Significance of Malignant Peritoneal Cytology on the Survival of Women with Early-Stage Cervical Cancer: A Japanese Gynecologic Oncology Group Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Eligibility Criteria
2.3. Clinical Information
2.4. Study Definition
2.5. Statistical Consideration
2.6. Systematic Review and Meta-Analysis
2.6.1. Aim
2.6.2. Article Retrieval
2.6.3. Inclusion and Exclusion Criteria
2.6.4. Meta-Analysis Plan
3. Results
3.1. Utilization of Peritoneal Cytology Testing
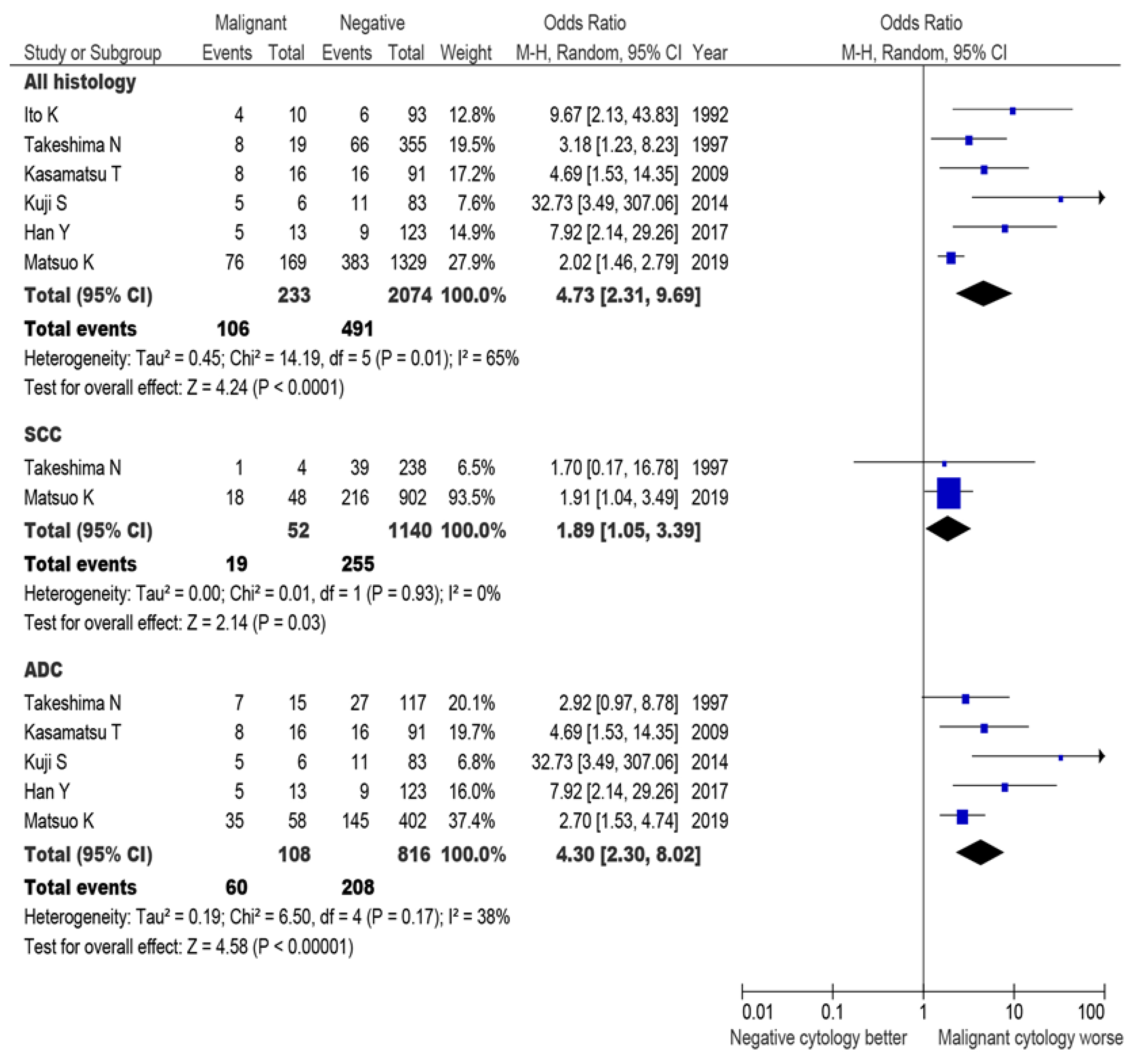
3.2. Risk Factors for Malignant Peritoneal Cytology
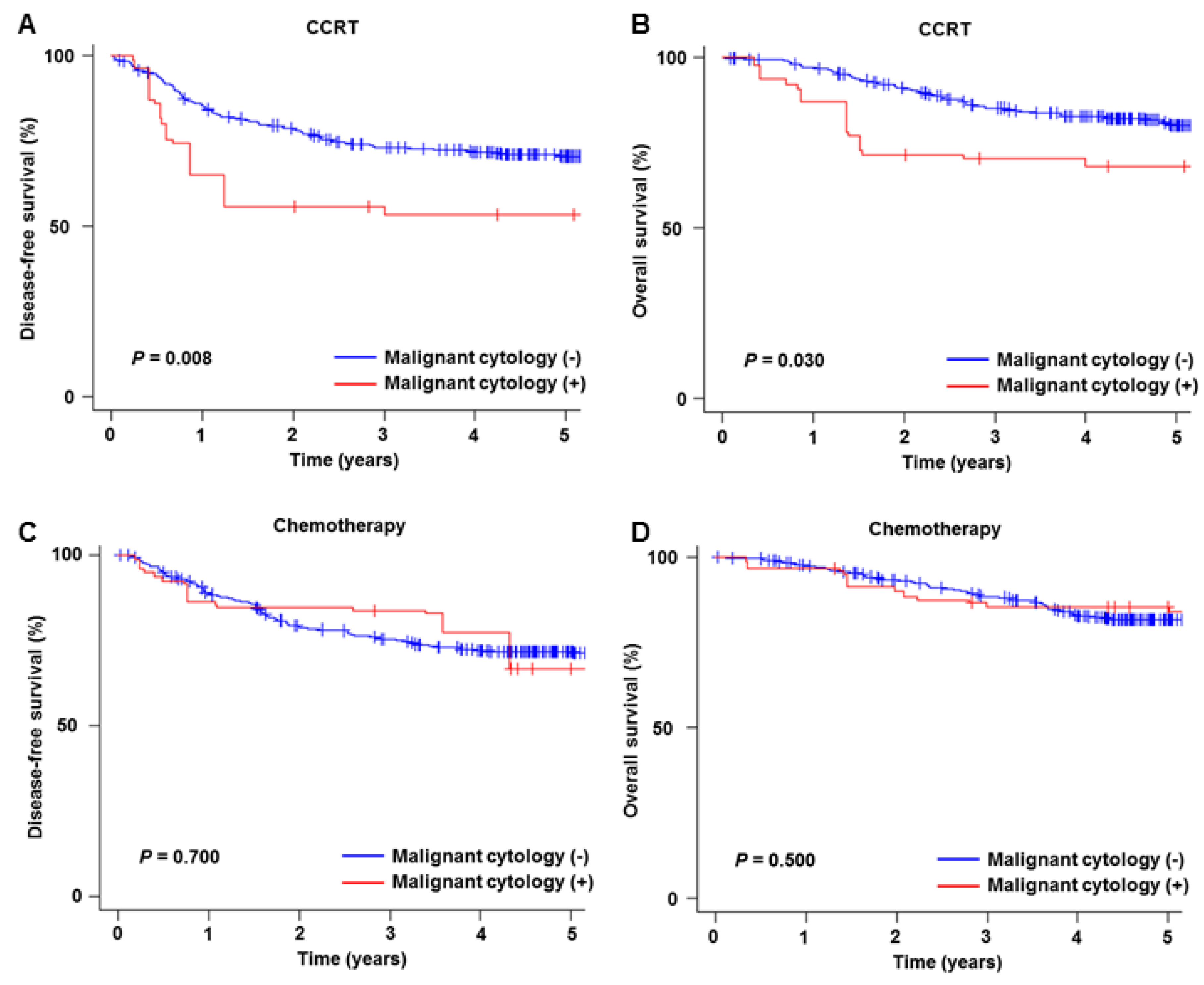
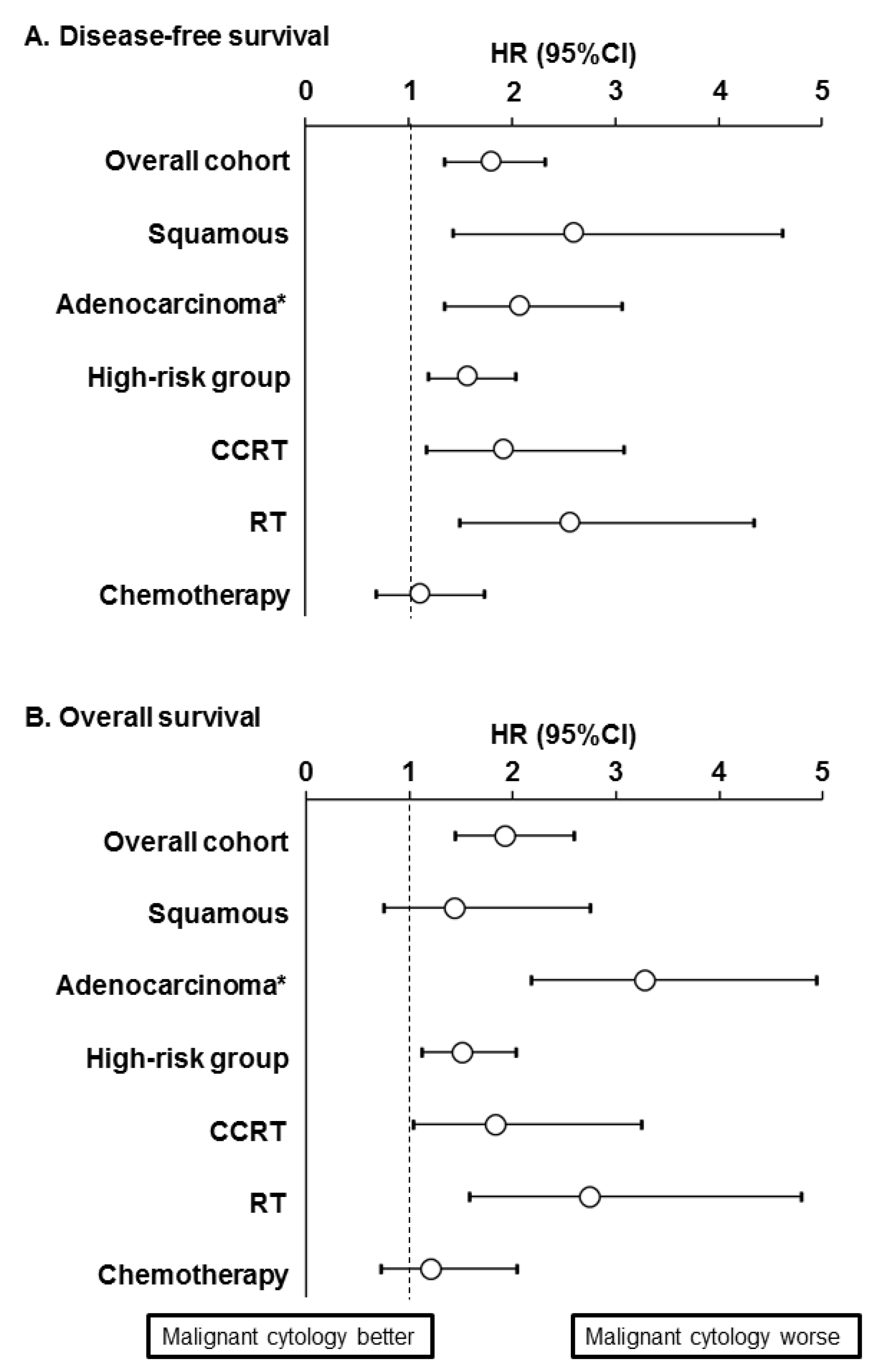
3.3. Prognostic Significance of Malignant Peritoneal Cytology
3.4. Sensitivity Analysis
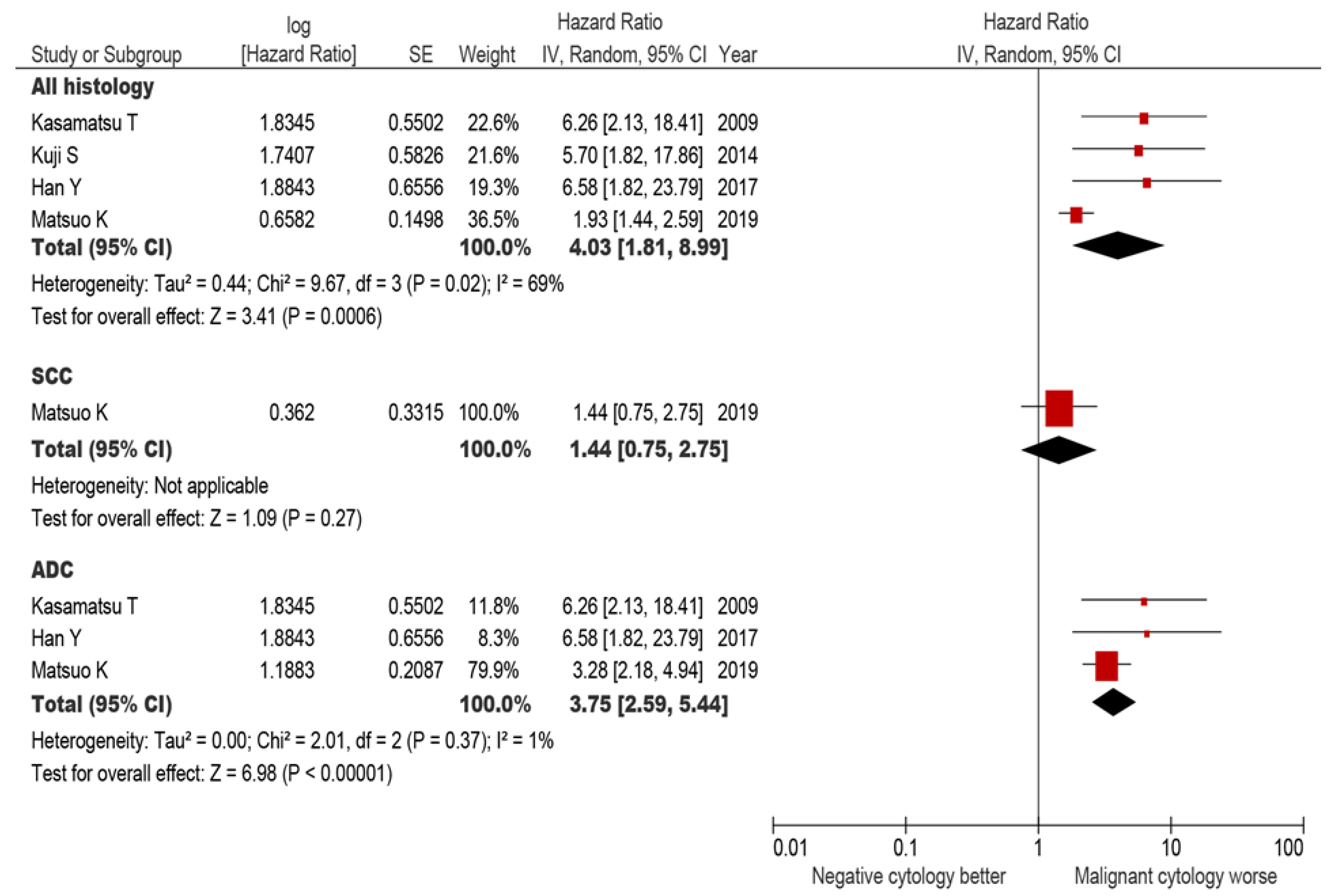
3.5. Systematic Review
3.5.1. Overview
3.5.2. All-Cause Mortality
3.5.3. Recurrence
4. Discussion
4.1. Prognostic Significance
4.2. Association for Historical Tumor Factors
4.3. Implication for Postoperative Therapy
4.4. Strengths and Limitations
4.5. Clinical Implication
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Financial Disclosure
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Cervical Cancer. National Comprehensive Cancer Network (US) NCCN Clinical Practice Guideline in Oncology. Version 4. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 9 October 2019).
- Peters, W.A., 3rd; Liu, P.Y.; Barrett, R.J., 2nd; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.; Bundy, B.N.; Fowler, W.C., Jr.; Stehman, F.B.; Sevin, B.; Creasman, W.T.; Major, F.; DiSaia, P.; Zaino, R. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1989, 35, 314–320. [Google Scholar] [CrossRef]
- Han, Y.; Li, N.; Zhang, R.; Li, X.; Sun, Y.; Wu, L. Role of positive peritoneal cytology in FIGO stage IB to IIB cervical adenocarcinoma. Int. J. Gynaecol. Obstet. 2017, 137, 150–156. [Google Scholar] [CrossRef]
- Kuji, S.; Hirashima, Y.; Komeda, S.; Tanaka, A.; Abe, M.; Takahashi, N.; Takekuma, M. The relationship between positive peritoneal cytology and the prognosis of patients with FIGO stage I/II uterine cervical cancer. J. Gynecol. Oncol. 2014, 25, 90–96. [Google Scholar] [CrossRef]
- Kasamatsu, T.; Onda, T.; Sasajima, Y.; Kato, T.; Ikeda, S.; Ishikawa, M.; Tsuda, H. Prognostic significance of positive peritoneal cytology in adenocarcinoma of the uterine cervix. Gynecol. Oncol. 2009, 115, 488–492. [Google Scholar] [CrossRef]
- Takeshima, N.; Katase, K.; Hirai, Y.; Yamawaki, T.; Yamauchi, K.; Hasumi, K. Prognostic value of peritoneal cytology in patients with carcinoma of the uterine cervix. Gynecol. Oncol. 1997, 64, 136–140. [Google Scholar] [CrossRef]
- Ito, K.; Noda, K. Peritoneal cytology in patients with uterine cervical carcinoma. Gynecol. Oncol. 1992, 47, 76–79. [Google Scholar] [CrossRef]
- Ditto, A.; Martinelli, F.; Carcangiu, M.; Lorusso, D.; Raspagliesi, F. Peritoneal cytology as prognostic factor in cervical cancer. Diagn. Cytopathol. 2015, 43, 705–709. [Google Scholar] [CrossRef]
- Kurita, T.; Matsuura, Y.; Koi, C.; Kagami, S.; Kawagoe, T.; Hachisuga, T. The Relationship between Positive Peritoneal Cytology and the Prognosis of Patients with Uterine Cervical Cancer. Acta Cytol. 2015, 59, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Zuna, R.E.; Behrens, A. Peritoneal washing cytology in gynecologic cancers: Long-term follow-up of 355 patients. J. Natl. Cancer Inst. 1996, 88, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, L.C.; Orr, J.W., Jr.; Hatch, K.D.; Shingleton, H.M.; Roberson, J. Peritoneal cytology in patients with squamous cell carcinoma of the cervix. Gynecol. Oncol. 1984, 19, 24–29. [Google Scholar] [CrossRef]
- Imachi, M.; Tsukamoto, N.; Matsuyama, T.; Nakano, H. Peritoneal cytology in patients with carcinoma of the uterine cervix. Gynecol. Oncol. 1987, 26, 202–207. [Google Scholar] [CrossRef]
- Weiser, E.B.; Bundy, B.N.; Hoskins, W.J.; Heller, P.B.; Whittington, R.R.; DiSaia, P.J.; Curry, S.L.; Schlaerth, J.; Thigpen, J.T. Extraperitoneal versus transperitoneal selective paraaortic lymphadenectomy in the pretreatment surgical staging of advanced cervical carcinoma (a Gynecologic Oncology Group study). Gynecol. Oncol. 1989, 33, 283–289. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, S.N.; Shim, S.H.; Lee, J.Y.; Lee, S.J.; Oh, I.K.; Kim, H.J.; Kang, S.B. The impact of positive peritoneal cytology on prognosis in patients with cervical cancer: A meta-analysis. Br. J. Cancer 2015, 113, 595–602. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Aoki, Y.; Sakamoto, M.; Takeshima, N.; Fujiwara, H.; Matsumoto, T.; Mikami, M.; Sugiyama, T. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: Systemic chemotherapy versus pelvic irradiation. Int. J. Cancer 2017, 141, 1042–1051. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Yamaguchi, S.; Kanao, H.; Nakanishi, T.; Saito, T.; Kamiura, S.; Iwata, T.; Mikami, M.; Sugiyama, T. Identifying a candidate population for ovarian conservation in young women with clinical stage IB-IIB cervical cancer. Int. J. Cancer 2018, 142, 1022–1032. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Yamaguchi, S.; Kigawa, J.; Tokunaga, H.; Tabata, T.; Kodama, J.; Kawana, K.; Mikami, M.; Sugiyama, T. Neoadjuvant Chemotherapy with Taxane and Platinum Followed by Radical Hysterectomy for Stage IB2-IIB Cervical Cancer: Impact of Histology Type on Survival. J. Clin. Med. 2019, 8, 156. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Yamaguchi, S.; Matoda, M.; Nakanishi, T.; Kikkawa, F.; Ohmichi, M.; Okamoto, A.; Sugiyama, T.; Mikami, M. Association of Radical Hysterectomy Surgical Volume and Survival for Early-Stage Cervical Cancer. Obstet. Gynecol. 2019, 133, 1086–1098. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Yokota, H.; Satoh, T.; Katabuchi, H.; Kodama, S.; Sasaki, H.; Matsumura, N.; Mikami, M.; Sugiyama, T. Effectiveness of adjuvant systemic chemotherapy for intermediate-risk stage IB cervical cancer. Oncotarget 2018, 8, 106866–106875. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Shimada, M.; Nakamura, K.; Takei, Y.; Ushijima, K.; Sumi, T.; Ohara, T.; Yahata, H.; Mikami, M.; Sugiyama, T. Predictors for pathological parametrial invasion in clinical stage IIB cervical cancer. Eur. J. Surg. Oncol. 2019, 45, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Shimada, M.; Saito, T.; Takehara, K.; Tokunaga, H.; Watanabe, Y.; Todo, Y.; Morishige, K.I.; Mikami, M.; Sugiyama, T. Risk stratification models for para-aortic lymph node metastasis and recurrence in stage IB-IIB cervical cancer. J. Gynecol. Oncol. 2018, 29, e11. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Shimada, M.; Mikami, M. Ovarian conservation for young women with clinical stage IB-IIB cervical cancer in Japan. J. Gynecol. Oncol. 2017, 28, e60. [Google Scholar] [CrossRef]
- FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynaecol. Obstet. 2014, 125, 97–98. [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Hershman, D.L.; Wright, J.D. Comparative effectiveness research in oncology methodology: Observational data. J. Clin. Oncol. 2012, 30, 4215–4222. [Google Scholar] [CrossRef]
- Austin, P.C. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Commun. Stat. Simul. Comput. 2009, 38, 1228–1234. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Yoshino, K.; Endo, M.; Kakigano, A.; Takiuchi, T.; Kimura, T. Conservative management of placenta percreta. Int. J. Gynaecol. Obstet. 2018, 140, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- RevMan 5. Cochrane Community. Available online: https://community.cochrane.org/help/tools-and-software/revman-5 (accessed on 9 November 2019).
- Gien, L.T.; Beauchemin, M.C.; Thomas, G. Adenocarcinoma: A unique cervical cancer. Gynecol. Oncol. 2010, 116, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ebina, Y.; Mikami, M.; Nagase, S.; Tabata, T.; Kaneuchi, M.; Tashiro, H.; Mandai, M.; Enomoto, T.; Kobayashi, Y.; Katabuchi, H.; et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int. J. Clin. Oncol. 2019, 24, 1–19. [Google Scholar] [CrossRef]
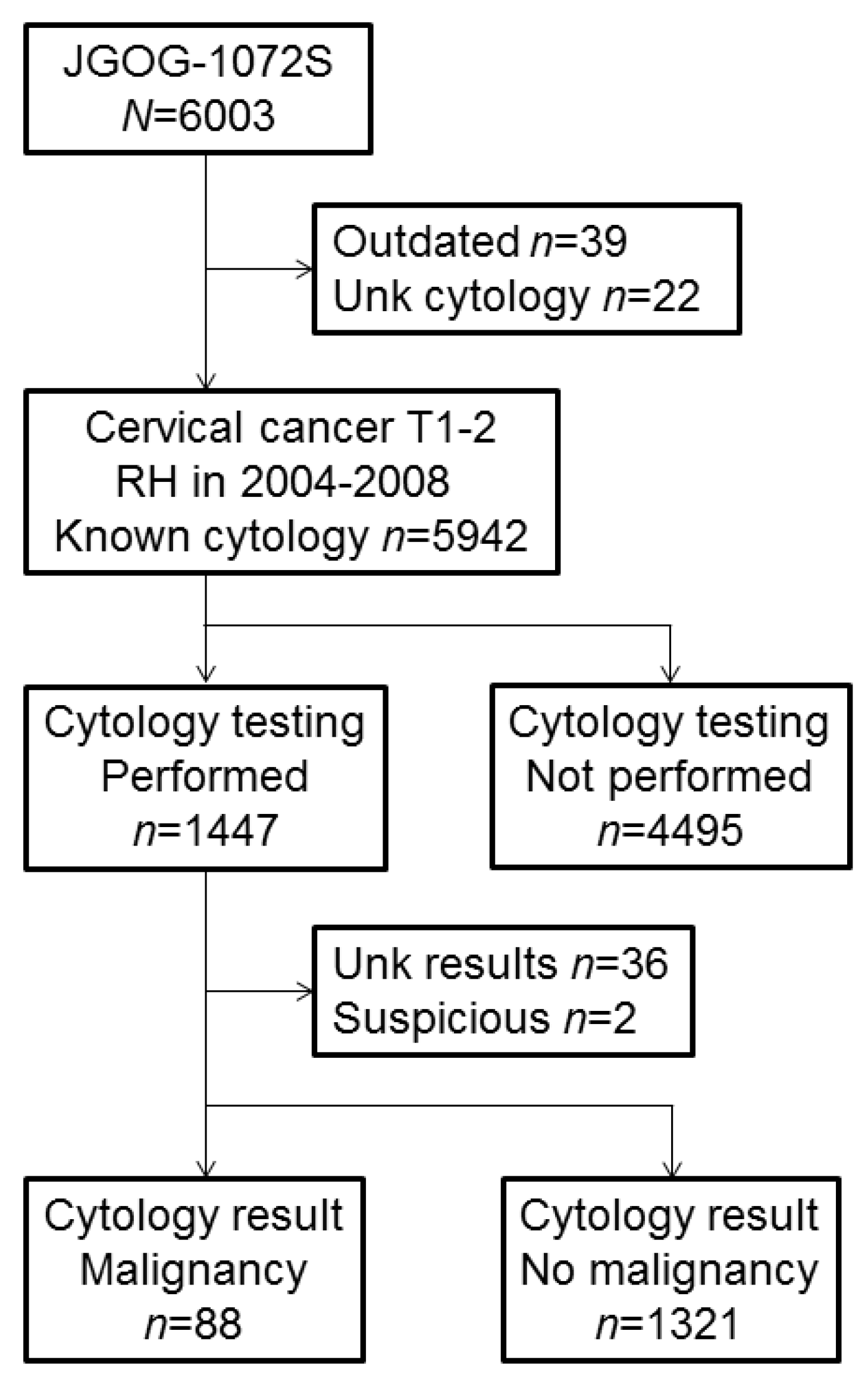
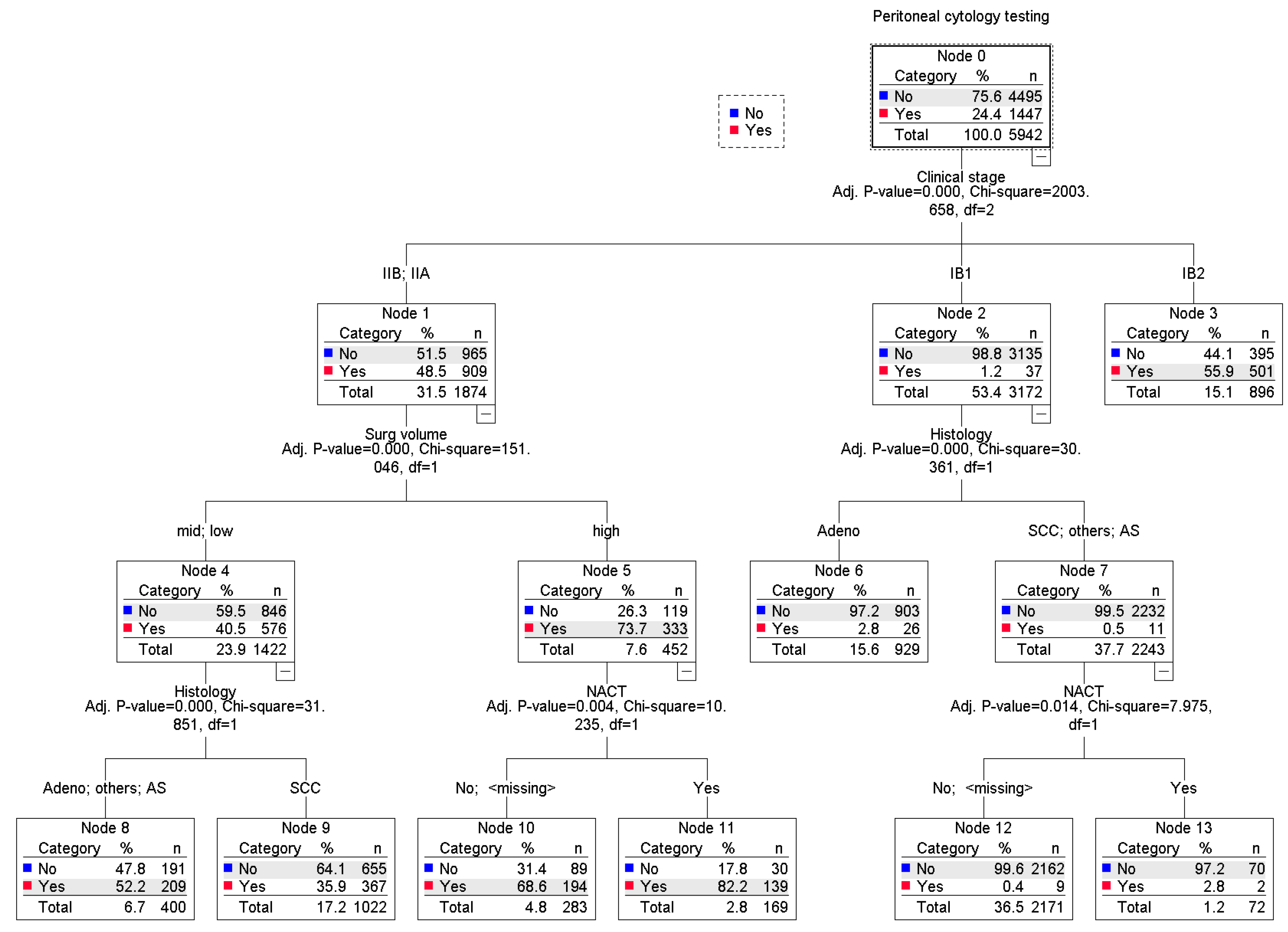
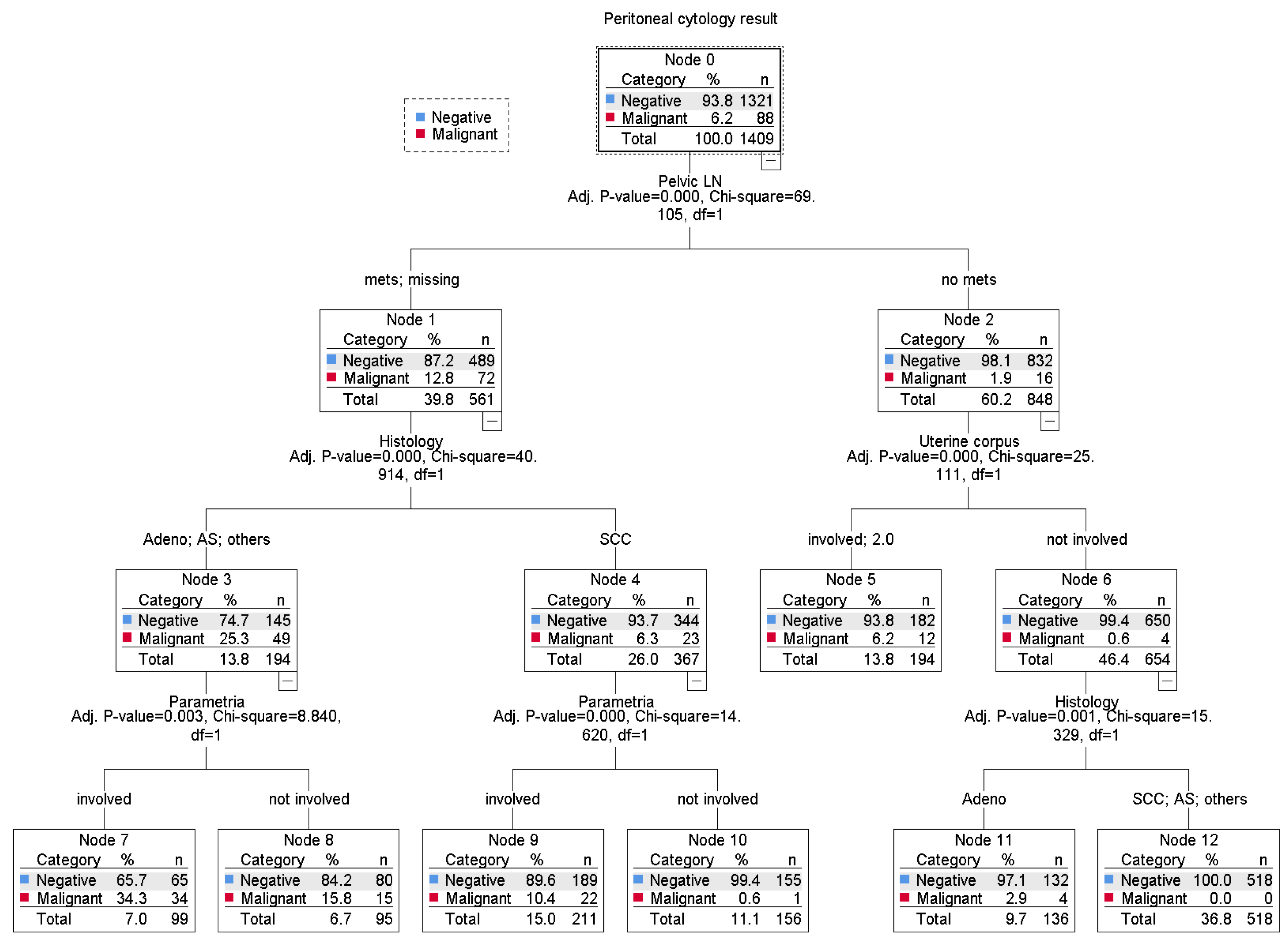
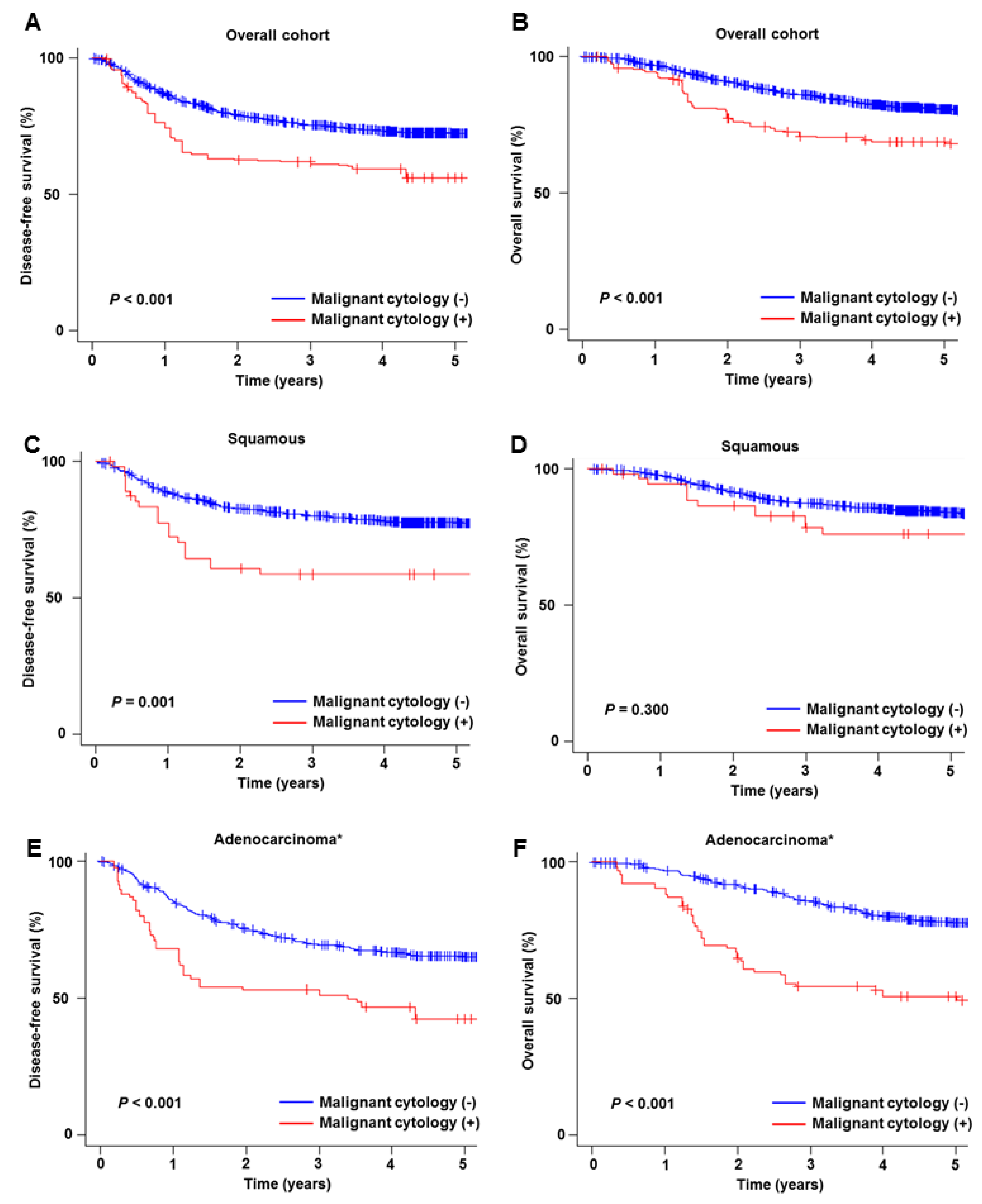
| Characteristic | Not Performed | Performed | P-Value |
|---|---|---|---|
| Number | n = 4495 | n = 1447 | |
| Age | 47.6 (±11.9) | 49.1 (±12.1) | <0.001 |
| Year | 0.467 * | ||
| 2004 | 825 (18.4%) | 239 (16.5%) | |
| 2005 | 884 (19.7%) | 289 (20.0%) | |
| 2006 | 892 (19.8%) | 298 (20.6%) | |
| 2007 | 957 (21.3%) | 335 (23.2%) | |
| 2008 | 937 (20.8%) | 286 (19.8%) | |
| Clinical stage | <0.001 | ||
| IB1 | 3,135 (69.7%) | 37 (2.6%) | |
| IB2 | 395 (8.8%) | 501 (34.6%) | |
| IIA | 312 (6.9%) | 290 (20.0%) | |
| IIB | 653 (14.5%) | 619 (42.8%) | |
| Histology | 0.872 | ||
| Squamous | 2,950 (65.6%) | 939 (64.9%) | |
| Adenocarcinoma | 1,118 (24.9%) | 361 (24.9%) | |
| Adenosquamous | 367 (8.2%) | 128 (8.8%) | |
| Others | 60 (1.3%) | 19 (1.3%) | |
| Surgical volume | <0.001 | ||
| Low | 501 (11.1%) | 148 (10.2%) | |
| Mid | 2,837 (63.1%) | 803 (55.5%) | |
| High | 1157 (25.7%) | 496 (34.3%) | |
| Neoadjuvant chemotherapy | <0.001 | ||
| No | 3,869 (86.8%) | 969 (67.2%) | |
| Yes | 586 (13.2%) | 472 (32.8%) |
| Characteristic | No Malignancy | Malignant Cells | P-Value | OR (95%CI) † | P-Value † |
|---|---|---|---|---|---|
| Number | n = 1321 | n = 88 | |||
| Age | 49.1 (±12.1) | 48.8 (±11.6) | 0.843 | ||
| Year | 0.821 * | ||||
| 2004 | 218 (16.5%) | 16 (18.2%) | |||
| 2005 | 268 (20.3%) | 15 (17.0%) | |||
| 2006 | 274 (20.7%) | 18 (20.5%) | |||
| 2007 | 298 (22.6%) | 26 (29.5%) | |||
| 2008 | 263 (19.9%) | 13 (14.8%) | |||
| Clinical stage | 0.039 | ||||
| IB1 | 0 | 0 | |||
| IB2 | 476 (36.0%) | 25 (28.4%) | |||
| IIA | 277 (21.0%) | 13 (14.8%) | |||
| IIB | 568 (43.0%) | 50 (56.8%) | |||
| Histology | <0.001 | <0.001 * | |||
| Squamous | 902 (68.3%) | 28 (31.8%) | 1 | ||
| Adenocarcinoma | 287 (21.7%) | 48 (54.5%) | 6.18 (3.49-10.9) | <0.001 | |
| Adenosquamous | 117 (8.9%) | 9 (10.2%) | 2.57 (1.08-6.13) | 0.034 | |
| Others | 15 (1.1%) | 3 (3.4%) | 5.89 (1.25-27.7) | 0.025 | |
| Parametrial involvement | <0.001 | ||||
| No | 897 (68.0%) | 29 (33.0%) | 1 | ||
| Yes | 423 (32.0%) | 59 (67.0%) | 1.87 (1.07-3.27) | 0.027 | |
| Node metastasis (pelvic) | <0.001 | ||||
| No | 832 (63.6%) | 16 (18.4%) | 1 | ||
| Yes | 482 (36.7%) | 71 (81.6%) | 6.51 (3.43-12.3) | <0.001 | |
| Node metastasis (para-aortic) | <0.001 | ||||
| No | 298 (23.8%) | 17 (20.7%) | |||
| Yes | 47 (3.8%) | 16 (19.5%) | |||
| Not examined * | 908 (72.5%) | 49 (59.8%) | |||
| Sampled lymph nodes | |||||
| Pelvic | 39 (IQR 29–51) | 36 (IQR 24–51) | 0.660 | ||
| Para-aortic | 10 (IQR 5–17) | 14 (IQR 6–29) | 0.055 | ||
| Deep stromal invasion | <0.001 | ||||
| No | 331 (27.3%) | 3 (4.1%) | |||
| Yes | 881 (72.7%) | 71 (95.9%) | |||
| Tumor size | 0.736 | ||||
| ≤4 cm | 533 (41.5%) | 34 (39.1%) | |||
| >4 cm | 751 (58.5%) | 53 (60.9%) | |||
| LVSI | <0.001 | ||||
| No | 403 (32.0%) | 7 (8.6%) | |||
| Yes | 856 (68.0%) | 74 (91.4%) | |||
| Uterine corpus invasion | <0.001 | ||||
| No | 999 (78.4%) | 35 (42.2%) | 1 | ||
| Yes | 276 (21.6%) | 48 (57.8%) | 2.74 (1.61-4.67) | <0.001 | |
| Ovarian metastasis | <0.001 | ||||
| No | 1237 (99.0%) | 68 (80.0%) | 1 | ||
| Yes | 13 (1.0%) | 17 (20.0%) | 5.72 (2.34-14.0) | <0.001 | |
| Surgical volume | 0.033 | ||||
| Low | 146 (11.1%) | 2 (2.3%) | |||
| Mid | 726 (55.0%) | 54 (61.4%) | |||
| High | 449 (34.0%) | 32 (36.4%) | |||
| Neoadjuvant chemotherapy | 0.014 | ||||
| No | 866 (65.8%) | 69 (78.4%) | |||
| Yes | 450 (34.2%) | 19 (21.6%) | |||
| Adjuvant therapy | 0.017 | ||||
| None | 253 (20.5%) | 5 (6.1%) | |||
| Radiotherapy alone | 262 (21.2%) | 20 (24.4%) | |||
| CCRT | 354 (28.6%) | 25 (30.5%) | |||
| Chemotherapy alone | 348 (28.2%) | 29 (35.4%) | |||
| Combined ** | 19 (1.5%) | 3 (3.7%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, K.; Shimada, M.; Matsuzaki, S.; Machida, H.; Nagase, Y.; Saito, T.; Kamiura, S.; Iwata, T.; Sugiyama, T.; Mikami, M. Significance of Malignant Peritoneal Cytology on the Survival of Women with Early-Stage Cervical Cancer: A Japanese Gynecologic Oncology Group Study. J. Clin. Med. 2019, 8, 1822. https://doi.org/10.3390/jcm8111822
Matsuo K, Shimada M, Matsuzaki S, Machida H, Nagase Y, Saito T, Kamiura S, Iwata T, Sugiyama T, Mikami M. Significance of Malignant Peritoneal Cytology on the Survival of Women with Early-Stage Cervical Cancer: A Japanese Gynecologic Oncology Group Study. Journal of Clinical Medicine. 2019; 8(11):1822. https://doi.org/10.3390/jcm8111822
Chicago/Turabian StyleMatsuo, Koji, Muneaki Shimada, Shinya Matsuzaki, Hiroko Machida, Yoshikazu Nagase, Toshiaki Saito, Shoji Kamiura, Takashi Iwata, Toru Sugiyama, and Mikio Mikami. 2019. "Significance of Malignant Peritoneal Cytology on the Survival of Women with Early-Stage Cervical Cancer: A Japanese Gynecologic Oncology Group Study" Journal of Clinical Medicine 8, no. 11: 1822. https://doi.org/10.3390/jcm8111822
APA StyleMatsuo, K., Shimada, M., Matsuzaki, S., Machida, H., Nagase, Y., Saito, T., Kamiura, S., Iwata, T., Sugiyama, T., & Mikami, M. (2019). Significance of Malignant Peritoneal Cytology on the Survival of Women with Early-Stage Cervical Cancer: A Japanese Gynecologic Oncology Group Study. Journal of Clinical Medicine, 8(11), 1822. https://doi.org/10.3390/jcm8111822








