Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience
Abstract
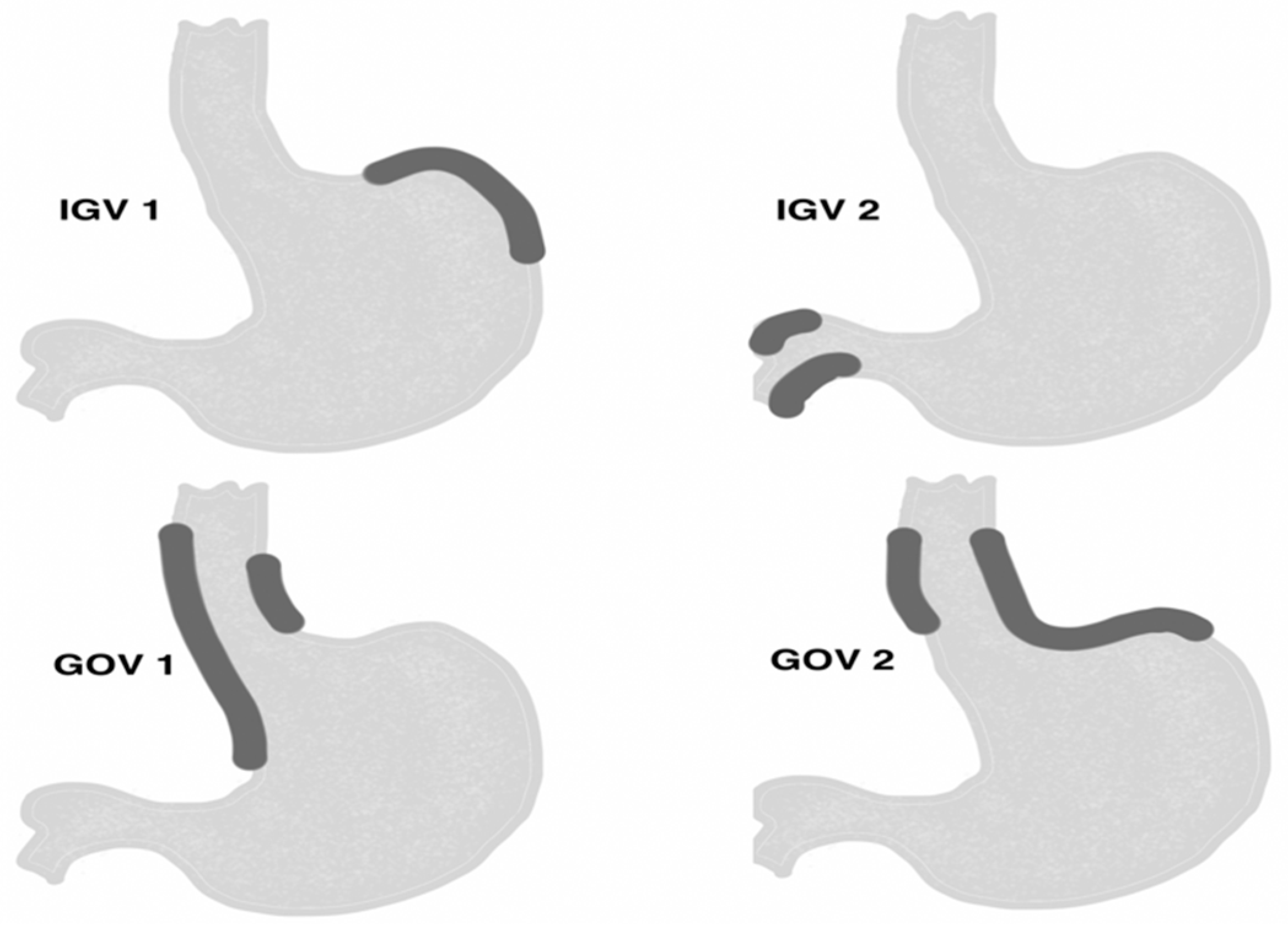
1. Introduction
- -
- periesophageal/perigastric, directly adjacent to the wall of the wall,
- -
- paraesophageal/paragastric, non-direct contact with the gastrointestinal wall;
- -
- perforating veins, connecting superficial vessels with the para- and peri- vessels (Figure 2).
2. Methods
- -
- conglomerate of gastric varices type GOV 2 and IGV 1 located in the gastric area, and varices IGV 2 located in the duodenum with a minimum diameter of 5 mm, visible in the endosonographic image.
- -
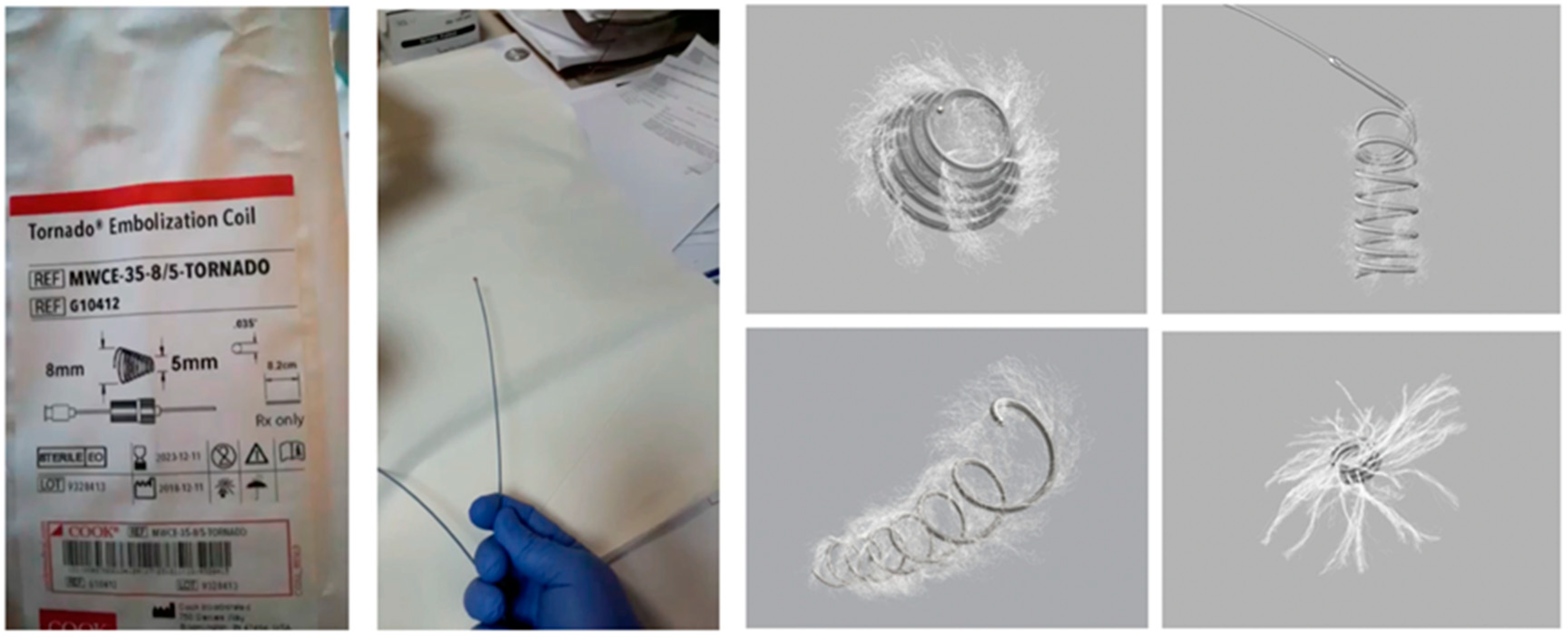
- in a case of varices’ diameter ranging from 5 to 10 mm, implantation of intravascular coils alone was performed. In a case of varices’ diameter above 10 mm, implantation of intravascular coils with cyanoacrylate injections was performed.
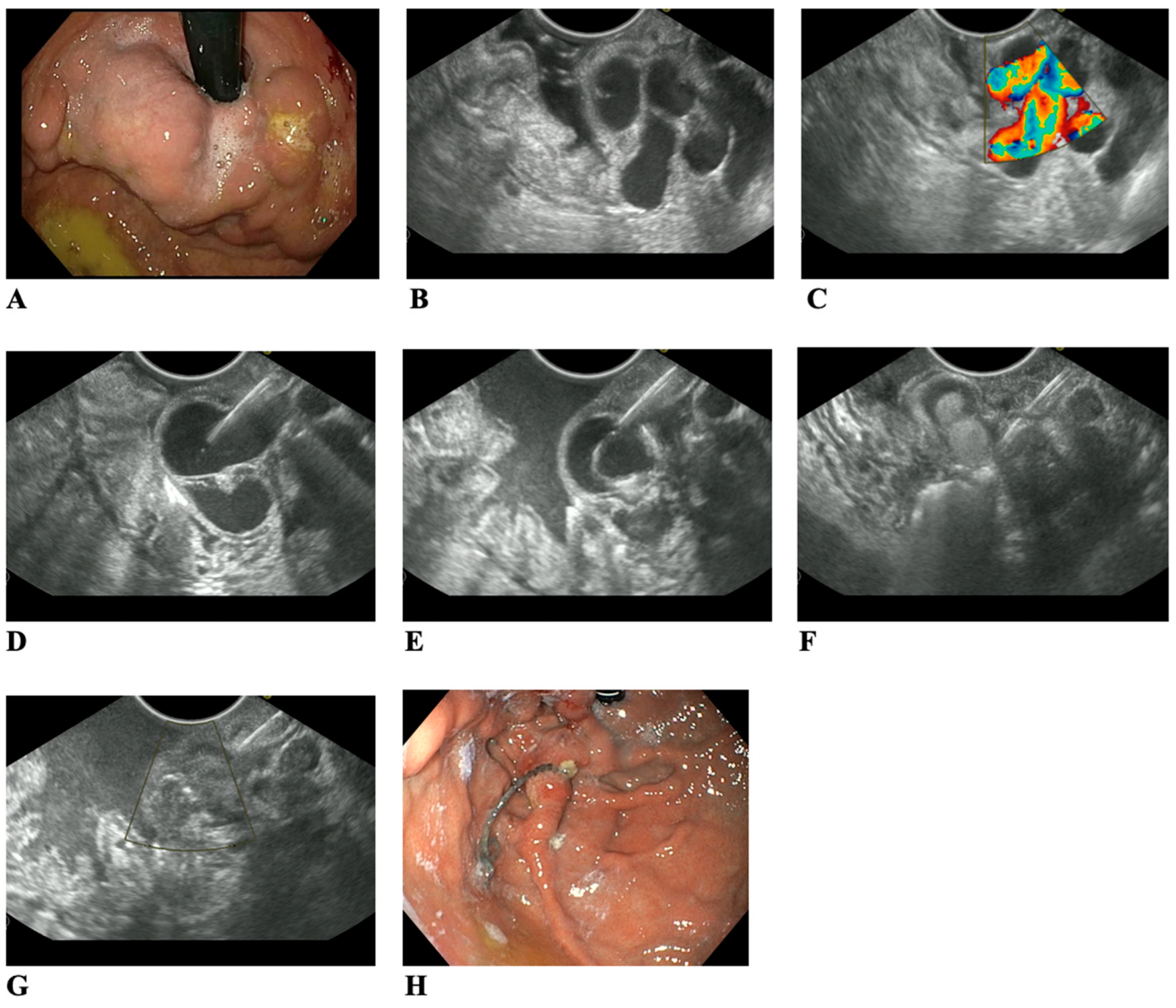
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Boregowda, U.; Umapathy, C.; Halim, N.; Desai, M.; Nanjappa, A.; Arekapudi, S.; Theethira, T.; Wong, H.; Roytman, M.; Saligram, S. Update on the management of gastrointestinal varices. World J. Gastrointest. Pharmacol. Ther. 2019, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wani, Z.A.; Bhat, R.A.; Bhadoria, A.S.; Maiwall, R.; Choudhury, A. Gastric varices: Classification, endoscopic and ultrasonographic management. J. Res. Med. Sci. 2015, 20, 1200–1207. [Google Scholar] [PubMed]
- Sarin, S.; Kumar, A. Gastric varices: Profile, classification, and management. Am. J. Gastrenterol. 1989, 84, 1244–1249. [Google Scholar]
- Wiechowska-Kozlowska, A.; Milkiewicz, P. Endoscopic ultrasonography in the assessment of portal hypertension. Gastroenterol. Klin. 2014, 14, 148–155. [Google Scholar]
- Soderlund, C.; Backman, L.; Erwald, R. Sclerotherapy of Esophageal Varices: An Endoscopic and Portographic Study. Hepatology 1984, 4, 877–884. [Google Scholar] [CrossRef] [PubMed]
- North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N. Engl. J. Med. 1988, 319, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W.; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar]
- Mishra, S.R.; Sharma, B.C.; Kumar, A.; Sarin, S.K. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: A randomized controlled trial. J. Hepatol. 2011, 54, 1161–1167. [Google Scholar] [CrossRef]
- Levy, M.J.; Song, L.M.W.K.; Kendrick, M.L.; Misra, S.; Gostout, C.J. EUS-guided coil embolization for refractory ectopic variceal bleeding (with videos). Gastrointest. Endosc. 2008, 67, 572–574. [Google Scholar] [CrossRef]
- Ibrahim, M.; Mostafa, I.; Deviere, J. New developments in managing variceal bleeding. Gastroenterology 2018, 154, 1964–1969. [Google Scholar] [CrossRef]
- de Franchis, R. Expanding consensus in portal hypertension report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.M.; Weilert, F.; Fredrick, R.T.; Kane, S.D.; Shah, J.N.; Hamerski, C.M.; Binmoeller, K.F. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: A large U.S. experience over 6 years (with video). Gastrointest. Endosc. 2016, 6, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Binmoeller, K.F.; Weilert, F.; Shah, J.N.; Kim, J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos). Gastrointest. Endosc. 2011, 74, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Romero-Castro, R.; Ellrichmann, M.; Ortiz-Moyano, C.; Subtil-Inigo, J.C.; Junquera-Florez, F.; Gornals, J.B.; Repiso-Ortega, A.; Vila-Costas, J.; Marcos-Sanchez, F.; Muñoz-Navas, M.; et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: A multicenter study (with videos). Gastrointest. Endosc. 2013, 78, 711–721. [Google Scholar] [CrossRef]
- Kim, T.; Shijo, H.; Kokawa, H.; Tokumitsu, H.; Kubara, K.; Ota, K.; Akiyoshi, N.; Iida, T.; Yokoyama, M.; Okumura, M. Risk factors for hemorrhage from gastric fundal varices. Hepatology 1997, 25, 307–312. [Google Scholar] [CrossRef]
- Sarin, S.; Lahoti, D.; Saxena, S. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertensionpatients. Hepatology 1992, 16, 1343–1349. [Google Scholar] [CrossRef]
- Saxena, P.; Lakhtakia, S. Endoscopic ultrasound guided vascular access and therapy (with videos). Endosc. Ultrasound 2015, 4, 168–175. [Google Scholar]
- Koziel, S.; Kobryń, K.; Paluszkiewicz, R.; Krawczyk, M.; Wroblewski, T. Endoscopic treatment of gastric varices bleeding with the use of n-butyl-2 cyanoacrylate. Prz. Gastroenterol. 2015, 10, 239–243. [Google Scholar] [CrossRef]
- Gotlib, J.P. Endoscopic obturation of esophageal and gastric varices with cyanoacrylate tissue adhesive. Can. J. Gastroenterol. 1990, 9, 637–638. [Google Scholar] [CrossRef]
- Cheng, L.F.; Wang, Z.Q.; Li, C.Z.; Cai, F.C.; Huang, Q.Y.; Linghu, E.Q.; Wen, L.I.; Chai, G.J.; Sun, G.H.; Mao, Y.P.; et al. Treatment of gastric varices by endoscopic sclerotherapy using butyl cyanoacrylate: 10 years’ experience of 635 cases. Chin. Med. J. 2007, 120, 2081–2085. [Google Scholar] [CrossRef]
- Kang, E.J.; Jeong, S.W.; Jang, J.Y.; Cho, J.Y.; Lee, S.H.; Kim, H.G.; Kim, S.G.; Kim, Y.S.; Cheon, Y.K.; Cho, Y.D.; et al. Long-term result of endoscopic HistoacrylR (N-butyl-2-cyanoacrylate) injection for treatment of gastric varices. World J. Gastroenterol. 2011, 17, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, J.Y. Prevention and Managment of Variceal Hemorrhage. Int. J. Hepatol. 2013, 2013, 434609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ASGE Technology Committe. Emerging Technologies for Endoscopic Hemostasis. Gastrointest. Endosc. 2012, 75, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yamazaki, K.; Toyota, J.; Karino, Y.; Ohmura, T.; Akaike, J. Observation of gastric variceal flow characteristics by endoscopic ultrasonography using color Doppler. Am. J. Gastroenterol. 2008, 103, 575–580. [Google Scholar] [CrossRef]
- LÔBO, M.R.D.A.; Chaves, D.M.; De Moura, D.T.H.; Ribeiro, I.B.; Ikari, E.; De Moura, E.G.H. Safety and efficacy of Eus-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: A randomized controlled trial. Arq. Gastroenterol. 2019, 56, 99–105. [Google Scholar] [CrossRef]
- Imamura, H.; Irisawa, A.; Shibukawa, G.; Takagi, T.; Hikichi, T.; Obara, K.; Ohira, H. Echo-endoscopic analysis of variceal hemodynamics in patient with isolated gastric varices. Endosc. Ultrasound 2014, 3, 238–244. [Google Scholar]
- Fujii-Lau, L.L.; Law, R.; Song, L.M.W.K.; Gostout, C.J.; Kamath, P.S.; Levy, M.J. Endoscopic ultrasound (EUS)-guided coil injection therapy of esophagogastric and ectopic varices. Surg. Endosc. 2015, 30, 1396–1404. [Google Scholar] [CrossRef]
- Seewald, S.; Ang, T.L.; Imazu, H.; Naga, M.; Omar, S.; Groth, S.; Seitz, U.; Zhong, Y.; Thonke, F.; Soehendra, N. A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices. Gastointest. Endosc. 2008, 68, 447–454. [Google Scholar] [CrossRef]

| Parameter | Type | Value | |
|---|---|---|---|
| Total number of patients | Male | 9 (56.0%) | Total: 16 |
| Female | 7 (44.0%) | ||
| Mean age (range) | 51 (29–75) | ||
| Portal hypertension etiology | Portal vein thrombosis (PVT) | 5 (31.0%) | |
| Isolated splenic vein thrombosis (SVT) | 4 (25.0%) | ||
| Alcoholic cirrhosis | 2 (12.5%) | ||
| Hepatitis C cirrhosis | 3 (19.0%) | ||
| Alcoholic cirrhosis + PVT | 2 (12.5%) | ||
| Varices type | GOV-2 | 8 (50.0%) | |
| IGV-1 | 6 (37.5%) | ||
| IGV-2 | 2 (12.5%) | ||
| History of bleeding | Primary prophylaxis (never bled) | 6 (35.0%) | |
| Secondary prophylaxis (recent hemorrhage) | 10 (65.0%) | ||
| Gastric varices | due to portal thrombosis | Child A—7 (77.8%) Child B—2 (22.2%) | Total: 9 |
| due to liver cirrhosis | Child B—6 (85.7%) Child C—1 (14.3%) | Total: 7 | |
| Nonselective betablockers (propranolol) | given in maximal tolerated doses | ||
| Parameter | Type | Value | |
|---|---|---|---|
| Previous varices treatment | EVL | 6 | |
| CYA glue injections | 4 | ||
| Mean varices size in mm (range) | 17 mm (5–45) | ||
| Method of treatment | Coils + CYA | 12 (75.0%) | |
| Coils alone | 4 (25.0%) | ||
| Mean coil number (range) | 1, 7 (1–3) | ||
| Mean CYA glue/Lipiodol mixture volume in mL (range) | 2 (1–9) | ||
| Technical success | 15 (94.0%) | ||
| Follow-up data (month; number of patients) | First follow-up (1–3 month) | 16 (100%) | |
| Second follow-up (3, 6, 12 month) | 13 (81.0%) | ||
| Mean follow-up in days (range) | 327 (182–443) | ||
| Therapeutic success | After first procedure | 12 (75.0%) | |
| Coils + CYA patients (n = 12) | 11 (92.0%) | ||
| Coils alone patients (n = 4) | 0 (0%) | ||
| After 2nd and 3rd (n = 5) | 5 (100%) | ||
| Adverse events | Pain | 3 (19.0%) | Total: 6 (37.5%) |
| Fever | 2 (12.5%) | ||
| Minor bleeding | 1 (6.0%) | ||
| Severe (embolization, serious hemorrhage, death) | 0 (0%) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozieł, S.; Pawlak, K.; Błaszczyk, Ł.; Jagielski, M.; Wiechowska-Kozłowska, A. Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience. J. Clin. Med. 2019, 8, 1786. https://doi.org/10.3390/jcm8111786
Kozieł S, Pawlak K, Błaszczyk Ł, Jagielski M, Wiechowska-Kozłowska A. Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience. Journal of Clinical Medicine. 2019; 8(11):1786. https://doi.org/10.3390/jcm8111786
Chicago/Turabian StyleKozieł, Sławomir, Katarzyna Pawlak, Łukasz Błaszczyk, Mateusz Jagielski, and Anna Wiechowska-Kozłowska. 2019. "Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience" Journal of Clinical Medicine 8, no. 11: 1786. https://doi.org/10.3390/jcm8111786
APA StyleKozieł, S., Pawlak, K., Błaszczyk, Ł., Jagielski, M., & Wiechowska-Kozłowska, A. (2019). Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience. Journal of Clinical Medicine, 8(11), 1786. https://doi.org/10.3390/jcm8111786





