Prognosis and Interplay of Cognitive Impairment and Sarcopenia in Older Adults Discharged from Acute Care Hospitals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcome
2.3. Exposure Variables
2.4. Covariates
2.5. Sample Selection
2.6. Analytic Approach
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fielding, R.A.; Rejeski, W.J.; Blair, S.; Church, T.; Espeland, M.A.; Gill, T.M.; Guralnik, J.M.; Hsu, F.-C.; Katula, J.; King, A.C.; et al. The Lifestyle Interventions and Independence for Elders Study: Design and methods. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2011, 66, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachex Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A. Sarcopenia, the last organ insufficiency. Eur. Geriatr. Med. 2016, 7, 195–196. [Google Scholar] [CrossRef]
- Bianchi, L.; Ferrucci, L.; Cherubini, A.; Maggio, M.; Bandinelli, S.; Savino, E.; Brombo, G.; Zuliani, G.; Guralnik, J.M.; Landi, F.; et al. The Predictive Value of the EWGSOP Definition of Sarcopenia: Results From the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, D.L.; Landi, F.; Volpato, S.; Corsonello, A.; Meloni, E.; Bernabei, R.; Onder, G. Association of Sarcopenia With Short- and Long-term Mortality in Older Adults Admitted to Acute Care Wards: Results From the CRIME Study. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2014, 69, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Wang, H.; Zhang, L.; Hao, Q.; Dong, B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: A prospective study. J. Cachexia Sarcopenia Muscle 2017, 8, 251–258. [Google Scholar] [CrossRef]
- Gariballa, S.; Alessa, A. Sarcopenia: Prevalence and prognostic significance in hospitalized patients. Clin. Nutr. 2013, 32, 772–776. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Biesemann, N.; Ried, J.S.; Ding-Pfennigdorff, D.; Dietrich, A.; Rudolph, C.; Hahn, S.; Hennerici, W.; Asbrand, C.; Leeuw, T.; Strübing, C. High throughput screening of mitochondrial bioenergetics in human differentiated myotubes identifies novel enhancers of muscle performance in aged mice. Sci. Rep. 2018, 8, 9408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Bossola, M.; Manes-Gravina, E.; Landi, F.; Bernabei, R.; Marzetti, E.; et al. Circulating Mitochondrial DNA at the Crossroads of Mitochondrial Dysfunction and Inflammation During Aging and Muscle Wasting Disorders. Rejuvenation Res. 2018, 21, 350–359. [Google Scholar] [CrossRef]
- Chang, K.-V.; Hsu, T.-H.; Wu, W.-T.; Huang, K.-C.; Han, D.-S. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Yamada, M.; Shirooka, H.; Nozaki, Y.; Fukutani, N.; Tashiro, Y.; Hirata, H.; Yamaguchi, M.; Tasaka, S.; Matsushita, T.; et al. Sarcopenia as a Risk Factor for Cognitive Deterioration in Community-Dwelling Older Adults: A 1-Year Prospective Study. J. Am. Med. Dir. Assoc. 2016, 17, 372.e5–372.e8. [Google Scholar] [CrossRef] [PubMed]
- Van Kan, G.A.; Cesari, M.; Gillette-Guyonnet, S.; Dupuy, C.; Vellas, B.; Rolland, Y. Association of a 7-year percent change in fat mass and muscle mass with subsequent cognitive dysfunction: The EPIDOS-Toulouse cohort. J. Cachex Sarcopenia Muscle 2013, 4, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Williams, L.J.; Jacka, F.N.; Stupka, N.; Brennan-Olsen, S.L.; Holloway, K.L.; Berk, M. Sarcopenia and the Common Mental Disorders: A Potential Regulatory Role of Skeletal Muscle on Brain Function? Curr. Osteoporos Rep. 2015, 13, 351–357. [Google Scholar] [CrossRef]
- Lauretani, F.; Meschi, T.; Ticinesi, A.; Maggio, M. “Brain-muscle loop” in the fragility of older persons: From pathophysiology to new organizing models. Aging Clin. Exp. Res. 2017, 29, 1305–1311. [Google Scholar] [CrossRef]
- Kwon, Y.N.; Yoon, S.S. Sarcopenia: Neurological Point of View. J. Bone Metab. 2017, 24, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- De Felice, F.G.; Lourenco, M.V. Brain metabolic stress and neuroinflammation at the basis of cognitive impairment in Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.; Manolagas, S.C. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fester, L.; Rune, G.M. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Res. 2015, 1621, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.; Cumming, R.G.; Waite, L.M.; Blyth, F.M.; Naganathan, V.; Le Couteur, D.G.; Seibel, M.J.; Handelsman, D.J. Longitudinal Relationships between Reproductive Hormones and Cognitive Decline in Older Men: The Concord Health and Ageing in Men Project. J. Clin. Endocrinol. Metab. 2015, 100, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, C.E.I.; Van Der Schouw, Y.T.; De Jong, F.H.; Pols, H.A.P.; Grobbee, D.E.; Lamberts, S.W.J. Endogenous oestrogens are related to cognition in healthy elderly women. Clin. Endocrinol. 2005, 63, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; De Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, C.; Scarano, E.; Barrea, L.; Zhukouskaya, V.V.; Savastano, S.; Mele, C.; Scacchi, M.; Aimaretti, G.; Colao, A.; Marzullo, P. Vitamin D and Neurological Diseases: An Endocrine View. Int. J. Mol. Sci. 2017, 18, 2482. [Google Scholar] [CrossRef]
- Goodwill, A.M.; Szoeke, C. A Systematic Review and Meta-Analysis of The Effect of Low Vitamin D on Cognition. J. Am. Geriatr. Soc. 2017, 65, 2161–2168. [Google Scholar] [CrossRef]
- Fusco, D.; Lattanzio, F.; Tosato, M.; Corsonello, A.; Cherubini, A.; Volpato, S.; Maraldi, C.; Ruggiero, C.; Onder, G. Development of CRIteria to assess appropriate Medication use among Elderly complex patients (CRIME) project: Rationale and methodology. Drugs Aging 2009, 26 (Suppl. 1), 3–13. [Google Scholar] [CrossRef]
- Hillman, T.; Nunes, Q.; Hornby, S.; Stanga, Z.; Neal, K.; Rowlands, B.; Allison, S.; Lobo, D. A practical posture for hand grip dynamometry in the clinical setting. Clin. Nutr. 2005, 24, 224–228. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Jackson, B.A.; Jaffe, M.W.; Moskowitz, R.W. Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914. [Google Scholar] [CrossRef] [PubMed]
- Lesher, E.L.; Berryhill, J.S. Validation of the geriatric depression scale-short form among inpatients. J. Clin. Psychol. 1994, 50, 256–260. [Google Scholar] [CrossRef]
- Schaeffner, E.S.; Ebert, N.; Delanaye, P.; Frei, U.; Gaedeke, J.; Jakob, O.; Kuhlmann, M.K.; Schuchardt, M.; Tölle, M.; Ziebig, R.; et al. Two Novel Equations to Estimate Kidney Function in Persons Aged 70 Years or Older. Ann. Intern. Med. 2012, 157, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Pourhassan, M.; Norman, K.; Muller, M.J.; Dziewas, R.; Wirth, R. Impact of Sarcopenia on One-Year Mortality among Older Hospitalized Patients with Impaired Mobility. J. Frailty Aging 2018, 7, 40–46. [Google Scholar]
- Landi, F.; Liperoti, R.; Fusco, D.; Mastropaolo, S.; Quattrociocchi, D.; Proia, A.; Tosato, M.; Bernabei, R.; Onder, G. Sarcopenia and Mortality among Older Nursing Home Residents. J. Am. Med. Dir. Assoc. 2012, 13, 121–126. [Google Scholar] [CrossRef]
- Arango-Lopera, V.E.; Arroyo, P.; Gutiérrez-Robledo, L.M.; Perez-Zepeda, M.U.; Cesari, M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging 2013, 17, 259–262. [Google Scholar] [CrossRef]
- Park, M.H.; Kwon, D.Y.; Jung, J.M.; Han, C.; Jo, I.; Jo, S.A. Mini-Mental Status Examination as predictors of mortality in the elderly. Acta Psychiatr. Scand. 2013, 127, 298–304. [Google Scholar] [CrossRef]
- Perna, L.; Wahl, H.W.; Mons, U.; Saum, K.U.; Holleczek, B.; Brenner, H. Cognitive impairment, all-cause and cause-specific mortality among non-demented older adults. Age Ageing 2015, 44, 445–451. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Chon, D.; Lee, K.-E.; Kim, J.-H.; Myeong, S.; Kim, S. The effects of frailty and cognitive impairment on 3-year mortality in older adults. Maturitas 2018, 107, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ayers, E.; Verghese, J. Diagnosing motoric cognitive risk syndrome to predict progression to dementia. Neurodegener. Dis. Manag. 2014, 4, 339–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, T.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Motoric Cognitive Risk Syndrome: Association with Incident Dementia and Disability. J. Alzheimer’s Dis. 2017, 59, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayers, E.; Verghese, J. Motoric cognitive risk syndrome and risk of mortality in older adults. Alzheimer’s Dement. 2016, 12, 556–564. [Google Scholar] [CrossRef]
- Marengoni, A.; Bandinelli, S.; Maietti, E.; Guralnik, J.; Zuliani, G.; Ferrucci, L.; Volpato, S. Combining Gait Speed and Recall Memory to Predict Survival in Late Life: Population-Based Study. J. Am. Geriatr. Soc. 2017, 65, 614–618. [Google Scholar] [CrossRef]
- Lozano-Montoya, I.; Correa-Pérez, A.; Abraha, I.; Soiza, R.L.; Cherubini, A.; O’Mahony, D.; Cruz-Jentoft, A.J. Nonpharmacological interventions to treat physical frailty and sarcopenia in older patients: A systematic overview—The Senator Project ONTOP Series. Clin. Interv. Aging 2017, 12, 721–740. [Google Scholar] [CrossRef]
- Landi, F.; Cherubini, A.; Cesari, M.; Calvani, R.; Tosato, M.; Sisto, A.; Martone, A.; Bernabei, R.; Marzetti, E. Sarcopenia and frailty: From theoretical approach into clinical practice. Eur. Geriatr. Med. 2016, 7, 197–200. [Google Scholar] [CrossRef]
- Park, H.; Park, J.H.; Na, H.R.; Hiroyuki, S.; Kim, G.M.; Jung, M.K.; Kim, W.K.; Park, K.W. Combined Intervention of Physical Activity, Aerobic Exercise, and Cognitive Exercise Intervention to Prevent Cognitive Decline for Patients with Mild Cognitive Impairment: A Randomized Controlled Clinical Study. J. Clin. Med. 2019, 8, 940. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Park, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients With Mild Cognitive Impairment: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2018, 19, 584–591. [Google Scholar] [CrossRef]
| All (N = 624) | No Sarcopenia and No Cognitive Impairment (N = 231) | Cognitive Impairment and No Sarcopenia (N = 234) | Sarcopenia and No Cognitive Impairment (N = 70) | Sarcopenia and Cognitive Impairment (N = 89) | P | |
|---|---|---|---|---|---|---|
| Age | 80.1 ± 7.0 | 77.6 ± 6.6 | 81.2 ± 7.0 | 80.6 ± 7.1 | 83.0 ± 6.2 | 0.001 |
| Gender (female) | 350 (56.1) | 118 (51.1) | 152 (65.0) | 27 (38.6) | 53 (59.6) | 0.001 |
| Dependency in at least 1 BADL | 168 (26.9) | 25 (10.8) | 93 (39.7) | 10 (14.3) | 40 (44.9) | 0.001 |
| Depression | 201 (32.2) | 63 (27.3) | 87 (37.2) | 24 (34.3) | 27 (30.3) | 0.006 |
| History of falls | 169 (27.1) | 46 (19.9) | 74 (31.6) | 14 (20.0) | 35 (39.3) | 0.001 |
| Urinary incontinence | 169 (27.1) | 43 (18.6) | 86 (36.8) | 9 (12.9) | 31 (34.8) | 0.001 |
| Delirium during stay | 19 (3.0) | 5 (2.2) | 12 (5.1) | – | 2 (2.2) | 0.092 |
| BMI < 20 kg/m2 | 28 (4.5) | 1 (0.4) | 7 (3.0) | 7 (10.0) | 13 (14.6) | 0.001 |
| Length of stay | 11.0 ± 6.5 | 10.5 ± 6.7 | 11.2 ± 6.1 | 12.4 ± 7.2 | 10.5 ± 6.0 | 0.162 |
| Number of medications at discharge | 5.3 ± 1.9 | 5.3 ± 1.9 | 5.4 ± 2.0 | 5.1 ± 2.1 | 5.0 ± 1.8 | 0.380 |
| Hypertension | 493 (79.0) | 198 (85.7) | 175 (74.8) | 53 (75.7) | 67 (75.3) | 0.019 |
| Coronary artery disease | 192 (30.8) | 66 (28.6) | 74 (31.6) | 25 (35.7) | 27 (30.3) | 0.702 |
| Atrial fibrillation | 101 (16.2) | 31 (13.4) | 44 (18.8) | 12 (17.1) | 14 (15.7) | 0.467 |
| Peripheral arterial disease | 51 (8.2) | 16 (6.9) | 25 (10.7) | 4 (5.7) | 6 (6.7) | 0.355 |
| Heart failure | 162 (26.0) | 51 (22.1) | 63 (26.9) | 16 (22.9) | 32 (36.0) | 0.075 |
| Cerebrovascular disease | 122 (19.6) | 32 (13.9) | 58 (24.8) | 6 (8.6) | 26 (29.2) | 0.001 |
| Parkinson | 35 (5.6) | 8 (3.5) | 16 (6.9) | 4 (5.7) | 7 (7.9) | 0.316 |
| Dementia | 82 (13.1) | – | 55 (23.5) | – | 27 (30.3) | 0.001 |
| Diabetes | 192 (30.8) | 69 (29.9) | 79 (33.8) | 18 (25.7) | 26 (29.2) | 0.570 |
| Chronic obstructive pulmonary disease | 240 (38.5) | 92 (39.8) | 88 (37.6) | 24 (34.3) | 36 (40.4) | 0.821 |
| Malignancies | 84 (13.5) | 32 (13.9) | 23 (9.8) | 17 (24.3) | 12 (13.5) | 0.021 |
| Chronic kidney disease | 345 (55.3) | 125 (54.1) | 126 (53.8) | 40 (57.1) | 54 (60.7) | 0.690 |
| Arthritis/Ostheoporosis | 286 (45.8) | 112 (48.5) | 108 (46.2) | 31 (44.3) | 35 (39.3) | 0.522 |
| Number of diagnoses | 5.2 ± 2.7 | 5.0 ± 2.8 | 5.3 ± 2.7 | 5.1 ± 2.6 | 5.7 ± 2.7 | 0.220 |
| Crude | Age- and Gender-Adjusted | Fully-Adjusted * | ||
|---|---|---|---|---|
| Mortality, n (%) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| All patients (N = 624) | ||||
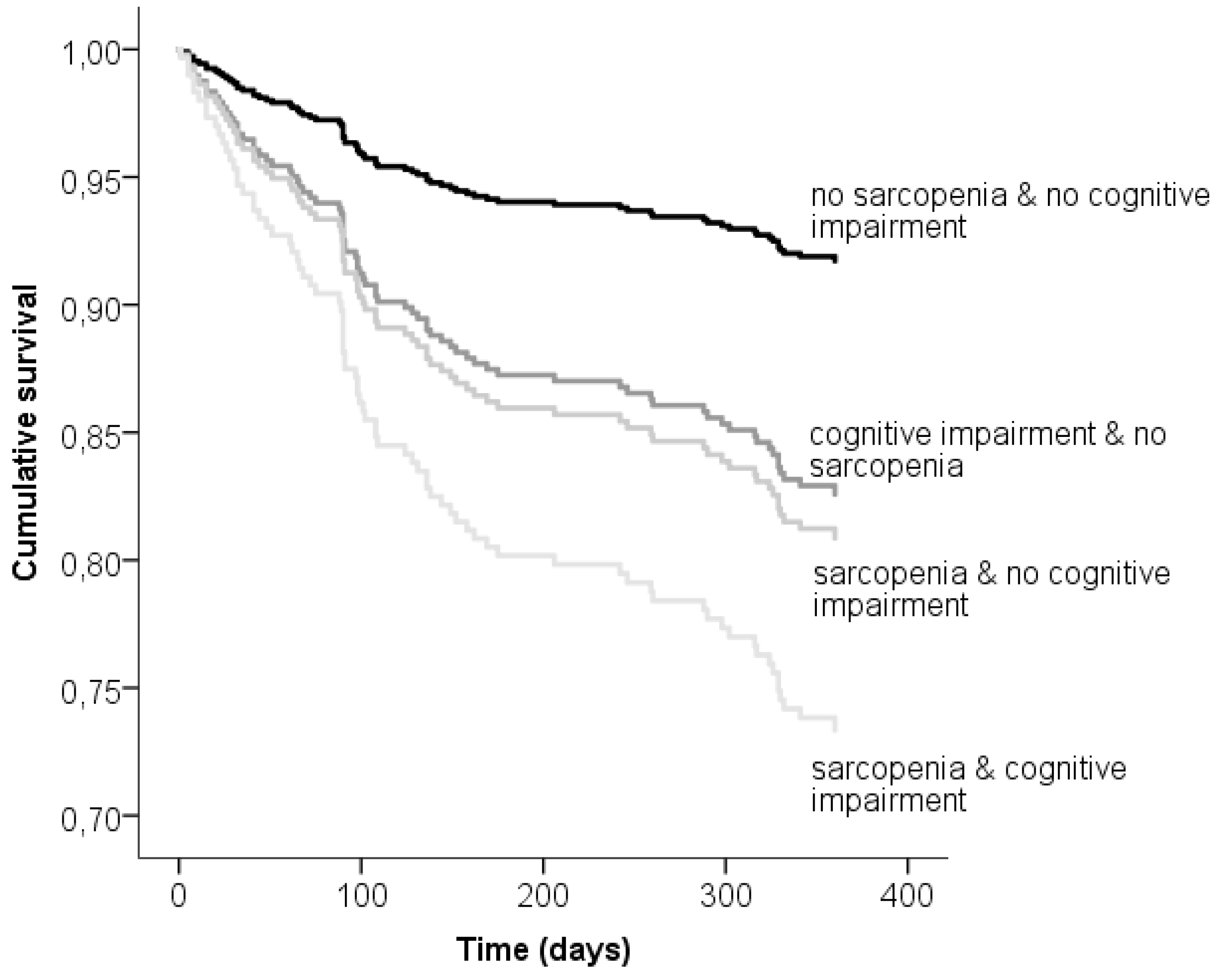
| No sarcopenia and no cognitive impairment | 17 (7.4) | 1.0 | 1.0 | 1.0 |
| Cognitive impairment and no sarcopenia | 31 (13.2) | 2.21 (1.22–4.01) | 2.15 (1.18–3.91) | 1.48 (0.77–2.77) |
| Sarcopenia and no cognitive impairment | 11 (15.7) | 2.46 (1.20–5.25) | 2.02 (0.98–4.14) | 1.79 (0.87–3.51) |
| Sarcopenia and cognitive impairment | 20 (22.5) | 3.60 (1.88–6.85) | 3.09 (1.60–5.98) | 2.12 (1.05–4.13) |
| Excluded patients with recorded diagnosis of dementia (N = 542) | ||||
| No sarcopenia and no cognitive impairment | 17 (7.6) | 1.0 | 1.0 | 1.0 |
| Cognitive impairment and no sarcopenia | 23 (12.8) | 2.10 (1.12–3.92) | 2.01 (1.06–3.80) | 1.50 (0.81–2.86) |
| Sarcopenia and no cognitive impairment | 11 (15.9) | 2.42 (1.14–5.18) | 1.95 (0.96–4.21) | 1.77 (0.80–3.70) |
| Sarcopenia and cognitive impairment | 14 (22.6) | 3.30 (1.62–6.69) | 2.80 (1.35–5.74) | 2.13 (1.06–4.17) |
| Excluded patients with BMI <20 kg/m2 (N = 608) | ||||
| No sarcopenia and no cognitive impairment | 17 (7.4) | 1.0 | 1.0 | 1.0 |
| Cognitive impairment and no sarcopenia | 28 (12.3) | 2.05 (1.12–3.75) | 1.88 (0.97–3.64) | 1.44 (0.76–2.69) |
| Sarcopenia and no cognitive impairment | 9 (14.3) | 2.10 (1.03–4.70) | 1.71 (0.71–3.65) | 1.69 (0.72–3.44) |
| Sarcopenia and cognitive impairment | 16 (21.1) | 3.27 (1.65–6.47) | 2.83 (1.23–5.17) | 2.18 (1.03–3.91) |
| Excluded patients with number of diagnoses >5 (N = 373) | ||||
| No sarcopenia and no cognitive impairment | 10 (6.5) | 1.0 | 1.0 | 1.0 |
| Cognitive impairment and no sarcopenia | 10 (7.3) | 1.34 (0.56–3.22) | 1.30 (0.53–3.18) | 1.03 (0.47–2.13) |
| Sarcopenia and no cognitive impairment | 6 (14.6) | 2.34 (0.95–6.43) | 1.99 (0.67–5.31) | 1.99 (0.84–5.66) |
| Sarcopenia and cognitive impairment | 10 (23.8) | 3.82 (1.59–9.19) | 3.47 (1.38–8.69) | 2.63 (1.09–6.32) |
| Excluded patients with dependency in more than 4 BADL (N = 572) | ||||
| No sarcopenia and no cognitive impairment | 16 (7.0) | 1.0 | 1.0 | 1.0 |
| Cognitive impairment and no sarcopenia | 24 (11.9) | 2.09 (1.11–3.93) | 2.08 (1.05–3.94) | 1.99 (0.99–3.34) |
| Sarcopenia and no cognitive impairment | 11 (16.2) | 2.96 (1.24–5.74) | 3.01 (1.28–5.56) | 2.14 (1.01–4.65) |
| Sarcopenia and cognitive impairment | 14 (19.2) | 3.08 (1.50–6.31) | 2.71 (1.14–4.51) | 2.88 (1.10–5.73) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengarini, E.; Giacconi, R.; Mancinelli, L.; Riccardi, G.R.; Castellani, D.; Vetrano, D.L.; Onder, G.; Volpato, S.; Ruggiero, C.; Fabbietti, P.; et al. Prognosis and Interplay of Cognitive Impairment and Sarcopenia in Older Adults Discharged from Acute Care Hospitals. J. Clin. Med. 2019, 8, 1693. https://doi.org/10.3390/jcm8101693
Zengarini E, Giacconi R, Mancinelli L, Riccardi GR, Castellani D, Vetrano DL, Onder G, Volpato S, Ruggiero C, Fabbietti P, et al. Prognosis and Interplay of Cognitive Impairment and Sarcopenia in Older Adults Discharged from Acute Care Hospitals. Journal of Clinical Medicine. 2019; 8(10):1693. https://doi.org/10.3390/jcm8101693
Chicago/Turabian StyleZengarini, Elisa, Robertina Giacconi, Lucia Mancinelli, Giovanni Renato Riccardi, Daniele Castellani, Davide Liborio Vetrano, Graziano Onder, Stefano Volpato, Carmelinda Ruggiero, Paolo Fabbietti, and et al. 2019. "Prognosis and Interplay of Cognitive Impairment and Sarcopenia in Older Adults Discharged from Acute Care Hospitals" Journal of Clinical Medicine 8, no. 10: 1693. https://doi.org/10.3390/jcm8101693
APA StyleZengarini, E., Giacconi, R., Mancinelli, L., Riccardi, G. R., Castellani, D., Vetrano, D. L., Onder, G., Volpato, S., Ruggiero, C., Fabbietti, P., Cherubini, A., Guarasci, F., Corsonello, A., & Lattanzio, F. (2019). Prognosis and Interplay of Cognitive Impairment and Sarcopenia in Older Adults Discharged from Acute Care Hospitals. Journal of Clinical Medicine, 8(10), 1693. https://doi.org/10.3390/jcm8101693






