Clinical Benefit of Long-Term Disease Control with Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients
Abstract
1. Introduction
2. Experimental Section
2.1. Patients and Treatments
2.2. Safety and Clinical Evaluation
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Safety and Tolerability
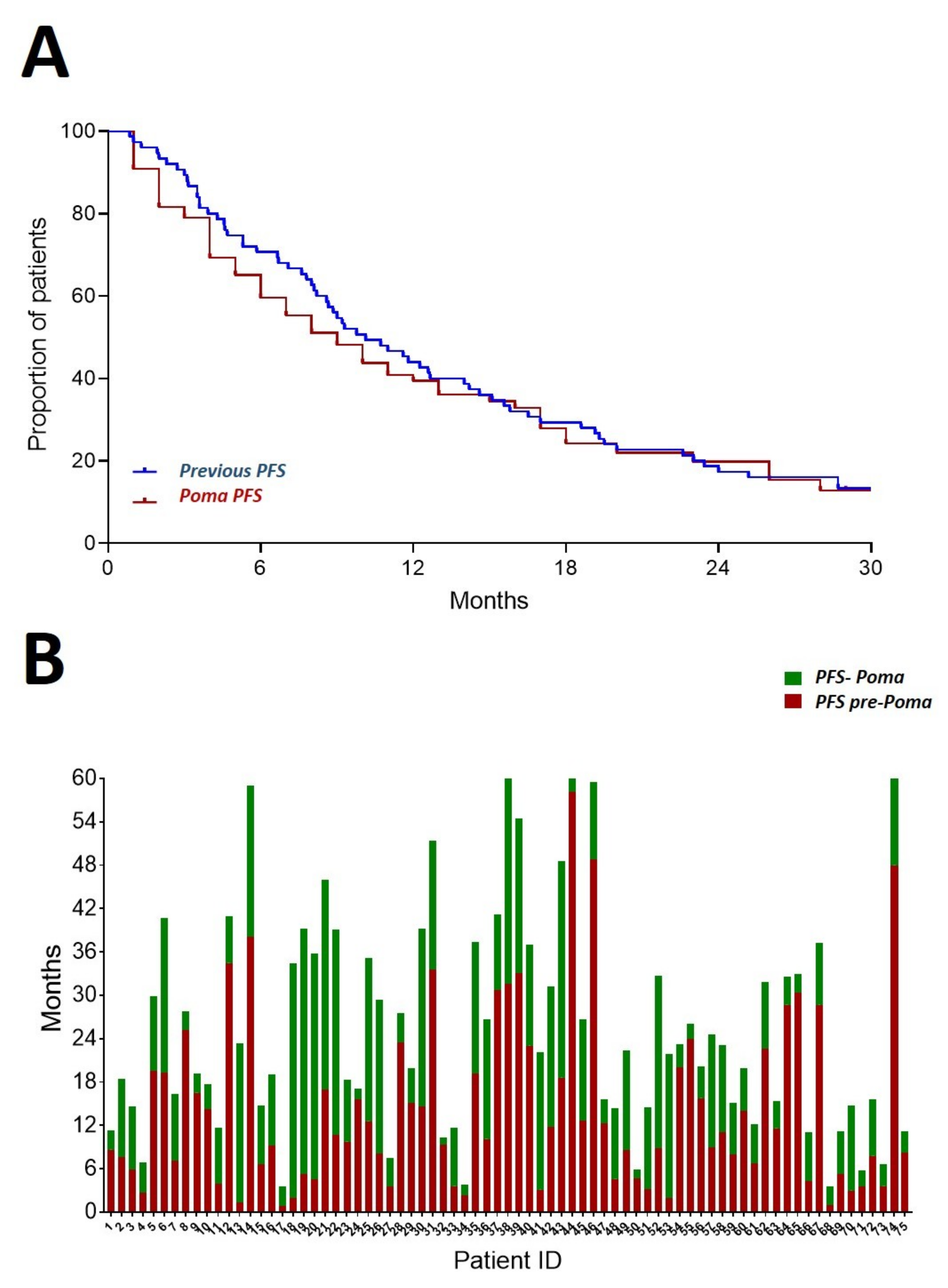
3.3. Efficacy
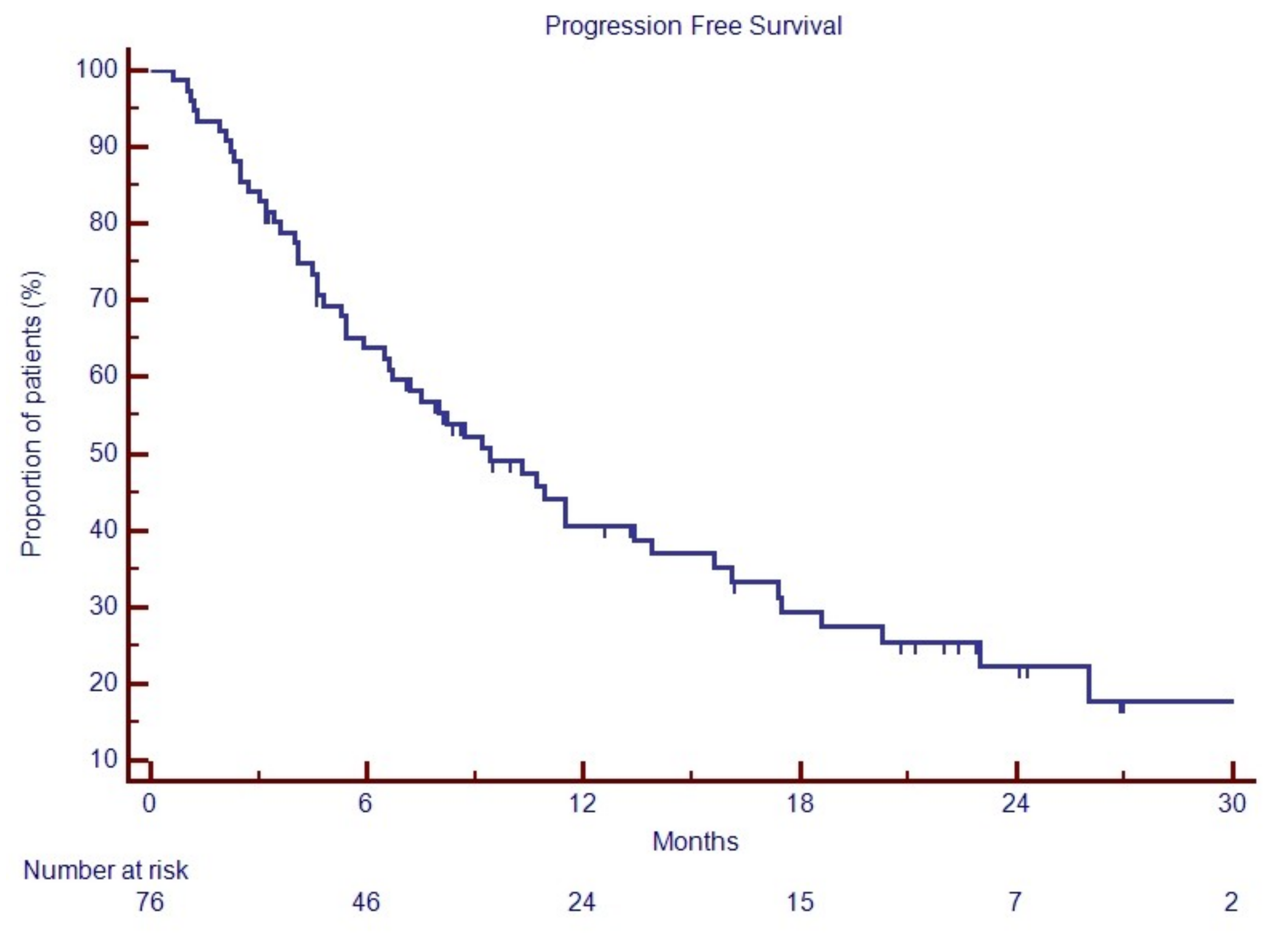
3.4. Progression Free Survival
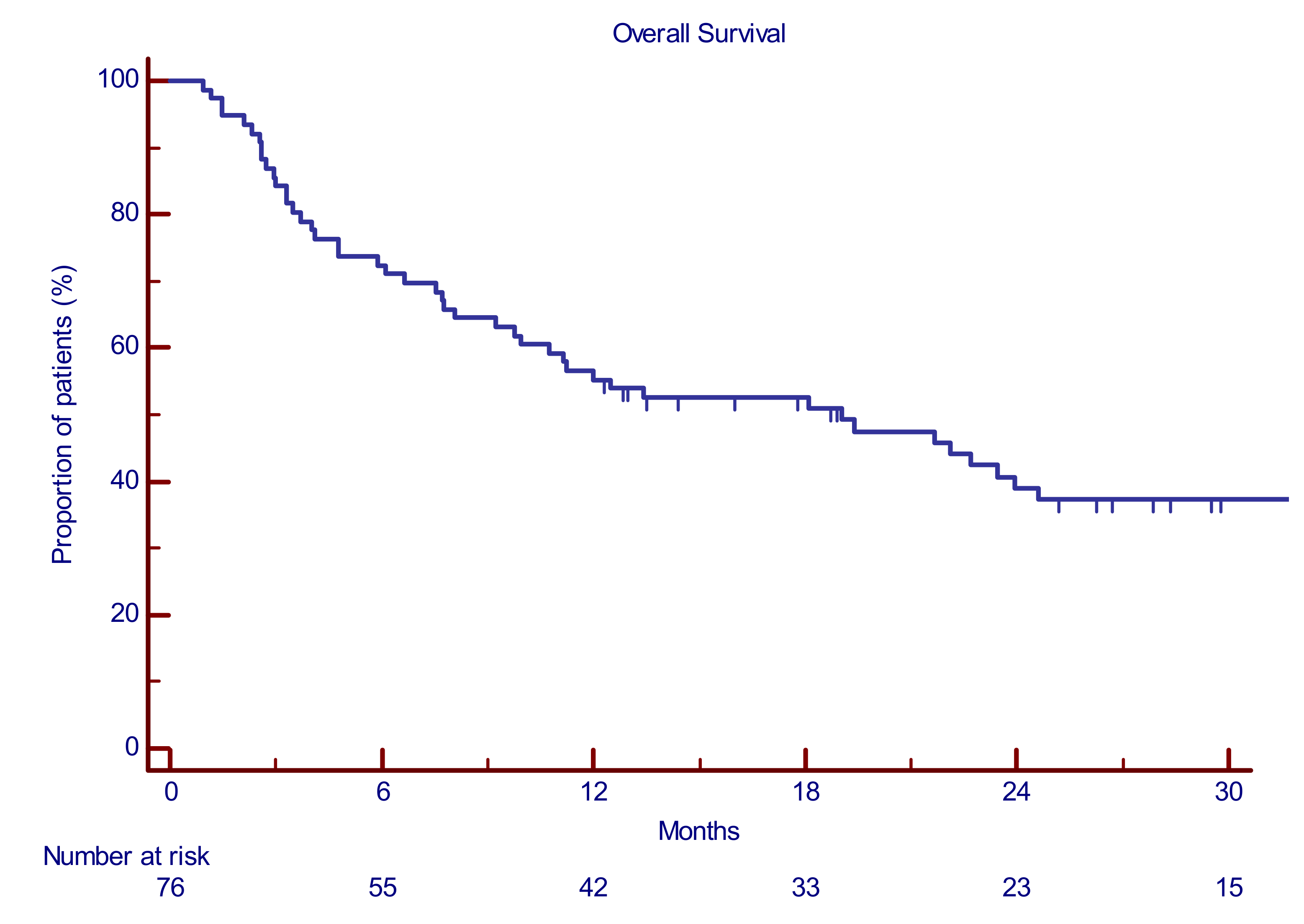
3.5. PomaD Can Prolong Overall Survival Independently from the Quality of the Achieved Best Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.K.; Lee, J.H.; Lahuerta, J.J.; Morgan, G.; Richardson, P.G.; Crowley, J.; Haessler, J.; Feather, J.; Hoering, A.; Moreau, P.; et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia 2012, 26, 149–157. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Spicka, I.; Oriol, A.; Hajek, R.; Rosinol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C.; Kyle, R.A.; Rajkumar, S.V.; Stewart, A.K.; Weber, D.; Richardson, P. Clinically relevant end points and new drug approvals for myeloma. Leukemia 2008, 22, 231–239. [Google Scholar] [CrossRef][Green Version]
- Nijhof, I.S.; van de Donk, N.W.C.J.; Zweegman, S.; Lokhorst, H.M. Current and New Therapeutic Strategies for Relapsed and Refractory Multiple Myeloma: An Update. Drugs 2018, 78, 19–37. [Google Scholar] [CrossRef]
- Pal, R.; Monaghan, S.A.; Hassett, A.C.; Mapara, M.Y.; Schafer, P.; Roodman, G.D.; Ragni, M.V.; Moscinski, L.; List, A.; Lentzsch, S. Immunomodulatory derivatives induce PU.1 down-regulation, myeloid maturation arrest, and neutropenia. Blood 2010, 115, 605–614. [Google Scholar] [CrossRef]
- Kumar, A.; Porwal, M.; Verma, A.; Mishra, A.K. Impact of pomalidomide therapy in multiple myeloma: A recent survey. J. Chemother. 2014, 26, 321–327. [Google Scholar] [CrossRef]
- Lacy, M.Q.; Allred, J.B.; Gertz, M.A.; Hayman, S.R.; Short, K.D.; Buadi, F.; Dispenzieri, A.; Kumar, S.; Greipp, P.R.; Lust, J.A.; et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: Comparison of 2 dosing strategies in dual-refractory disease. Blood 2011, 118, 2970–2975. [Google Scholar] [CrossRef]
- Lacy, M.Q.; Hayman, S.R.; Gertz, M.A.; Dispenzieri, A.; Buadi, F.; Kumar, S.; Greipp, P.R.; Lust, J.A.; Russell, S.J.; Dingli, D.; et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J. Clin. Oncol. 2009, 27, 5008–5014. [Google Scholar] [CrossRef] [PubMed]
- Conticello, C.; Parisi, M.; Romano, A.; Calafiore, V.; Ancora, F.; La Fauci, A.; Consoli, M.L.; Di Raimondo, F. Pomalidomide experience: An effective therapeutic approach with immunomodulatory drugs in a patient with relapsed-refractory multiple myeloma. Future Oncol. 2017, 13, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Palumbo, A.; Corradini, P.; Cavo, M.; Delforge, M.; di Raimondo, F.; Weisel, K.C.; Oriol, A.; Hansson, M.; Vacca, A.; et al. Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): A phase 3b study in refractory multiple myeloma. Blood 2016, 128, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Ailawadhi, S.; Mikhael, J.R.; LaPlant, B.R.; Laumann, K.M.; Kumar, S.; Roy, V.; Dingli, D.; Bergsagel, P.L.; Buadi, F.K.; Rajkumar, S.V.; et al. Pomalidomide-dexamethasone in refractory multiple myeloma: Long-term follow-up of a multi-cohort phase II clinical trial. Leukemia 2018, 32, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Maciocia, N.; Melville, A.; Cheesman, S.; Sharpley, F.; Ramasamy, K.; Streetly, M.; Jenner, M.; Benjamin, R.; Schey, S.; Maciocia, P.; et al. Real-world use of pomalidomide and dexamethasone in double refractory multiple myeloma suggests benefit in renal impairment and adverse genetics: A multi-centre UK experience. Br. J. Haematol. 2017, 176, 908–917. [Google Scholar] [CrossRef]
- Sriskandarajah, P.; Pawlyn, C.; Mohammed, K.; Dearden, C.E.; Davies, F.E.; Morgan, G.J.; Boyd, K.D.; Kaiser, M.F. The efficacy and tolerability of pomalidomide in relapsed/refractory myeloma patients in a “real-world” study: The Royal Marsden Hospital experience. Leuk. Lymphoma 2017, 58, 494–497. [Google Scholar] [CrossRef]
- Gueneau, P.; Chretien, M.L.; Cransac-Miet, A.; Aho, L.S.; Lafon, I.; Favennec, C.; Guy, J.; Caillot, D.; Boulin, M. Efficacy, safety, and cost of pomalidomide in relapsed and refractory multiple myeloma. Eur. J. Haematol. 2018, 100, 518–525. [Google Scholar] [CrossRef]
- Charlinski, G.; Grzasko, N.; Jurczyszyn, A.; Janczarski, M.; Szeremet, A.; Waszczuk-Gajda, A.; Bernatowicz, P.; Swiderska, A.; Guzicka-Kazimierczak, R.; Lech-Maranda, E.; et al. The efficacy and safety of pomalidomide in relapsed/refractory multiple myeloma in a “real-world” study: Polish Myeloma Group experience. Eur. J. Haematol. 2018, 101, 354–361. [Google Scholar] [CrossRef]
- Scott, A.; Weber, N.; Tiley, C.; Taylor, K.; Taper, J.; Harrison, S.; Chan, K.L.; Stark, R.; Lee, C.; Morris, K.; et al. ‘Real-world’ Australian experience of pomalidomide for relapsed and refractory myeloma. Leuk. Lymphoma 2018, 59, 1514–1516. [Google Scholar] [CrossRef]
- Jandial, A.; Mishra, K.; Lad, D.; Prakash, G.; Khadwal, A.; Malhotra, P. Real world experience with “generic” pomalidomide in relapsed refractory multiple myeloma. Leuk. Lymphoma 2019, 60, 1102–1104. [Google Scholar] [CrossRef]
- Matsumura-Kimoto, Y.; Kuroda, J.; Kaneko, H.; Kamitsuji, Y.; Fuchida, S.I.; Nakaya, A.; Shibayama, H.; Uoshima, N.; Yokota, I.; Uchiyama, H.; et al. Pomalidomide with or without dexamethasone for relapsed/refractory multiple myeloma in Japan: A retrospective analysis by the Kansai Myeloma Forum. Int. J. Hematol. 2018, 107, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Mele, G.; Pastore, D.; di Renzo, N.; Fragasso, A.; Guarini, A.; Mazza, P.; Musto, P.; Pavone, V.; Tarantini, G.; Curci, P.; et al. Real world Italian experience of pomalidomide plus low-dose dexamethasone in the relapsed and refractory myeloma setting: Extended follow-up of a retrospective multicenter study by the ‘Rete Ematologica Pugliese E Basilicata’. Leuk. Lymphoma 2019, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chim, C.S.; Kumar, S.K.; Orlowski, R.Z.; Cook, G.; Richardson, P.G.; Gertz, M.A.; Giralt, S.; Mateos, M.V.; Leleu, X.; Anderson, K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2018, 32, 252–262. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Common Terminology Criteria for Adverse Events. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 15 October 2019).
- Laubach, J.; Garderet, L.; Mahindra, A.; Gahrton, G.; Caers, J.; Sezer, O.; Voorhees, P.; Leleu, X.; Johnsen, H.E.; Streetly, M.; et al. Management of relapsed multiple myeloma: Recommendations of the International Myeloma Working Group. Leukemia 2016, 30, 1005–1017. [Google Scholar] [CrossRef]
- Conticello, C.; Romano, A.; del Fabro, V.; Martino, E.A.; Calafiore, V.; Sapienza, G.; Leotta, V.; Parisi, M.S.; Markovic, U.; Garibaldi, B.; et al. Feasibility, Tolerability and Efficacy of Carfilzomib in Combination with Lenalidomide and Dexamethasone in Relapsed Refractory Myeloma Patients: A Retrospective Real-Life Survey of the Sicilian Myeloma Network. J. Clin. Med. 2019, 8, 877. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dimopoulos, M.A.; Kastritis, E.; Terpos, E.; Nahi, H.; Goldschmidt, H.; Hillengass, J.; Leleu, X.; Beksac, M.; Alsina, M.; et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 2017, 31, 2443–2448. [Google Scholar] [CrossRef]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef]
- Fonseca, R.; Bergsagel, P.L.; Drach, J.; Shaughnessy, J.; Gutierrez, N.; Stewart, A.K.; Morgan, G.; van Ness, B.; Chesi, M.; Minvielle, S.; et al. International Myeloma Working Group molecular classification of multiple myeloma: Spotlight review. Leukemia 2009, 23, 2210–2221. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Blade, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet. Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Parisi, M.S.; Calafiore, V.; Martino, E.; Del Fabro, V.; Romano, A.; Sapienza, G.; Conticello, C.; Di Raimondo, F. Relevant reduction of pomalidomide-related neutropenia by intensive use of myeloid growth factor. Available online: https://library.ehaweb.org/eha/2018/stockholm/215134/marina.parisi.relevant.reduction.of.pomalidomide-related.neutropenia.by.html (accessed on 15 October 2019).
- Romano, A.; Conticello, C.; Cavalli, M.; Vetro, C.; di Raimondo, C.; di Martina, V.; Schinocca, E.; la Fauci, A.; Parrinello, N.L.; Chiarenza, A.; et al. Salvage therapy of multiple myeloma: The new generation drugs. BioMed Res. Int. 2014, 2014, 456037. [Google Scholar] [CrossRef]
- Romano, A.; Chiarenza, A.; Consoli, U.; Conticello, C.; Forte, S.; Uccello, G.; Vetro, C.; Cavalli, M.; Coppolino, F.; Palumbo, G.A.; et al. Intravenous injection of bortezomib, melphalan and dexamethasone in refractory and relapsed multiple myeloma. Ann. Oncol. 2013, 24, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Tessoulin, B.; Jullien, M.; Blin, N.; Gastinne, T.; Mahe, B.; Dubruille, V.; Bonnet, A.; Lok, A.; Chevallier, P.; et al. Pomalidomide, cyclophosphamide, and dexamethasone for relapsed/refractory multiple myeloma patients in a real-life setting: A single-center retrospective study. Ann. Hematol. 2019, 98, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Parikh, K.; Abouzaid, S.; Purnomo, L.; McGuiness, C.B.; Hussein, M.; Wade, R.L. Real-World Treatment Patterns, Time to Next Treatment, and Economic Outcomes in Relapsed or Refractory Multiple Myeloma Patients Treated with Pomalidomide or Carfilzomib. J. Manag. Care Spec. Pharm. 2017, 23, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Therneau, T.M.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Rajkumar, S.V.; Fonseca, R.; Witzig, T.E.; Lust, J.A.; Larson, D.R.; et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin. Proc. 2004, 79, 867–874. [Google Scholar] [CrossRef]
- Usami, E.; Kimura, M.; Takenaka, S.; Iwai, M.; Teramachi, H.; Yoshimura, T. Tolerability and safety of real-world use of pomalidomide in patients with relapsed/refractory multiple myeloma. Mol. Clin. Oncol. 2019, 10, 293–298. [Google Scholar] [CrossRef]
| Study Design | Nation | Number of Patients Involved | Percentage of Double Refractory Patients | ORR (%) | Median PFS (Months) | Median OS (Months) | References |
|---|---|---|---|---|---|---|---|
| Multi-centric (5) | UK | 85 (70) | 72.9 | 52.9 | 5.2 (13.2) | 13.7 (13.2) | Macrocia, BJH 2017 [15] |
| Uni-centric | UK | 39 | 33 | 41 | 5.2 | 13.1 | Sriskandarajah, leuk&lymph 2016 [16] |
| Uni-centric | France | 63 | 19 | 51 | 6.4-26.8-nr | Gueneau, Eur J Hematol 2018 [17] | |
| Multi-centric | Poland | 50 | 26 | 31.6–75 (plus Bortezomib) | 9,5 | 14 | Charlinski, Eur J Hematol 2018 [18] |
| Multi-centric | Australia | 151 (87) | 69 | 32 | 3.4 | 7.5 | Scott leuk&lymphoma 2018 [19] |
| Uni-centric | India | 24 | 75 | 50 | 6 | Jandial Leukemia & lymphoma 2018 [20] | |
| Kansai Myeloma Forum (multi-centric) | Japan | 108 | 54 | 31.3 | 4.4 (median Time to Treatment Failure) | Nr | Matsumura-Kimoto Int JH 2018 [21] |
| Multi-centric | Italy | 103 (94) | 33 % refractory | 51% | 30 (mTTNT) | 16 | Mele Leukemia and Lymphoma 2019 [22] |
| Uni-centric | Italy | 76 | 43 | 44 | 9.4 | 19.02 | present study |
| Age | |
| Median (range) | 63 (43-83) |
| <61 years, N (%) | 29 (38.1%) |
| 61–71 years, N (%) | 32 (42.1%) |
| >71 years, N (%) | 15 (19.7%) |
| Gender | |
| Male, N (%) | 43 (56.5%) |
| Female, N (%) | 33 (43.4%) |
| Paraprotein (isotype) | |
| secreting, N (%) | 66 (86.8%) |
| micromolecolar, N (%) | 7 (9.2%) |
| non secreting, N (%) | 3 (3.9%) |
| Kappa–light chain, N (%) | 44 (60.2%) |
| Lambda–light chain, N (%) | 29 (39.7%) |
| ECOG (Performance Status at baseline) | |
| 0–1, N (%) | 37 (48.6%) |
| 2, N (%) | 29 (38.1%) |
| 3 or more, N (%) | 10 (13.1%) |
| Durie and Salmon Stage at Baseline | |
| IA, N (%) | 7 (9.2%) |
| IIA, N (%) | 19 (25%) |
| IIIA, N (%) | 41 (53.9%) |
| IIB, N (%) | 1 (1.3%) |
| IIIB, N (%) | 8 (10.5%) |
| ISS Stage at Baseline | |
| I, N (%) | 18 (23.6%) |
| II, N (%) | 21 (27.6%) |
| III, N (%) | 37 (48.6%) |
| Risk Class at Relapse According to IMWG (26pts) | |
| High, N (%) | 9 (34.6%) |
| Standard, N (%) | 17 (65.4%) |
| Creatinine Clearance | |
| <30 mL/min, N (%) | 4 (5.2%) |
| 30–50 mL/min, N (%) | 13 (17.1%) |
| >50 mL/min, N (%) | 59 (77.6%) |
| Bone Lesions | |
| At least 3, N (%) | 55 (72.3%) |
| Less than 3, N (%) | 21 (27.6%) |
| Extramedullary Lesions | |
| Yes, N (%) | 10 (13.1%) |
| No, N (%) | 66 (86.8%) |
| Exposure/Tolerability | |
|---|---|
| mean duration, months (range) | 7.2 (2–21) |
| dose reduction, N (%) | 11 (14%) |
| dose interruption, N (%) | 18 (23%) |
| deaths (no treatment-related), N (%) | 28 (50%) |
| hematological adverse events (grade 3–4), N (%) | 39 (51%) |
| neutropenia, N (%) | 15 (19%) |
| anemia, N (%) | 17 (22%) |
| thrombocytopenia, N (%) | 7 (8%) |
| non-hematological adverse events (grade 3–4), N (%) | 25 (32%) |
| infection, N (%) | 7 (9%) |
| glucose metabolism alteration, N (%) | 5 (6.5%) |
| sepsis, N (%) | 4 (5%) |
| pneumonia, N (%) | 4 (5%) |
| fatigue, N (%) | 2 (2.6%) |
| diffuse erythema, N (%) | 2 (2.6%) |
| thromboembolism, N (%) | 2 (2.6%) |
| diarrhea, N (%) | 1 (1.3%) |
| hyponatremia, N (%) | 2 (2.6%) |
| neuropathy, N (%) | 2 (2.6%) |
| melena, N (%) | 1 (1.3%) |
| atrial flutter, N (%) | 1 (1.3%) |
| acute renal failure, N (%) | 1 (1.3%) |
| Within First 6 Cycles, N (%) | Best Response, N (%) | ||
|---|---|---|---|
| CR | 3 (4) | 4 (5) | ORR 44% DCR 89% |
| VGPR | 3 (4) | 3 (4) | |
| PR | 19 (25) | 27 (35) | |
| MR | 10(13) | 5 (7) | |
| SD | 33 (43) | 28 (38) | |
| PD | 8 (11) | 8 (11) |
| N | PFS@ 18 Months (% Survival) | p-Value | OS@ 18 Months (% Survival) | p-Value | ||
|---|---|---|---|---|---|---|
| age | ≤65 | 49 | 27.8 | 0.98 | 57.1 | 0.64 |
| >65 | 27 | 36.1 | 56.8 | |||
| sex | Male | 43 | 28.9 | 0.86 | 57.2 | 0.33 |
| Female | 33 | 30.3 | 55.4 | |||
| extramedullary disease | No | 66 | 29.4 | 0.86 | 54.3 | 0.77 |
| Yes | 10 | 30.0 | 70.1 | |||
| baseline LDH | normal | 47 | 18.9 | 0.21 | 70.1 | 0.43 |
| increased | 29 | 32.5 | 58.1 | |||
| G-CSF | No | 36 | 23.1 | 0.21 | 63.2 | 0.95 |
| Yes | 40 | 36.1 | 49.1 | |||
| prior lines | 3 | 22 | 49.6 | 0.0001 | 57.3 | 0.53 |
| more than 3 | 54 | 17.6 | 53.2 | |||
| last therapy before Pd start | lenalidomide | 49 | 37.2 | 0.77 | 60.2 | 0.12 |
| bortezomib | 20 | 25.0 | 65.1 | |||
| double refractory | no | 43 | 43.3 | 0.002 | 67.7 | 0.08 |
| yes | 33 | 10.8 | 44.0 | |||
| disease control | <6 months | 40 | 20.6 | 0.003 | 15.9 | <0.0001 |
| >6 months | 36 | 35.3 | 81.2 | |||
| disease control | <12 months | 47 | 0.0 | 0.0003 | 10.3 | <0.0001 |
| >12 months | 29 | 29.5 | 88.3 | |||
| ClCr | <50 mL/min | 13 | 21.6 | 0.12 | 56.2 | 0.81 |
| >50mL/min | 63 | 28.5 | 55.8 | |||
| Response at 6 cycles | less than PR | 41 | 25.1 | 0.03 | 43.1 | 0.01 |
| At least PR | 35 | 63.9 | 72.3 |
| PFS, HR, (95% CI) | p-Value | OS, HR, (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| double refractory | no | 2.85 | 0.0004 | NA | |
| yes | (1.60–5.11) | ||||
| disease control | <6 months | 0.43 | 0.02 | 0.43 (0.25–0.84) | 0.02 |
| >6 months | (0.25–0.84) | ||||
| disease control | <12 months | 0.54 (0.21–1.45) | 0.22 | NA | |
| >12 months | |||||
| prior lines | 3 | 0.48 | 0.06 | NA | |
| more than 3 | (0.22–1.03) | ||||
| response at 6 cycles | less than PR | 0.53 | 0.22 | NA | |
| At least PR | (0.20–1.44) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, M.S.; Leotta, S.; Romano, A.; Del Fabro, V.; Martino, E.A.; Calafiore, V.; Giubbolini, R.; Markovic, U.; Leotta, V.; Di Giorgio, M.A.; et al. Clinical Benefit of Long-Term Disease Control with Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients. J. Clin. Med. 2019, 8, 1695. https://doi.org/10.3390/jcm8101695
Parisi MS, Leotta S, Romano A, Del Fabro V, Martino EA, Calafiore V, Giubbolini R, Markovic U, Leotta V, Di Giorgio MA, et al. Clinical Benefit of Long-Term Disease Control with Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients. Journal of Clinical Medicine. 2019; 8(10):1695. https://doi.org/10.3390/jcm8101695
Chicago/Turabian StyleParisi, Marina Silvia, Salvatore Leotta, Alessandra Romano, Vittorio Del Fabro, Enrica Antonia Martino, Valeria Calafiore, Rachele Giubbolini, Uros Markovic, Valerio Leotta, Mary Ann Di Giorgio, and et al. 2019. "Clinical Benefit of Long-Term Disease Control with Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients" Journal of Clinical Medicine 8, no. 10: 1695. https://doi.org/10.3390/jcm8101695
APA StyleParisi, M. S., Leotta, S., Romano, A., Del Fabro, V., Martino, E. A., Calafiore, V., Giubbolini, R., Markovic, U., Leotta, V., Di Giorgio, M. A., Tibullo, D., Di Raimondo, F., & Conticello, C. (2019). Clinical Benefit of Long-Term Disease Control with Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients. Journal of Clinical Medicine, 8(10), 1695. https://doi.org/10.3390/jcm8101695







