| Hematological cancer | 2.28 | 26 | 11.39 | (7.44–16.69) |
| Hodgkin lymphoma (C81) | 0.05 | 1 | 21.85 | (0.55–121.75) |
| Non-Hodgkin lymphoma (C82–C85, C96) | 1.28 | 13 | 10.14 | (5.40–17.34) |
| Multiple myeloma (C90) | 0.47 | 10 | 21.12 | (10.13–38.85) |
| Leukemia (C91–C95) | 0.48 | 2 | 4.16 | (0.50–15.01) |
| Other (remaining cancer codes) | 7.40 | 23 | 3.11 | (1.97–4.66) |
| Cancer (ICD-10) | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Expected | Observed | SIR | 95% CI | Expected | Observed | SIR | 95% CI | |
| Overall cancer (C00–C96) | 34.96 | 61 | 1.74 | (1.33–2.24) | 25.79 | 53 | 2.06 | (1.54–2.69) |
| Stomach (C16) | 6.10 | 5 | 0.82 | (0.27–1.91) | 2.93 | 4 | 1.36 | (0.37–3.49) |
| Colon and rectum (C18–C20) | 5.00 | 7 | 1.40 | (0.56–2.88) | 3.38 | 2 | 0.59 | (0.07–2.14) |
| Liver (C22) | 3.35 | 3 | 0.89 | (0.18–2.62) | 1.38 | 1 | 0.73 | (0.02–4.04) |
| Gallbladder, etc. (C23–C24) | 1.05 | 4 | 3.82 | (1.04–9.78) | 0.92 | 1 | 1.08 | (0.03–6.04) |
| Pancreas (C25) | 1.08 | 1 | 0.93 | (0.02–5.17) | 0.95 | 2 | 2.12 | (0.26–7.64) |
| Lung (C33–C34) | 6.29 | 14 | 2.23 | (1.22–3.74) | 2.38 | 5 | 2.10 | (0.68–4.90) |
| Breast (C50) | 3.57 | 3 | 0.84 | (0.17–2.46) | ||||
| Ovary (C56) | 0.53 | 2 | 3.75 | (0.45–13.54) | ||||
| Prostate (C61) | 3.88 | 1 | 0.26 | (0.01–1.44) | ||||
| Kidney (C64) | 0.77 | 3 | 3.90 | (0.80–11.40) | 0.39 | 1 | 2.57 | (0.06–14.31) |
| Bladder (C670 | 1.14 | 1 | 0.88 | (0.02–4.90) | 0.25 | 3 | 12.05 | (2.48–35.21) |
| Thyroid (C73) | 0.84 | 1 | 1.19 | (0.03–6.63) | 4.13 | 1 | 0.24 | (0.01–1.35) |
| Hematological cancer | 1.25 | 12 | 9.59 | (4.96–16.76) | 1.03 | 14 | 13.58 | (7.42–22.78) |
| Hodgkin lymphoma (C81) | 0.02 | 1 | 48.19 | (1.22–268.50) | ||||
| Non-Hodgkin lymphoma (C82–C85, C96) | 0.70 | 6 | 8.60 | (3.16–18.72) | 0.59 | 7 | 11.95 | (4.81–24.63) |
| Multiple myeloma (C90) | 0.25 | 5 | 20.01 | (6.50–46.70) | 0.22 | 5 | 22.30 | (7.24–52.04) |
| Leukemia (C91–C95) | 0.28 | 1 | 3.58 | (0.09–19.93) | 0.20 | 1 | 4.99 | (0.13–27.81) |
| Other (remaining cancer codes) | 4.19 | 9 | 2.15 | (0.98–4.08) | 3.23 | 14 | 4.34 | (2.37–7.28) |
| Cancer (ICD-10) | MPA | GPA | EGPA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expected | Observed | SIR | 95% CI | Expected | Observed | SIR | 95% CI | Expected | Observed | SIR | 95% CI | |
| Overall cancer (C00–C96) | 26.89 | 38 | 1.41 | (1.00–1.94) | 17.19 | 49 | 2.85 | (2.11–3.77) | 15.98 | 27 | 1.69 | (1.11–2.46) |
| Stomach (C16) | 4.16 | 5 | 1.20 | (0.39–2.81) | 2.61 | 2 | 0.77 | (0.09–2.77) | 2.36 | 2 | 0.85 | (0.10–3.07) |
| Colon and rectum (C18–C20) | 3.87 | 4 | 1.03 | (0.28–2.65) | 2.40 | 5 | 2.08 | (0.68–4.86) | – | |||
| Liver (C22) | 2.18 | 3 | 1.38 | (0.28–4.03) | 1.39 | 1 | 0.72 | (0.02–4.02) | – | |||
| Gallbladder, etc. (C23–C24) | 0.95 | 2 | 2.10 | (0.25–7.60) | 0.55 | 1 | 1.80 | (0.05–10.05) | 0.46 | 2 | 4.32 | (0.52–15.60) |
| Pancreas (C25) | 0.97 | 2 | 2.07 | (0.25–7.48) | 0.57 | 1 | 1.74 | (0.04–9.70) | – | |||
| Lung (C33–C34) | 4.09 | 7 | 1.71 | (0.69–3.53) | 2.40 | 10 | 4.17 | (2.00–7.66) | 2.03 | 2 | 0.99 | (0.12–3.56) |
| Breast (C50) | – | 0.97 | 1 | 1.03 | (0.03–5.74) | 1.13 | 2 | 1.78 | (0.22–6.42) | |||
| Ovary (C56) | – | 0.15 | 1 | 6.83 | (0.17–38.05) | 0.15 | 1 | 6.55 | (0.17–36.50) | |||
| Prostate (C61) | – | – | 0.89 | 1 | 1.12 | (0.03–6.25) | ||||||
| Kidney (C64) | 0.51 | 2 | 3.92 | (0.47–14.15) | 0.34 | 1 | 2.93 | (0.07–16.31) | 0.33 | 1 | 3.07 | (0.08–17.12) |
| Bladder (C67) | 0.65 | 3 | 4.62 | (0.95–13.51) | – | 0.33 | 1 | 3.07 | (0.08–17.10) | |||
| Thyroid (C73) | – | 1.38 | 1 | 0.72 | (0.02–4.02) | 1.68 | 1 | 0.59 | (0.02–3.31) | |||
| Hematological cancer | 1.02 | 6 | 5.86 | (2.15–12.75) | 0.65 | 12 | 18.40 | (9.51–32.15) | 0.61 | 8 | 13.20 | (5.70–26.01) |
| Hodgkin lymphoma (C81) | – | – | 0.01 | 1 | 72.78 | (1.84–405.58) | ||||||
| Non-Hodgkin lymphoma (C82–C85, C96) | 0.57 | 1 | 1.75 | (0.04–9.75) | 0.37 | 7 | 19.08 | (7.67–39.31) | 0.34 | 5 | 14.56 | (4.73–33.99) |
| Multiple myeloma (C90) | 0.22 | 5 | 22.54 | (7.32–52.59) | 0.13 | 3 | 22.29 | (4.60–65.15) | 0.12 | 2 | 17.10 | (2.07–61.78) |
| Leukemia (C91–C95) | – | 0.14 | 2 | 14.52 | (1.76–52.47) | – | ||||||
| Other (remaining cancer codes) | 3.37 | 4 | 1.19 | (0.32–3.03) | 2.11 | 13 | 6.15 | (3.27–10.51) | 1.91 | 6 | 3.13 | (1.15–6.82) |
| Crude Odds Ratio a | Adjusted Odds Ratio | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| Type of insurance | ||||||
| National Health Insurance | 1.0 (ref) | 1.0 (ref) | ||||
| Medical Aid | 1.11 | (0.38–3.28) | 0.8508 | 1.47 | (0.47–4.57) | 0.5092 |
| Glucocorticoid usage ≥ 1 year | 4.36 | (1.61–11.84) | 0.0039 | 4.20 | (1.47–12.01) | 0.0075 |
| Cyclophosphamide | 1.78 | (1.08–2.93) | 0.0239 | 1.75 | (1.03–2.96) | 0.0385 |
| Rituximab | 0.78 | (0.24–2.49) | 0.6731 | 0.56 | (0.17–1.92) | 0.3591 |
| Azathioprine/mizoribine | 1.33 | (0.77–2.30) | 0.3087 | 0.94 | (0.50–1.75) | 0.8347 |
| Methotrexate | 2.27 | (1.11–4.63) | 0.0247 | 2.39 | (1.12–5.13) | 0.0247 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.S.; Han, M.; Yoo, J.; Jung, S.M.; Song, J.J.; Park, Y.-B.; Jung, I.; Lee, S.-W. Risk of Cancers in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: Results from the Korea National Health Insurance Claims Database 2010–2018. J. Clin. Med. 2019, 8, 1871. https://doi.org/10.3390/jcm8111871
Ahn SS, Han M, Yoo J, Jung SM, Song JJ, Park Y-B, Jung I, Lee S-W. Risk of Cancers in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: Results from the Korea National Health Insurance Claims Database 2010–2018. Journal of Clinical Medicine. 2019; 8(11):1871. https://doi.org/10.3390/jcm8111871
Chicago/Turabian StyleAhn, Sung Soo, Minkyung Han, Juyoung Yoo, Seung Min Jung, Jason Jungsik Song, Yong-Beom Park, Inkyung Jung, and Sang-Won Lee. 2019. "Risk of Cancers in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: Results from the Korea National Health Insurance Claims Database 2010–2018" Journal of Clinical Medicine 8, no. 11: 1871. https://doi.org/10.3390/jcm8111871
APA StyleAhn, S. S., Han, M., Yoo, J., Jung, S. M., Song, J. J., Park, Y.-B., Jung, I., & Lee, S.-W. (2019). Risk of Cancers in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: Results from the Korea National Health Insurance Claims Database 2010–2018. Journal of Clinical Medicine, 8(11), 1871. https://doi.org/10.3390/jcm8111871





