Long-Term Outcome and Rejection After Allogeneic Uterus Transplantation in Cynomolgus Macaques
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. MHC Typing
2.3. Uterus Transplantation
2.4. Intraoperative Indocyanine Green Fluorescent Angiography
2.5. Immunosuppressive Management
2.6. Flow Cytometry
2.7. Mixed Lymphocyte Reaction (MLR)
2.8. Donor-Specific Antibodies in Plasma of Macaques after Transplantation
2.9. Postoperative Assessment
2.10. Histological Evaluation, Immunohistochemistry and in Situ Hybridization
3. Results
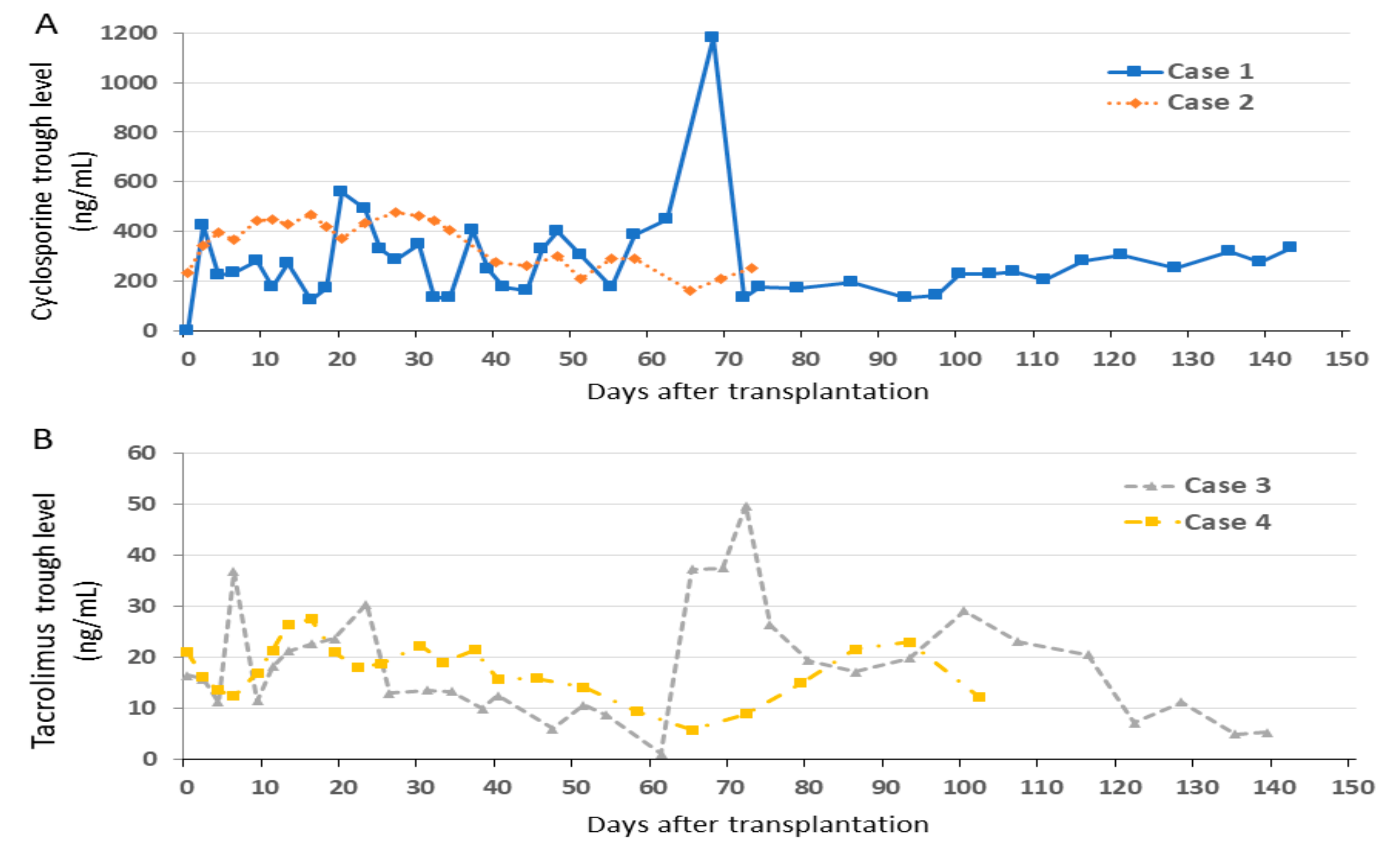
3.1. Changes of Trough Levels of Immunosuppressants
3.2. Overall Outcome
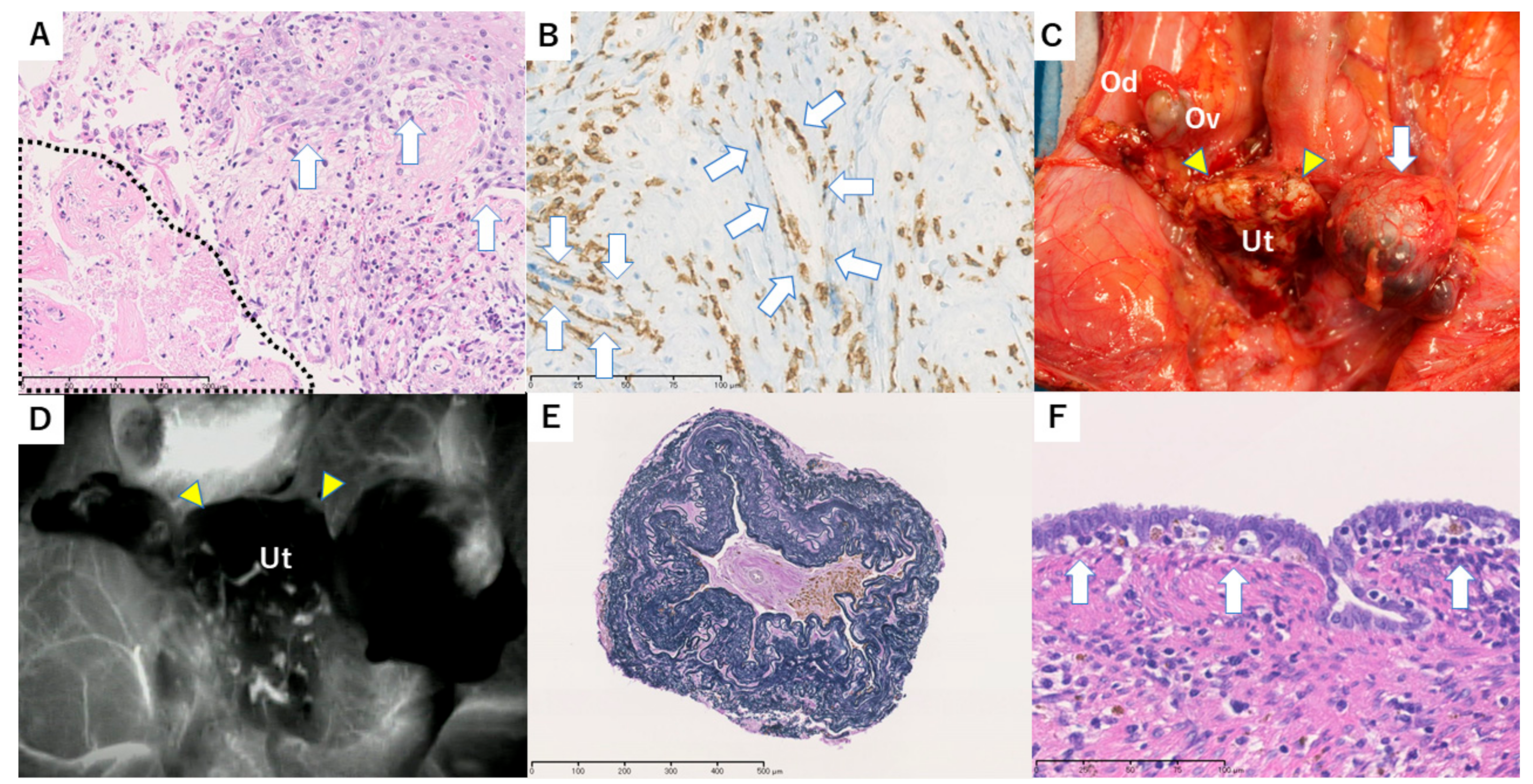
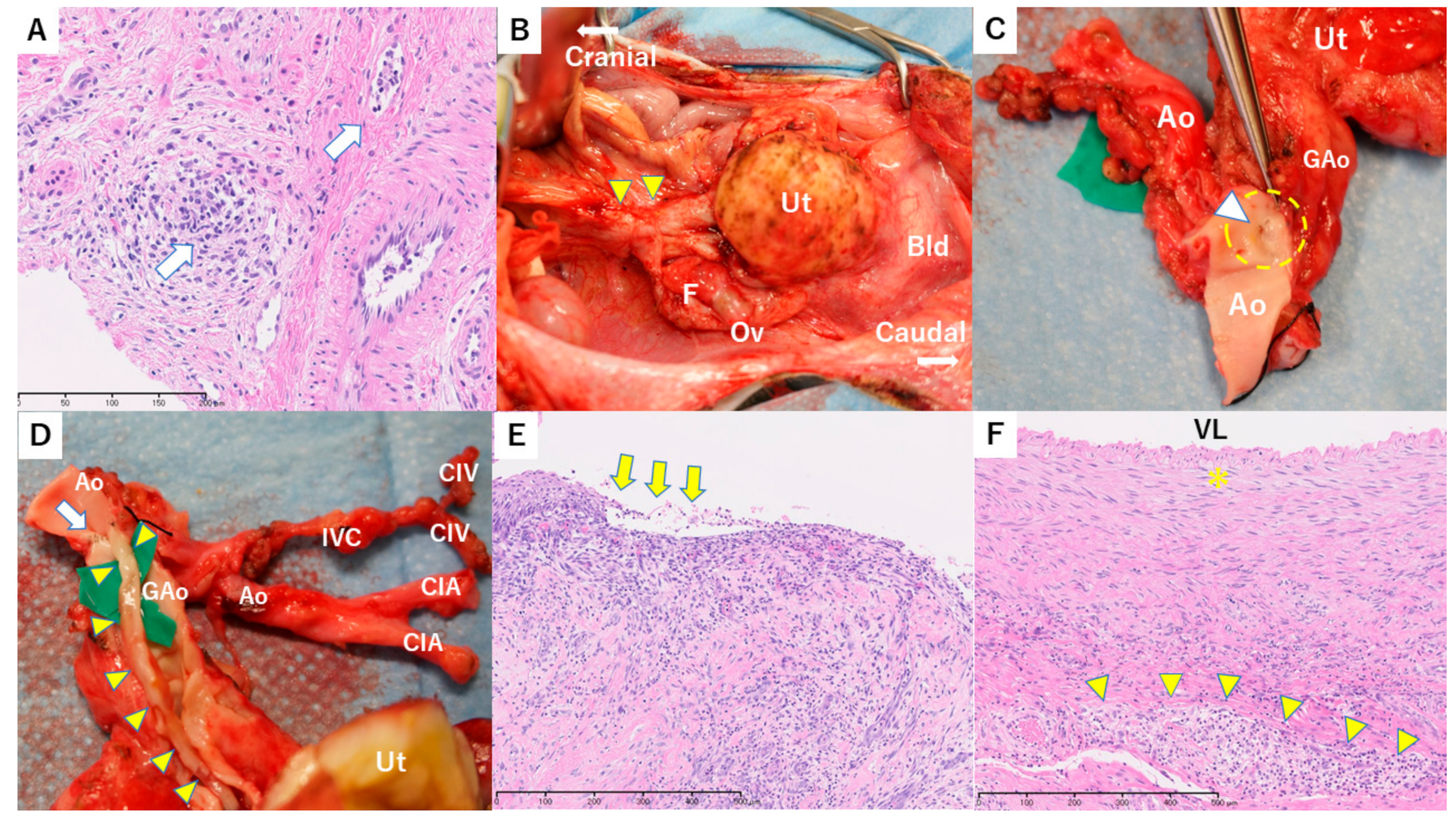
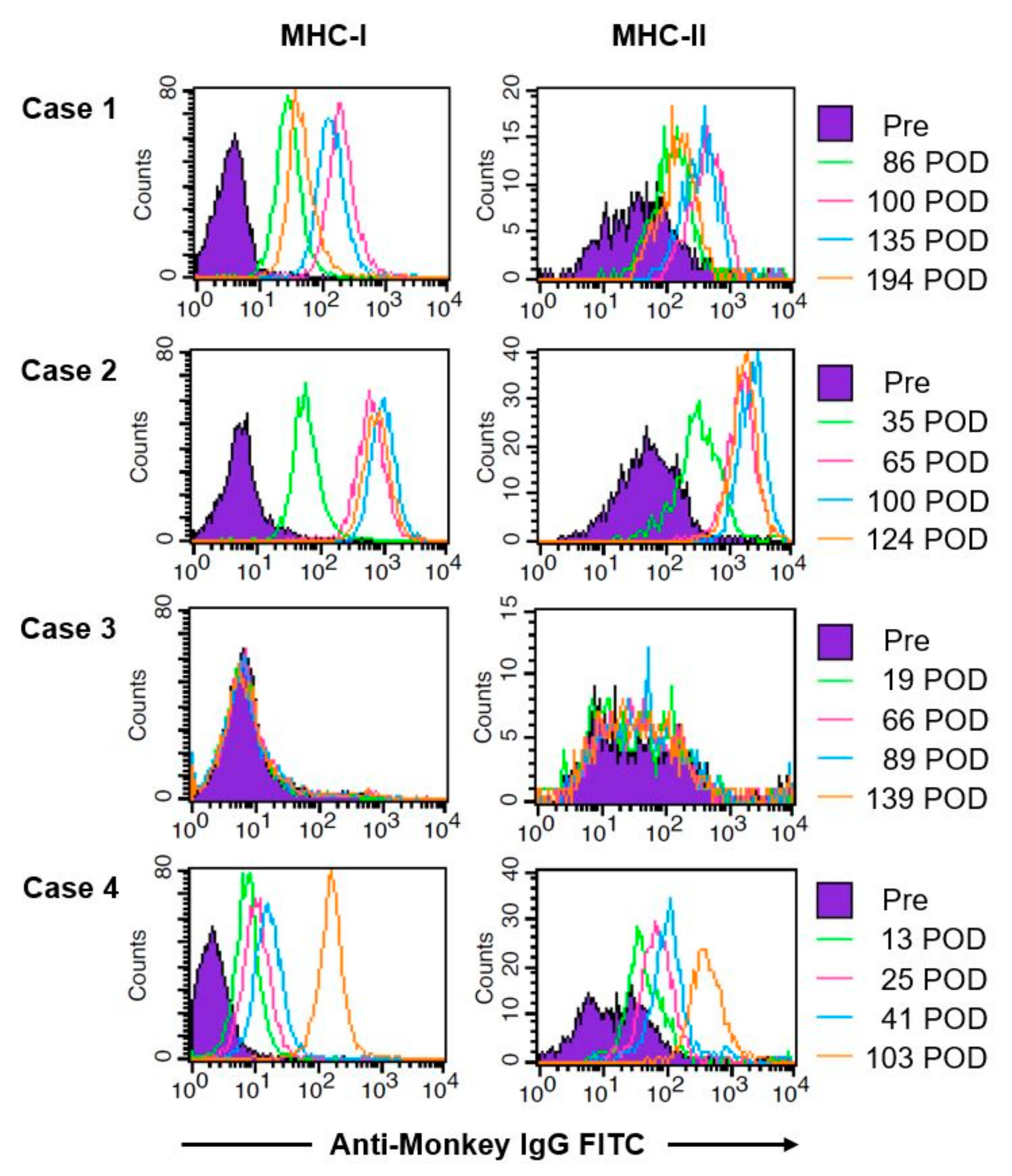
3.3. Case 1
3.4. Case 2
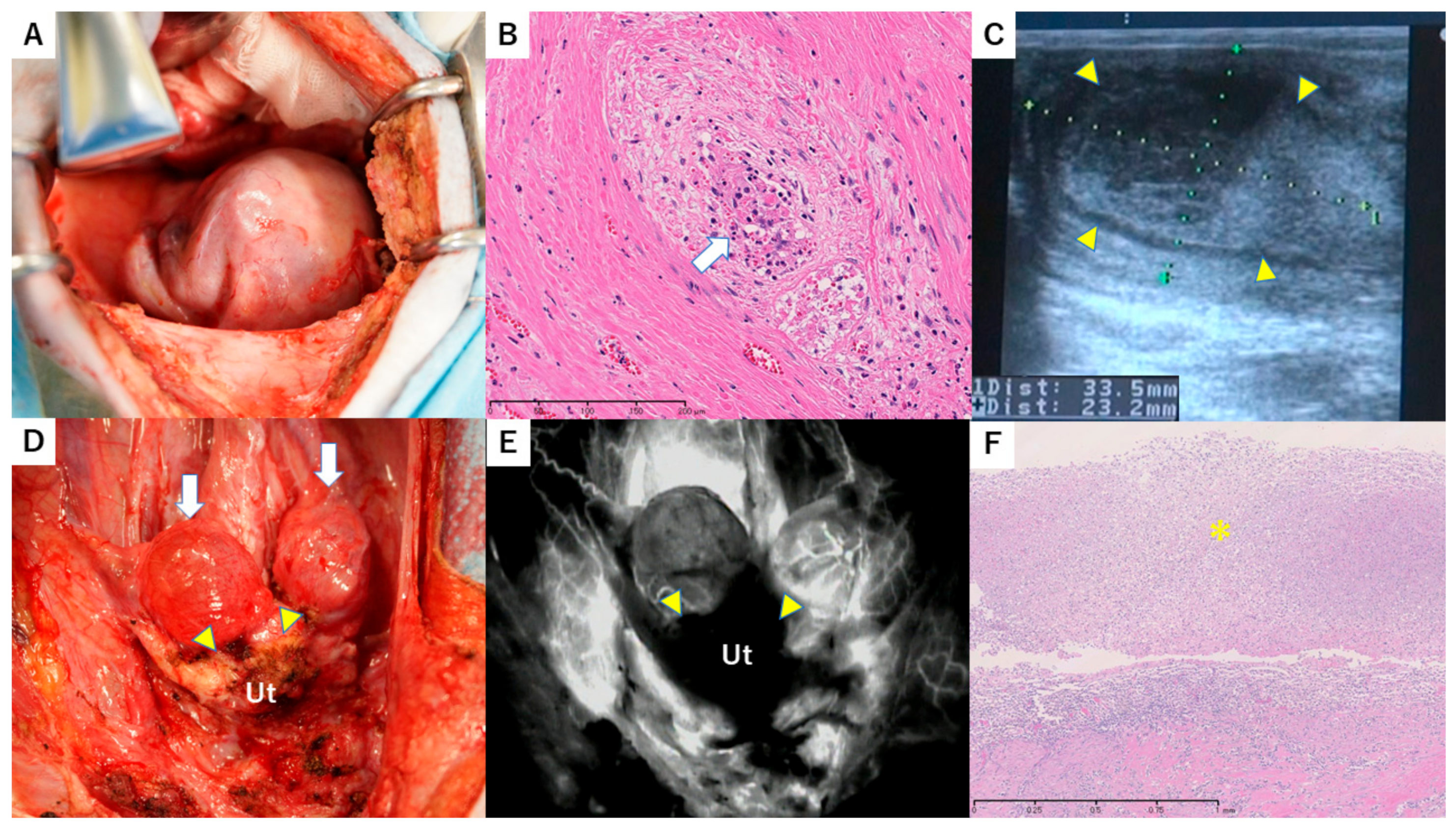
3.5. Case 3
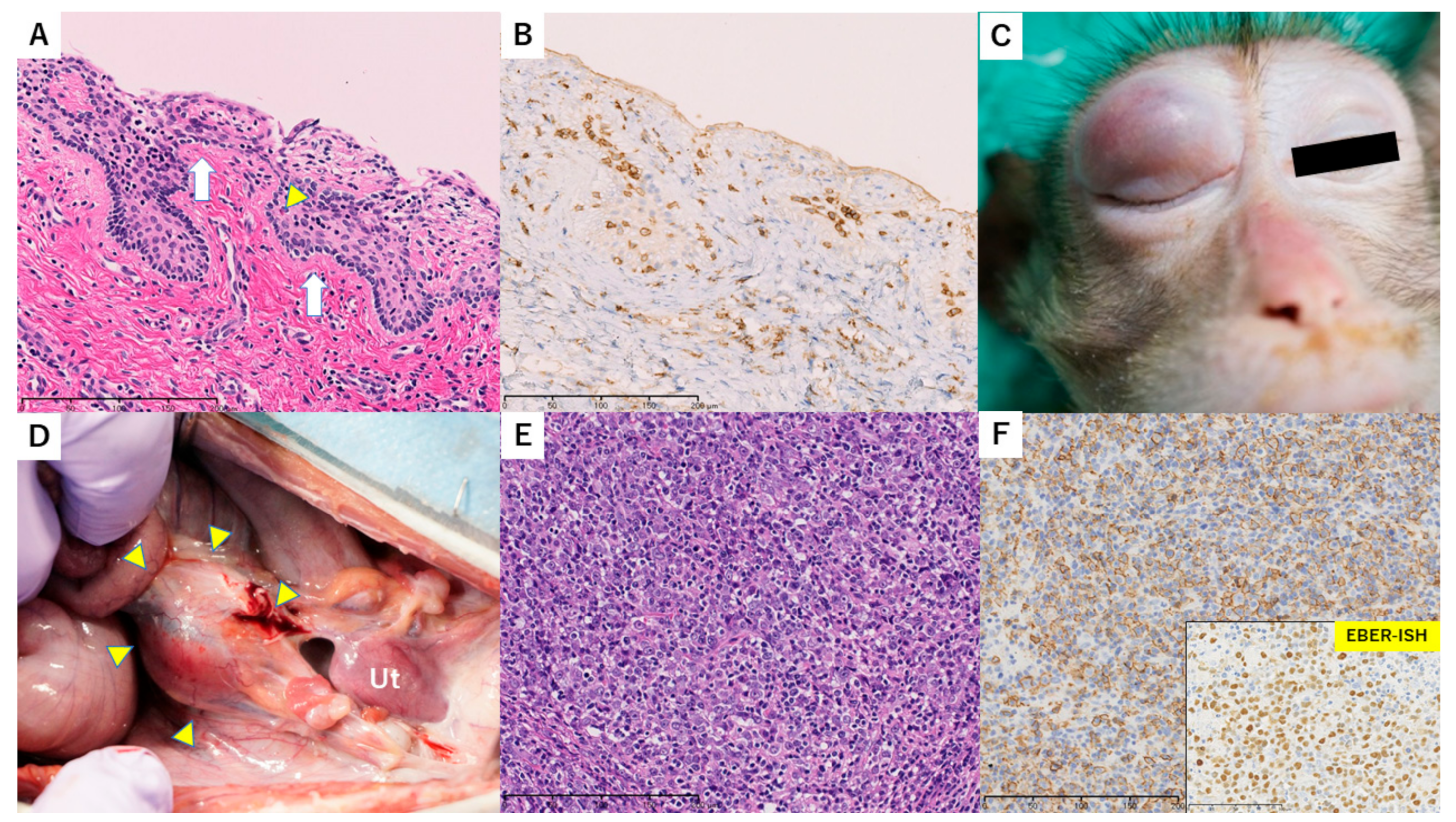
3.6. Case 4
4. Recovery of Cyclicity
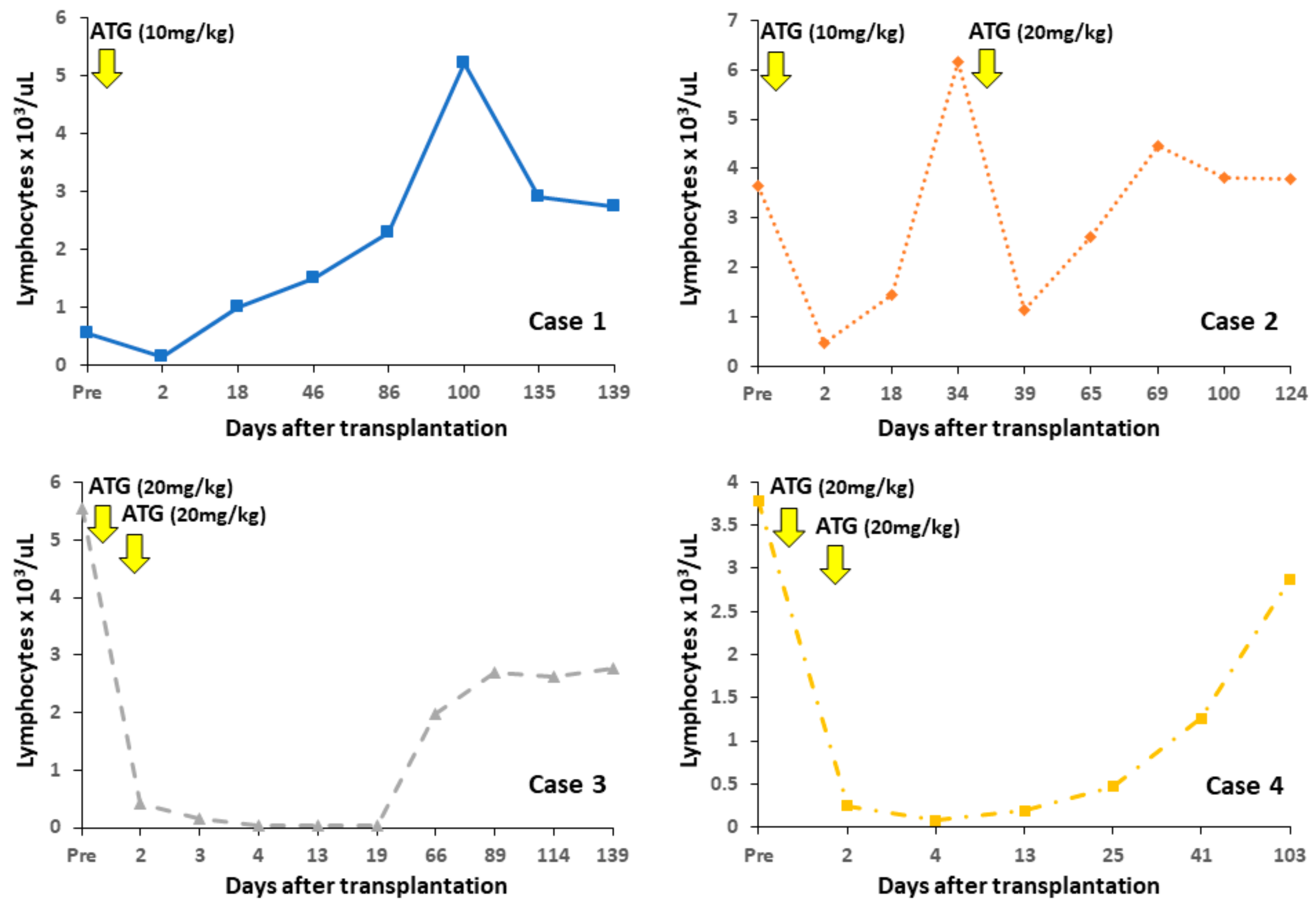
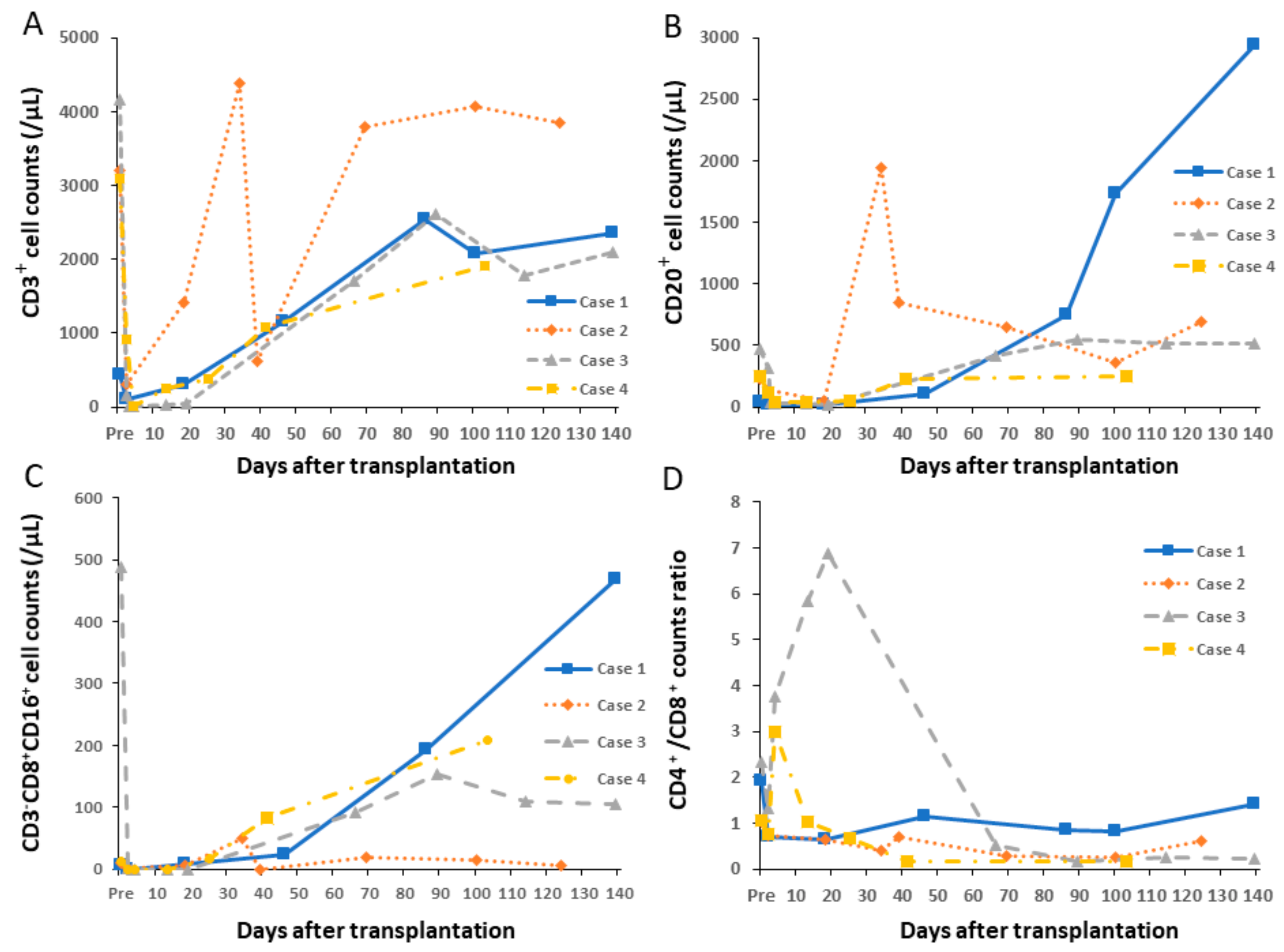
4.1. Changes in Peripheral Lymphocyte Counts
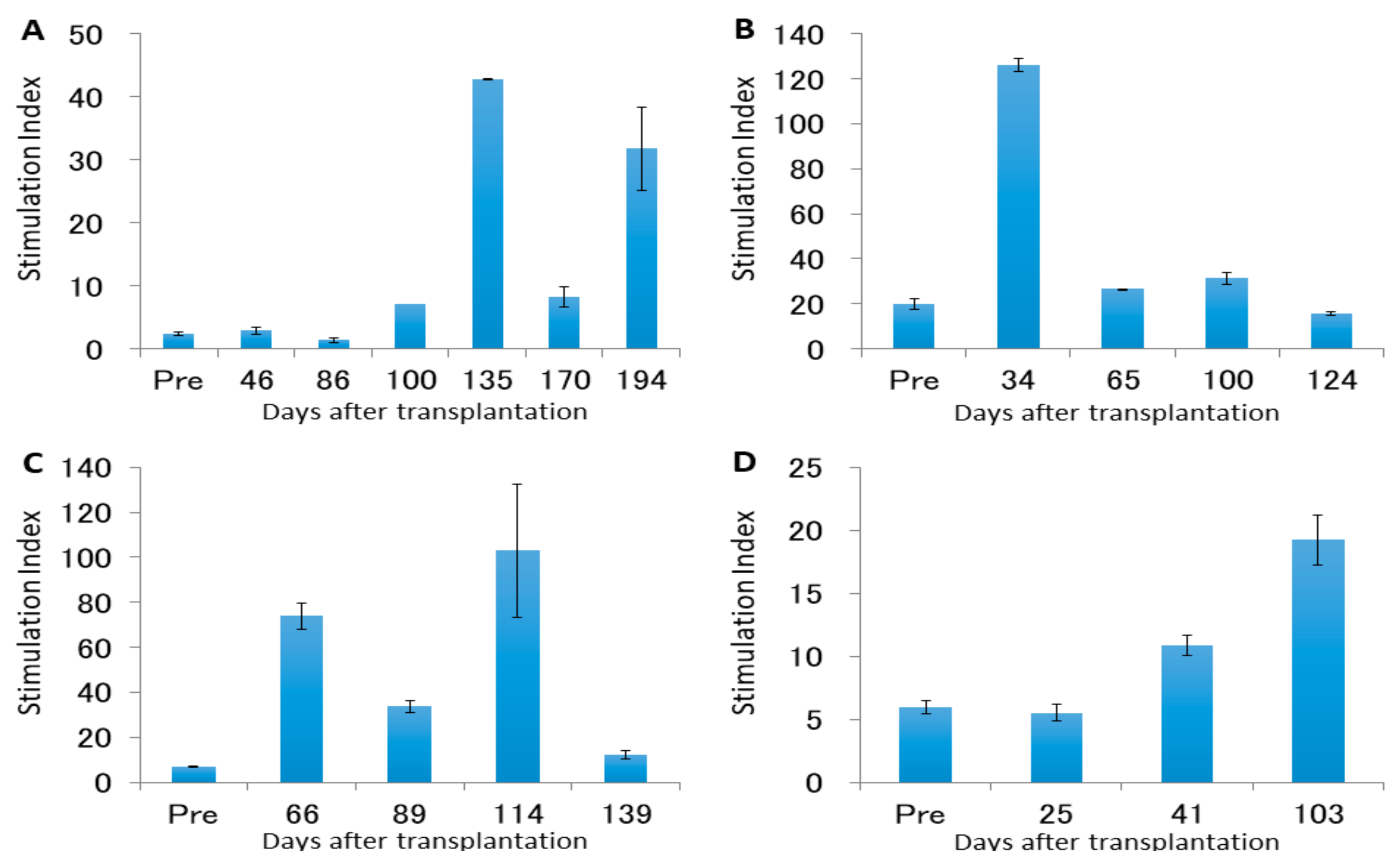
4.2. Leukocyte Proliferation Against Donor or Third-Party Antigens
4.3. Antidonor Antibody Production
5. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
Details of Ethics Approval
References
- Brannstrom, M.; Johannesson, L.; Bokstrom, H.; Kvarnstrom, N.; Molne, J.; Dahm-Kahler, P.; Enskog, A.; Milenkovic, M.; Ekberg, J.; Diaz-Garcia, C.; et al. Livebirth after uterus transplantation. Lancet 2015, 385, 607–616. [Google Scholar] [CrossRef]
- Ejzenberg, D.; Andraus, W.; Mendes, L.R.B.C.; Ducatti, L.; Song, A.; Tanigawa, R.; Rocha-Santos, V.; Arantes, R.M.; Soares, J.M., Jr.; Serafini, P.C.; et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet 2019, 392, 2697–2704. [Google Scholar] [CrossRef]
- Kisu, I.; Banno, K.; Mihara, M.; Suganuma, N.; Aoki, D. Current status of uterus transplantation in primates and issues for clinical application. Fertil. Steril. 2013, 100, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Brannstrom, M. Uterus transplantation and beyond. J. Mater. Sci. Mater. Med. 2017, 28, 70. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garcia, C.; Johannesson, L.; Shao, R.; Bilig, H.; Brannstrom, M. Pregnancy after allogeneic uterus transplantation in the rat: Perinatal outcome and growth trajectory. Fertil. Steril. 2014, 102, 15451552. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, E.R.; Ramirez Nessetti, D.K.; Nessetti, M.B.; Khatamee, M.; Wolfson, M.R.; Shaffer, T.H.; Ramirez, V.Z.; Ramirez, H.A. Pregnancy and outcome of uterine allotransplantation and assisted reproduction in sheep. J. Minim. Invasive Gynecol. 2011, 18, 238–245. [Google Scholar] [CrossRef]
- Tzakis, A.G. Nonhuman primates as models for transplantation of the uterus. Fertil. Steril. 2013, 100, 61. [Google Scholar] [CrossRef]
- Kisu, I.; Banno, K.; Mihara, M.; Aoki, D. Uterus transplantation in nonhuman primates. Fertil. Steril. 2013, 100, e3. [Google Scholar] [CrossRef]
- Kisu, I.; Banno, K.; Matoba, Y.; Adachi, M.; Aoki, D. Basic research on uterus transplantation in nonhuman primates in Japan. J. Obstet. Gynaecol. Res. 2018, 44, 1871–1881. [Google Scholar] [CrossRef]
- Kisu, I.; Mihara, M.; Banno, K.; Hara, H.; Yamamoto, T.; Araki, J.; Iida, T.; Hayashi, Y.; Moriguchi, H.; Aoki, D. A new surgical technique of uterine auto-transplantation in cynomolgus monkey: Preliminary report about two cases. Arch. Gynecol. Obstet. 2012, 285, 129–137. [Google Scholar] [CrossRef]
- Tryphonopoulos, P.; Tzakis, A.G.; Tekin, A.; Johannesson, L.; Rivas, K.; Morales, P.R.; Wagner, J.; Molne, J.; Enskog, A.; Diaz-Garcia, C.; et al. Allogeneic uterus transplantation in baboons: Surgical technique and challenges to Long-Term graft survival. Transplantation 2014, 98, e51–e56. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Kisu, I.; Hara, H.; Iida, T.; Araki, J.; Shim, T.; Narushima, M.; Yamamoto, T.; Moriguchi, H.; Kato, Y.; et al. Uterine autotransplantation in cynomolgus macaques: The first case of pregnancy and delivery. Hum. Reprod. 2012, 27, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- Kisu, I.; Mihara, M.; Banno, K.; Hara, H.; Masugi, Y.; Araki, J.; Iida, T.; Yamada, Y.; Kato, Y.; Shiina, T.; et al. Uterus allotransplantation in cynomolgus macaque: A preliminary experience with Non-Human primate models. J. Obstet. Gynaecol. Res. 2014, 40, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Obara, H.; Kisu, I.; Kato, Y.; Yamada, Y.; Matsubara, K.; Emoto, K.; Adachi, M.; Matoba, Y.; Umene, K.; Nogami, Y.; et al. Surgical technique for allogeneic uterus transplantation in macaques. Sci. Rep. 2016, 6, 35989. [Google Scholar] [CrossRef] [PubMed]
- Shiina, T.; Yamada, Y.; Aarnink, A.; Suzuki, S.; Masuya, A.; Ito, S.; Ido, D.; Yamanaka, H.; Iwatani, C.; Tsuchiya, H.; et al. Discovery of novel MHC-Class I alleles and haplotypes in Filipino cynomolgus macaques (Macaca fascicularis) by pyrosequencing and Sanger sequencing: Mafa-Class I polymorphism. Immunogenetics 2015, 67, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS Cell-Derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef] [PubMed]
- De Groot, N.G.; Otting, N.; Robinson, J.; Blancher, A.; Lafont, B.A.; Marsh, S.G.; O’Connor, D.H.; Shiina, T.; Walter, L.; Watkins, D.I.; et al. Nomenclature report on the major histocompatibility complex genes and alleles of Great Ape, Old and New World monkey species. Immunogenetics 2012, 64, 615–631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holcomb, C.L.; Rastrou, M.; Williams, T.C.; Goodridge, D.; Lazaro, A.M.; Tilanus, M.; Erlich, H.A. Next-generation sequencing can reveal in Vitro-Generated PCR crossover products: Some artifactual sequences correspond to HLA alleles in the IMGT/HLA database. Tissue Antigens 2014, 83, 32–40. [Google Scholar] [CrossRef]
- Shiina, T.; Suzuki, S.; Ozaki, Y.; Taira, H.; Kikkawa, E.; Shigenari, A.; Oka, A.; Umemura, T.; Joshita, S.; Takahashi, O.; et al. Super high resolution for single Molecule-Sequence-Based typing of classical HLA loci at the 8-Digit level using next generation sequencers. Tissue Antigens 2012, 80, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Kisu, I.; Banno, K.; Mihara, M.; Lin, L.Y.; Tsuji, K.; Yanokura, M.; Hara, H.; Araki, J.; Iida, T.; Abe, T.; et al. Indocyanine green fluorescence imaging for evaluation of uterine blood flow in cynomolgus macaque. PLoS ONE 2012, 7, e35124. [Google Scholar] [CrossRef]
- Mihara, M.; Kisu, I.; Hara, H.; Iida, T.; Yamamoto, T.; Araki, J.; Hayashi, Y.; Moriguchi, H.; Narushima, M.; Banno, K.; et al. Uterus autotransplantation in cynomolgus macaques: Intraoperative evaluation of uterine blood flow using indocyanine green. Hum. Reprod. 2011, 26, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Kisu, I.; Banno, K.; Yanokura, M.; Nogami, Y.; Umene, K.; Tsuji, K.; Masuda, K.; Ueki, A.; Kobayashi, Y.; Aoki, D. Indocyanine green fluorescence imaging in the pregnant cynomolgus macaque: Childbearing is supported by a unilateral uterine artery and vein alone? Arch. Gynecol. Obstet. 2013, 288, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Molne, J.; Broecker, V.; Ekberg, J.; Nilsson, O.; Dahm-Kahler, P.; Brannstrom, M. Monitoring of Human Uterus Transplantation with Cervical Biopsies: A Provisional Scoring System for Rejection. Am. J. Transplant. 2017, 17, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Enskog, A.; Mölne, J.; Diaz-Garcia, C.; Hanafy, A.; Dahm-Kähler, P.; Tekin, A.; Tryphonopoulos, P.; Morales, P.; Rivas, K.; et al. Preclinical report on allogeneic uterus transplantation in Non-Human primates. Hum. Reprod. 2013, 28, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kisu, I.; Kato, Y.; Obara, H.; Matsubara, K.; Matoba, Y.; Banno, K.; Aoki, D. Emerging problems in uterus transplantation. BJOG 2018, 125, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Fageeh, W.; Raffa, H.; Jabbad, H.; Marzouki, A. Transplantation of the human uterus. Int. J. Gynaecol. Obstet. 2002, 76, 245–251. [Google Scholar] [CrossRef]
- Chmel, R.; Novackova, M.; Janousek, L.; Matecha, J.; Pastor, Z.; Maluskova, J.; Cekal, M.; Kristek, J.; Olausson, M.; Fronek, J. Revaluation and lessons learned from the first 9 cases of a Czech uterus transplantation trial: Four deceased donor and 5 living donor uterus transplantations. Am. J. Transplant. 2019, 19, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Puntambekar, S.; Telang, M.; Kulkarni, P.; Puntambekar, S.; Jadhav, S.; Panse, M.; Sathe, R.; Agarkhedkar, N.; Warty, N.; Kade, S.; et al. Laparoscopic-Assisted Uterus Retrieval from Live Organ Donors for Uterine Transplant: Our Experience of Two Patients. J. Minim. Invasive Gynecol. 2018, 25, 622–631. [Google Scholar] [CrossRef]
- Adachi, M.; Kisu, I.; Nagai, T.; Emoto, K.; Banno, K.; Umene, K.; Nogami, Y.; Tsuchiya, H.; Itagaki, I.; Kawamoto, I.; et al. Evaluation of allowable time and histopathological changes in warm ischemia of the uterus in cynomolgus monkey as a model for uterus transplantation. Acta Obstet. Gynecol. Scand. 2016, 95, 991–998. [Google Scholar] [CrossRef]
- Kisu, I.; Umene, K.; Adachi, M.; Emoto, K.; Nogami, Y.; Banno, K.; Itagaki, I.; Kawamoto, I.; Nakagawa, T.; Narita, H.; et al. Allowable warm ischemic time and morphological and biochemical changes in uterine ischemia/reperfusion injury in cynomolgus macaque: A basic study for uterus transplantation. Hum. Reprod. 2017, 32, 2026–2035. [Google Scholar] [CrossRef]
- Brannstrom, M.; Johannesson, L.; Dahm-Kahler, P.; Enskog, A.; Molne, J.; Kvarnstrom, N.; Diaz-Garcia, C.; Hanafy, A.; Lundmark, C.; Marcickiewicz, J.; et al. First clinical uterus transplantation trial: A Six-Month report. Fertil. Steril. 2014, 101, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Cho, C.W.; Park, H.; Choi, G.S.; Park, J.B.; Kim, S.J. Technique for orthotopic liver transplantation in cynomolgus monkeys (Macaca fascicularis). Ann. Surg. Treat Res. 2018, 94, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, F.; Nagatomi, I.; Ishikawa, H.; Nakanishi, T.; Maeda, M.; Hirose, J.; Fukahori, H.; Ooshima, S.; Noto, T.; Higashi, Y.; et al. Efficacy of oral treatment with tacrolimus in the renal transplant model in cynomolgus monkeys. J. Pharmacol. Sci. 2008, 108, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, G.; Bigaud, M.; Menninger, K.; Riesen, S.; Quesniaux, V.; Schuurman, H.J.; Audet, M.; Blancher, A.; Mihatsch, M.J.; Nickeleit, V. Acute and chronic vascular rejection in nonhuman primate kidney transplantation. Am. J. Transplant. 2006, 6, 1285–1296. [Google Scholar] [CrossRef]
- Kato, Y.; Griesemer, A.D.; Wu, A.; Sondermeijer, H.P.; Weiner, J.I.; Duran-Struuck, R.; Martinez, M.; Slate, A.R.; Romanov, A.; Lefkowitch, J.H.; et al. Novel H-Shunt Venovenous Bypass for Liver Transplantation in Cynomolgus Macaques. Comp. Med. 2017, 67, 436–441. [Google Scholar] [PubMed]
- Préville, X.; Flacher, M.; LeMauff, B.; Beauchard, S.; Davelu, P.; Tiollier, J.; Revillard, J.P. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation 2001, 71, 460–468. [Google Scholar] [CrossRef]
| Case | Survival Days | Recovery of Menstruation | Rejection Episode | DSA Production | MLR Stimulation Indexes (Pre-Transplant; Maximum) | Cause of Sacrifice | Major Pathological Findings in Autopsy |
|---|---|---|---|---|---|---|---|
| 1 | 196 | Temporary | Severe | Positive | 2.40; 40.0 | Graft atrophy, chronic rejection | Atrophic uterus with hyalinized fibrosis and no endometrium Grafted vessels with fibrous occlusion and lymphocytes infiltration |
| DSA2 | 126 | Temporary | Severe | Positive | 19.9; 125.0 | Graft atrophy, chronic rejection | Atrophic uterus with hyalinized fibrosis and uterine infection Uterine stroma with lymphocytes infiltration and capillary endotheliitis |
| 3 | 140 | None | Mild– Moderate | Negative | 7.0; 33.7 | Lower limbs paralysis | Normal-sized uterus with mild lymphocytes inflammation and a small number of Civatte bodies in the cervix Nodular tumors diagnosed as PTLD (diffuse large B-cell lymphoma) |
| 4 | 104 | None | Severe | Positive | 5.9; 19.3 | Swollen graft, chronic rejection | Whole uterus with severe endotheliitis, cervix with epithelial desquamation, and corpus with obstructive vasculitis and focal necrosis Feeding vessels in grafted aorta adventitia with severe endotheliitis and obstruction |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisu, I.; Ishigaki, H.; Emoto, K.; Kato, Y.; Yamada, Y.; Matsubara, K.; Obara, H.; Masugi, Y.; Matoba, Y.; Adachi, M.; et al. Long-Term Outcome and Rejection After Allogeneic Uterus Transplantation in Cynomolgus Macaques. J. Clin. Med. 2019, 8, 1572. https://doi.org/10.3390/jcm8101572
Kisu I, Ishigaki H, Emoto K, Kato Y, Yamada Y, Matsubara K, Obara H, Masugi Y, Matoba Y, Adachi M, et al. Long-Term Outcome and Rejection After Allogeneic Uterus Transplantation in Cynomolgus Macaques. Journal of Clinical Medicine. 2019; 8(10):1572. https://doi.org/10.3390/jcm8101572
Chicago/Turabian StyleKisu, Iori, Hirohito Ishigaki, Katsura Emoto, Yojiro Kato, Yohei Yamada, Kentaro Matsubara, Hideaki Obara, Yohei Masugi, Yusuke Matoba, Masataka Adachi, and et al. 2019. "Long-Term Outcome and Rejection After Allogeneic Uterus Transplantation in Cynomolgus Macaques" Journal of Clinical Medicine 8, no. 10: 1572. https://doi.org/10.3390/jcm8101572
APA StyleKisu, I., Ishigaki, H., Emoto, K., Kato, Y., Yamada, Y., Matsubara, K., Obara, H., Masugi, Y., Matoba, Y., Adachi, M., Banno, K., Saiki, Y., Itagaki, I., Kawamoto, I., Iwatani, C., Nakagawa, T., Tsuchiya, H., Sasamura, T., Urano, H., ... Shiina, T. (2019). Long-Term Outcome and Rejection After Allogeneic Uterus Transplantation in Cynomolgus Macaques. Journal of Clinical Medicine, 8(10), 1572. https://doi.org/10.3390/jcm8101572







