The Relationship between Sleep Bruxism and Obstructive Sleep Apnea Based on Polysomnographic Findings
Abstract
:1. Introduction
2. Material and Methods
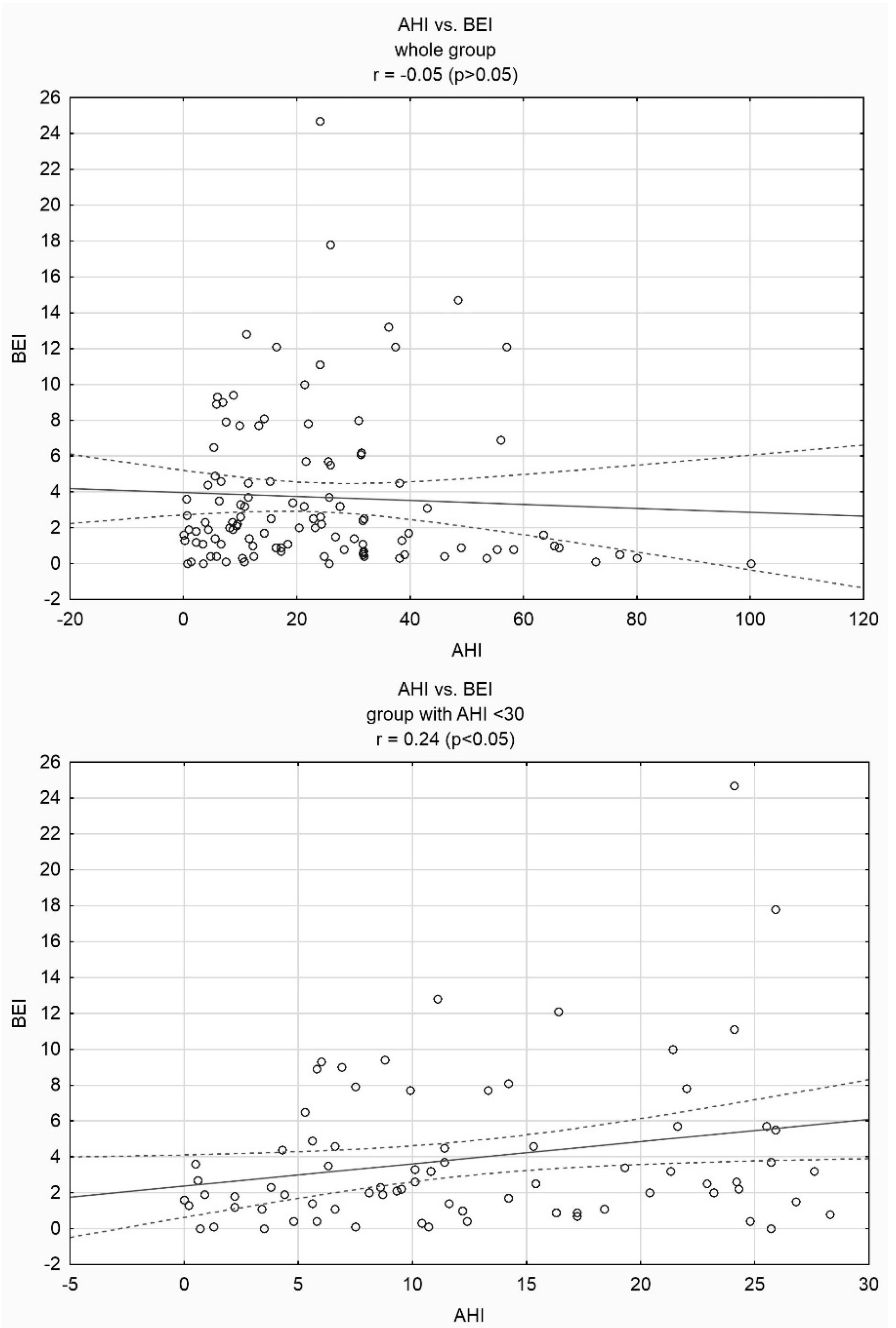
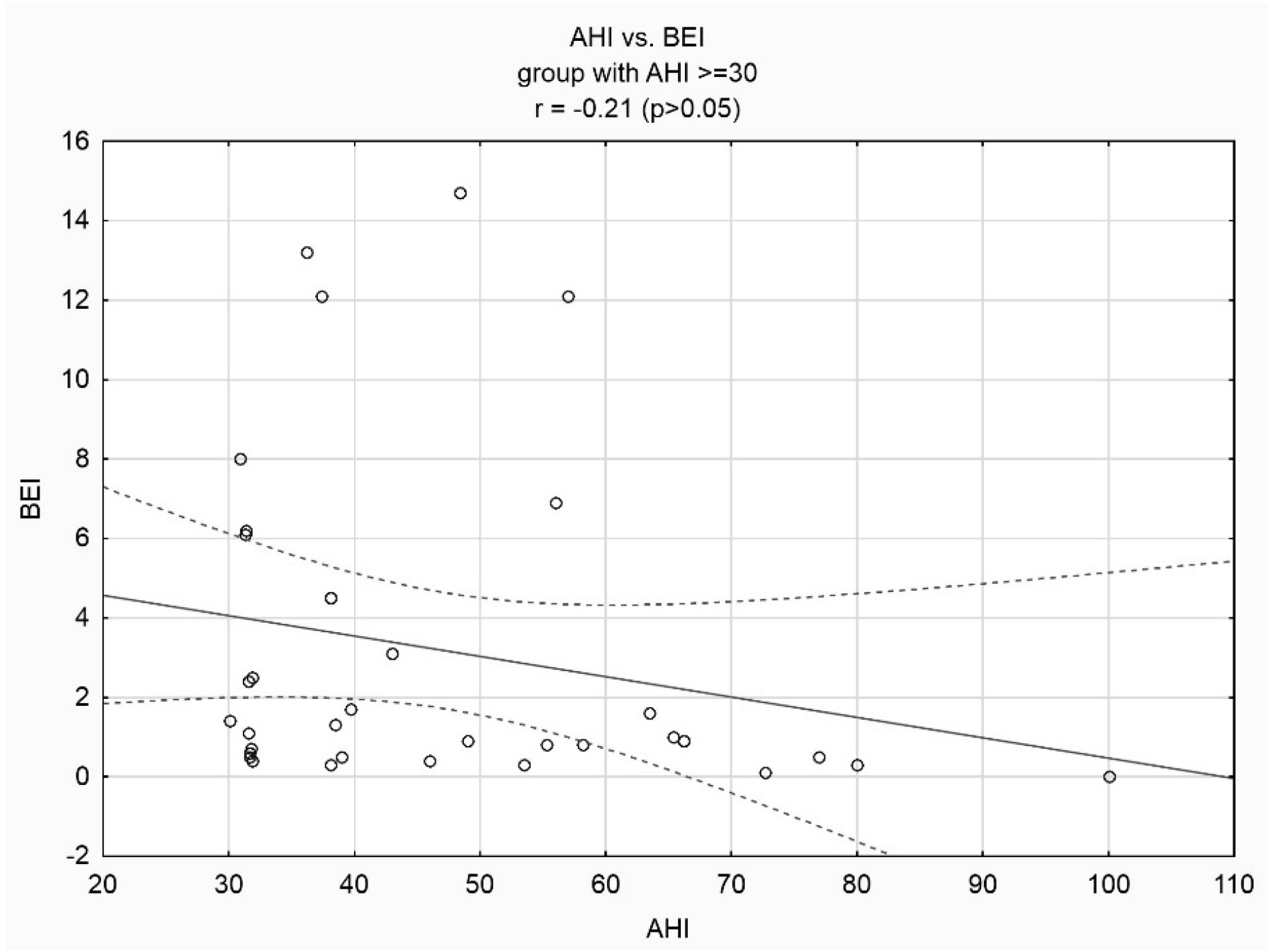
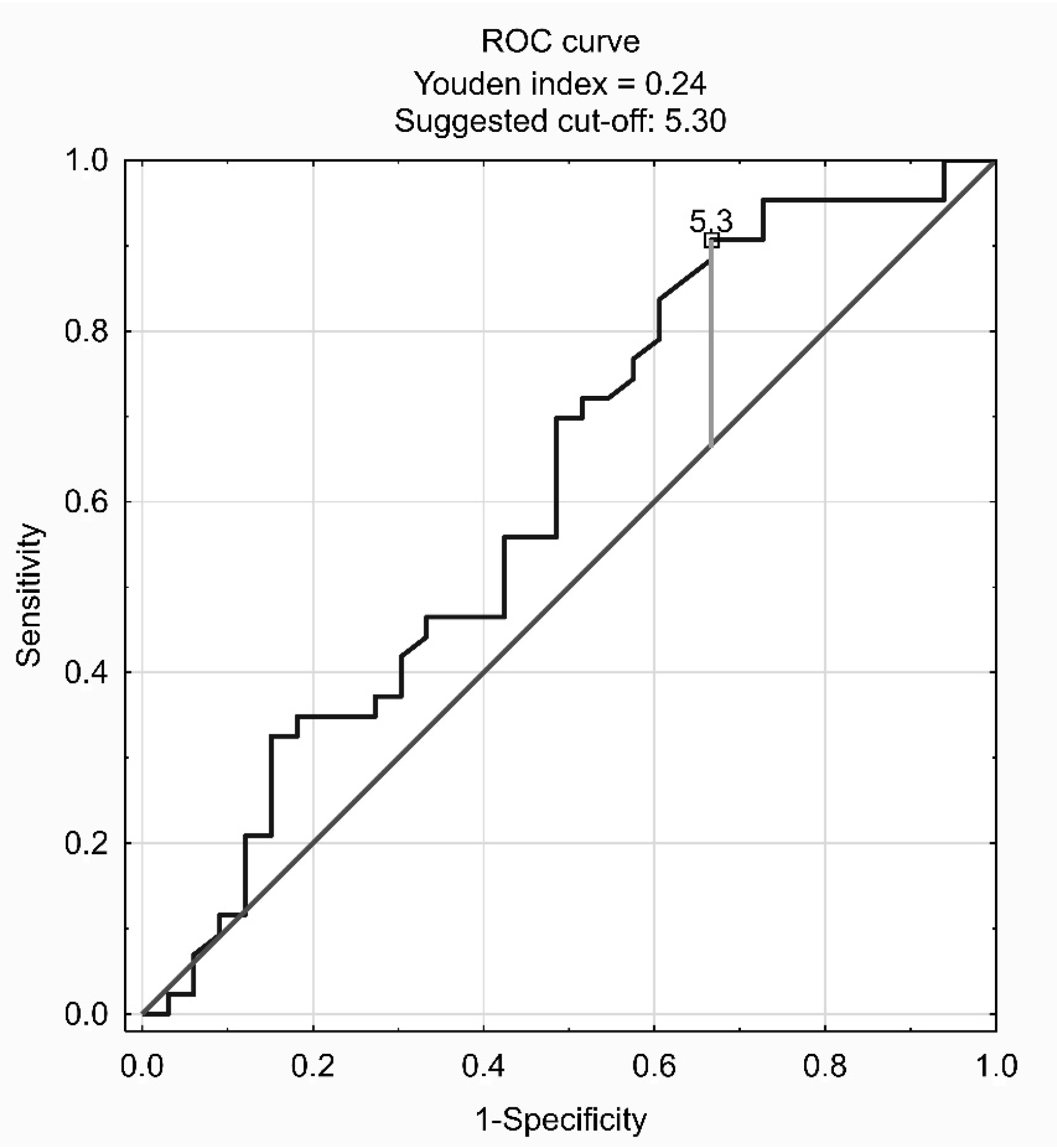
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hou, H.; Zhao, Y.; Yu, W.; Dong, H.; Xue, X.; Ding, J.; Xing, W.; Wang, W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010405. [Google Scholar] [CrossRef] [PubMed]
- Munoz, R.; Duran-Cantolla, J.; Martinez-Vila, E.; Gallego, J.; Rubio, R.; Aizpuru, F.; De La Torre, G. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke 2006, 37, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Vasheghani-Farahani, A.; Kazemnejad, F.; Sadeghniiat-Haghighi, K.; Saadat, S.; Tavakolipoor, P.; Yazdani, T.; Alidoosti, M.; Ghasem-Amooeian, V.; Ashaf, H. Obstructive sleep apnea and severity of coronary artery disease. Caspian J. Intern. Med. 2018, 9, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.l.; Sahadevan, J.; Redline, S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care. Med. 2006, 173, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Finn, L.; Peppard, P.E. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008, 31, 1071–1078. [Google Scholar]
- Hollowell, D.E.; Bhandary, P.R.; Funsten, A.W.; Suratt, P.M. Respiratory related recruitment of the masseter: Response to hypercapnia and loading. J. Appl. Physiol. 1991, 70, 2508–2513. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Klasser, G.D.; Rei, N.; Lavigne, G.J. Sleep bruxism etiology: The evolution of a changing paradigm. J. Can. Dent. Assoc. 2015, 81, f2. [Google Scholar]
- Lavigne, G.; Manzini, C.; Huynh, N.T. Sleep bruxism. In Principles and Practice of Sleep Medicine, 5th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 1128–1139. [Google Scholar]
- Kuhn, M.; Türp, J.C. Risk factors for bruxism. Swiss. Dent. J. 2018, 128, 118–124. [Google Scholar]
- Hosoya, H.; Kitaura, H.; Hashimoto, T.; Ito, M.; Kinbara, M.; Deguchi, T.; Irokawa, T.; Ohisa, N.; Ogawa, H.; Takano-Yamamoto, T. Relationship between sleep bruxism and sleep respiratory events in patients with obstructive sleep apnea syndrome. Sleep Breath. 2014, 18, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Jokubauskas, L.; Baltrušaitytė, A. Relationship between obstructive sleep apnoea syndrome and sleep bruxism: A systematic review. J. Oral Rehabil. 2017, 44, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yamaguchi, T.; Mikami, S.; Watanabe, K.; Gotouda, A.; Okada, K.; Hishikawa, R.; Shibuya, E.; Shibuya, Y.; Lavigne, G. Weak association between sleep bruxism and obstructive sleep apnea. A sleep laboratory study. Sleep Breath. 2016, 20, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, T.T.; Lowe, A.A.; Miyamoto, K.; Fleetham, J.A.; Ryan, C.F. Sleep bruxism in patients with sleep-disordered breathing. Arch. Oral Biol. 2000, 45, 889–896. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [PubMed]
- Lavigne, G.J.; Rompre, P.H.; Montplaisir, J.Y. Sleep bruxism: Validity of clinical research diagnostic criteria in a controlled polysomnographic study. J. Dent. Res. 1996, 75, 546–552. [Google Scholar] [CrossRef]
- Sousa, H.C.S.; Lima, M.D.M.; Dantas, N.N.B.; Tobias, R.Q.; Moura, M.S.; Moura, L.F.A.D. Prevalence and associated factors to sleep bruxism in adolescents from Teresina, Piauí. Rev. Bras. Epidemiol. 2018, 21, e180002. [Google Scholar] [CrossRef]
- Manfredini, D.; Guarda-Nardini, L.; Marchese-Ragona, R.; Lobbezoo, F. Theories on possible temporal relationships between sleep bruxism and obstructive sleep apnea events. An expert opinion. Sleep Breath. 2015, 19, 1459–1465. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Li, K.K.; Guilleminault, C. Risk factors for sleep bruxism in the general population. Chest 2001, 119, 53–61. [Google Scholar] [CrossRef]
- Kato, T.; Velly, A.M.; Nakane, T.; Masuda, Y.; Maki, S. Age is associated with self-reported sleep bruxism, independently of tooth loss. Sleep Breath. 2012, 16, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.R.; Dipalo, F.; Gold, M.S.; O’Hearn, D. The symptoms and signs of upper airway resistance syndrome: A link to the functional somatic syndromes. Chest 2003, 123, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.; Lavigne, G. Sleep bruxism; an overview of an oromandibular sleep movement disorder. Sleep Med. Rev. 2000, 4, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.W.Y.; Yap, A.U.; Chua, A.P.; Wong, J.C.M.; Parot, M.V.J.; Tan, K.B.C. Prevalence of Sleep Bruxism and Its Association with Obstructive Sleep Apnea in Adult Patients: A Retrospective Polysomnographic Investigation. J. Oral Facial Pain Headache 2018, 33, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Mengatto, C.M.; Dalberto Cda, S.; Scheeren, B.; Barros, S.G. Association between sleep bruxism and gastroesophageal reflux disease. J. Prosthet. Dent. 2013, 110, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Nakata, A.; Takahashi, M.; Ikeda, T.; Hojou, M.; Araki, S. Perceived psychosocial job stress and sleep bruxism among male and female workers. Community Dent. Oral Epidemiol. 2008, 36, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Bisulli, F.; Vignatelli, L.; Naldi, I.; Licchetta, L.; Provini, F.; Plazzi, G.; Di Vito, L.; Ferioli, S.; Montagna, P.; Tinuper, P. Increased frequency of arousal parasomnias in families with nocturnal frontal lobe epilepsy: A common mechanism? Epilepsia 2010, 51, 1852–1860. [Google Scholar] [CrossRef]
- Winocur, E.; Uziel, N.; Lisha, T.; Goldsmith, C.; Eli, I. Self-reported bruxism—Associations with perceived stress, motivation for control, dental anxiety and gagging. J. Oral Rehabil. 2011, 38, 3–11. [Google Scholar] [CrossRef]
- Agashe, S.; Petak, S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc. J. 2018, 14, 251–256. [Google Scholar]
- López-Pintor, R.M.; Casañas, E.; González-Serrano, J.; Serrano, J.; Ramírez, L.; de Arriba, L.; Hernández, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef]
- Dumais, I.E.; Lavigne, G.J.; Carram, M.C.; Rompré, P.H.; Huynh, N.T. Could transient hypoxia be associated with rhythmic masticatory muscle activity in sleep bruxism in the absence of sleep-disordered breathing? A preliminary report. J. Oral Rehabil. 2015, 42, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Serra-Negra, J.; Carboncini, F.; Lobbezoo, F. Current concepts of bruxism. Int. J. Prosthodont. 2017, 30, 437–438. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| SE (%) | 80.48 ± 10.37 | 52.40 | 96.80 |
| SL (min) | 21.92 ± 20.70 | 0.00 | 112.60 |
| WASO (min) | 55.88 ± 38.96 | 3.00 | 172.50 |
| N1 (% of TST) | 5.93 ± 4.99 | 0.20 | 21.20 |
| N2 (% of TST) | 47.72 ± 9.79 | 26.20 | 72.90 |
| N3 (% of TST) | 24.61 ± 9.65 | 2.60 | 52.70 |
| REM (% of TST) | 21.75 ± 7.72 | 4.10 | 48.90 |
| BEI (n/hour) | 3.70 ± 4.27 | 0.0 | 24.70 |
| Phasic BEI (n/hour) | 1.93 ± 3.13 | 0.0 | 19.30 |
| Tonic BEI (n/hour) | 1.13 ± 1.30 | 0.0 | 6.90 |
| Mixed BEI (n/hour) | 0.66 ± 0.79 | 0.0 | 4.00 |
| AHI (n/hour) | 23.28 ± 19.98 | 0.0 | 100.10 |
| ODI (n/hour) | 22.92 ± 19.60 | 0.0 | 83.40 |
| Mean SatO2 (%) | 92.68 ± 2.19 | 83.30 | 96.80 |
| Minimal SatO2(%) | 81.76± 7.39 | 54.00 | 93.00 |
| Cheyne-Stokes (% of TST) | 0.69 ± 2.41 | 0.0 | 18.20 |
| Mean desaturation (%) | 4.78± 2.28 | 3.0 | 19.80 |
| Parameter | % | n | |
|---|---|---|---|
| AHI (n/hour) | <5 | 13.63 | 15 |
| ≥5<15 | 30.0 | 33 | |
| ≥15<30 | 25.44 | 28 | |
| ≥30 | 30.90 | 34 | |
| BEI (n/hour) | <2 | 50 | 55 |
| ≥2<4 | 20 | 22 | |
| ≥4 | 30 | 33 | |
| Parameter | BEI (n/hour) | Phasic BEI (n/hour) | Tonic BEI (n/hour) | Mixed BEI (n/hour) |
|---|---|---|---|---|
| AHI (n/hour) | 0.24 | 0.27 | 0.03 | 0.15 |
| SL (min) | −0.11 | −0.08 | −0.07 | −0.16 |
| WASO (min) | −0.06 | −0.01 | −0.15 | −0.08 |
| SE (%) | 0.17 | 0.16 | 0.14 | 0.07 |
| N1 (% of TST) | 0.10 | 0.10 | −0.07 | 0.19 |
| N2 (% of TST) | −0.01 | −0.01 | −0.00 | −0.01 |
| N3 (% of TST) | 0.08 | 0.06 | 0.10 | 0.05 |
| REM (% of TST) | −0.15 | −0.12 | −0.09 | −0.17 |
| Arousal index (n/hour) | 0.45 | 0.52 | −0.08 | 0.34 |
| Cheyne-Stokes (% of TST) | 0.09 | 0.07 | −0.13 | −0.05 |
| ODI (n/hour) | 0.20 | 0.23 | 0.02 | 0.14 |
| SatO2 (%) | −0.02 | −0.06 | 0.09 | 0.02 |
| Min SatO2 (%) | −0.20 | −0.26 | 0.08 | −0.11 |
| Mean desaturation (%) | −0.04 | 0.03 | −0.07 | 0.0 |
| Parameter | Models for BEI | |||
|---|---|---|---|---|
| Model 1 | ||||
| Rc | SEM of RC | p | R2 | |
| intercept | 0.92 | 0.69 | 0.024 | 0.488 |
| AHI | 0.12 | 0.03 | 0.029 | |
| Arousal index | 0.23 | 0.17 | 0.076 | |
| Male gender | 1.73 | 0.72 | 0.034 | |
| Age | 0.03 | 0.03 | 0.115 | |
| Diabetes | 1.59 | 1.21 | 0.039 | |
| Coronary artery disease | 1.25 | 1.66 | 0.201 | |
| Model 2 | ||||
| Rc | SEM of RC | p | R2 | |
| intercept | 1.28 | 0.68 | 0.013 | 0.471 |
| AHI | 0.09 | 0.03 | 0.030 | |
| Arousal index | 0.24 | 0.15 | 0.071 | |
| Male gender | 1.80 | 0.82 | 0.045 | |
| Age | 0.04 | 0.03 | 0.285 | |
| Diabetes | 1.43 | 1.07 | 0.036 | |
| Model 3 | ||||
| Rc | SEM of RC | p | R2 | |
| intercept | 1.65 | 1.03 | 0.007 | 0.458 |
| AHI | 0.08 | 0.03 | 0.034 | |
| Arousal index | 0.25 | 0.17 | 0.070 | |
| Male gender | 1.97 | 0.91 | 0.035 | |
| Diabetes | 1.47 | 0.94 | 0.040 | |
| Model 4 | ||||
| Rc | SEM of RC | p | R2 | |
| intercept | 1.83 | 1.06 | 0.004 | 0.423 |
| AHI | 0.10 | 0.04 | 0.044 | |
| Male gender | 2.22 | 0.96 | 0.024 | |
| Diabetes | 1.47 | 1.06 | 0.047 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martynowicz, H.; Gac, P.; Brzecka, A.; Poreba, R.; Wojakowska, A.; Mazur, G.; Smardz, J.; Wieckiewicz, M. The Relationship between Sleep Bruxism and Obstructive Sleep Apnea Based on Polysomnographic Findings. J. Clin. Med. 2019, 8, 1653. https://doi.org/10.3390/jcm8101653
Martynowicz H, Gac P, Brzecka A, Poreba R, Wojakowska A, Mazur G, Smardz J, Wieckiewicz M. The Relationship between Sleep Bruxism and Obstructive Sleep Apnea Based on Polysomnographic Findings. Journal of Clinical Medicine. 2019; 8(10):1653. https://doi.org/10.3390/jcm8101653
Chicago/Turabian StyleMartynowicz, Helena, Pawel Gac, Anna Brzecka, Rafal Poreba, Anna Wojakowska, Grzegorz Mazur, Joanna Smardz, and Mieszko Wieckiewicz. 2019. "The Relationship between Sleep Bruxism and Obstructive Sleep Apnea Based on Polysomnographic Findings" Journal of Clinical Medicine 8, no. 10: 1653. https://doi.org/10.3390/jcm8101653
APA StyleMartynowicz, H., Gac, P., Brzecka, A., Poreba, R., Wojakowska, A., Mazur, G., Smardz, J., & Wieckiewicz, M. (2019). The Relationship between Sleep Bruxism and Obstructive Sleep Apnea Based on Polysomnographic Findings. Journal of Clinical Medicine, 8(10), 1653. https://doi.org/10.3390/jcm8101653








