A Population Pharmacokinetic Model of Intravenous Dexmedetomidine for Mechanically Ventilated Children after Neurosurgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Protocol
2.3. Blood Sampling and Drug Assays for PKs
2.4. Efficacy and Safety Assessment
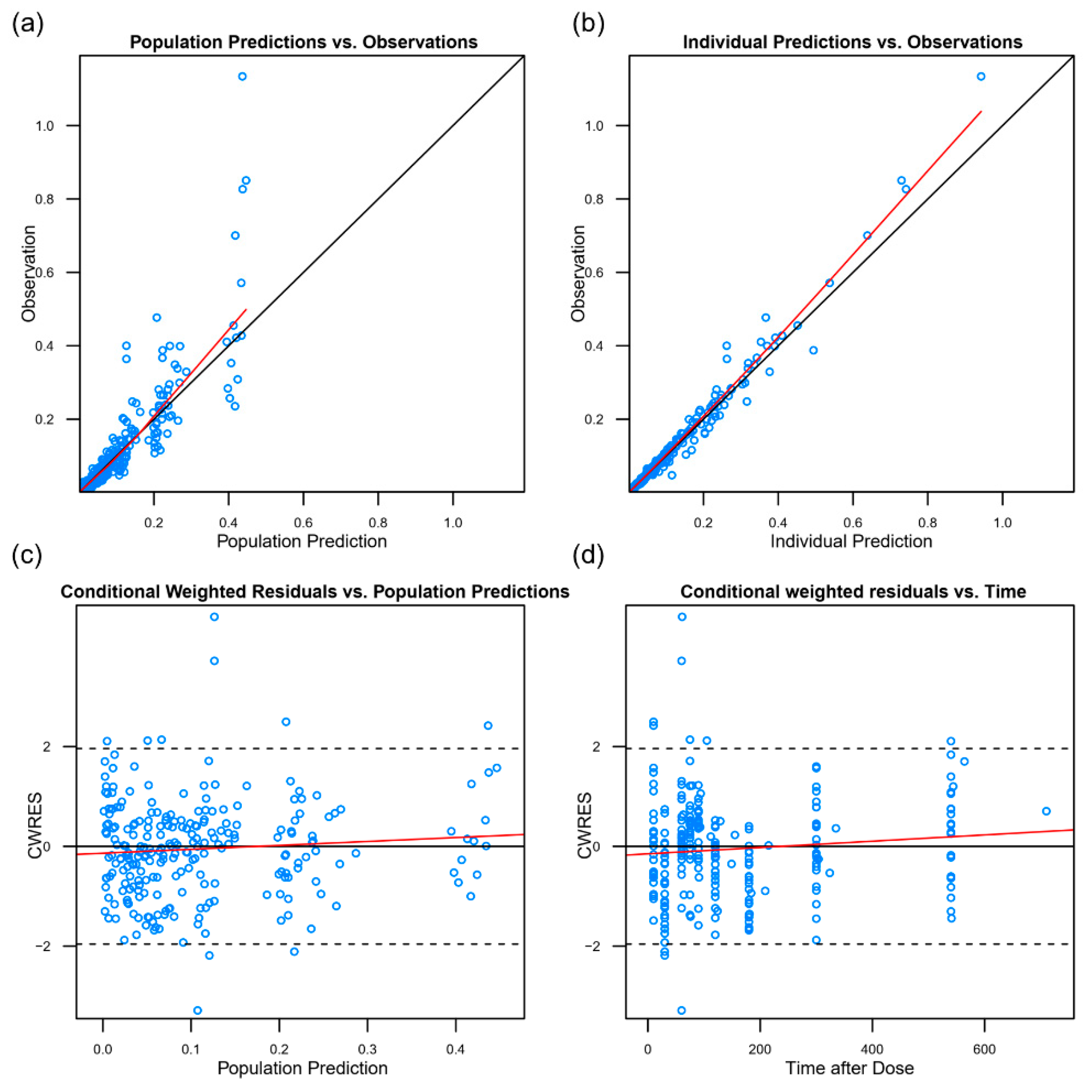
2.5. Population PK Analysis
2.6. Statistics
3. Results
3.1. Overall
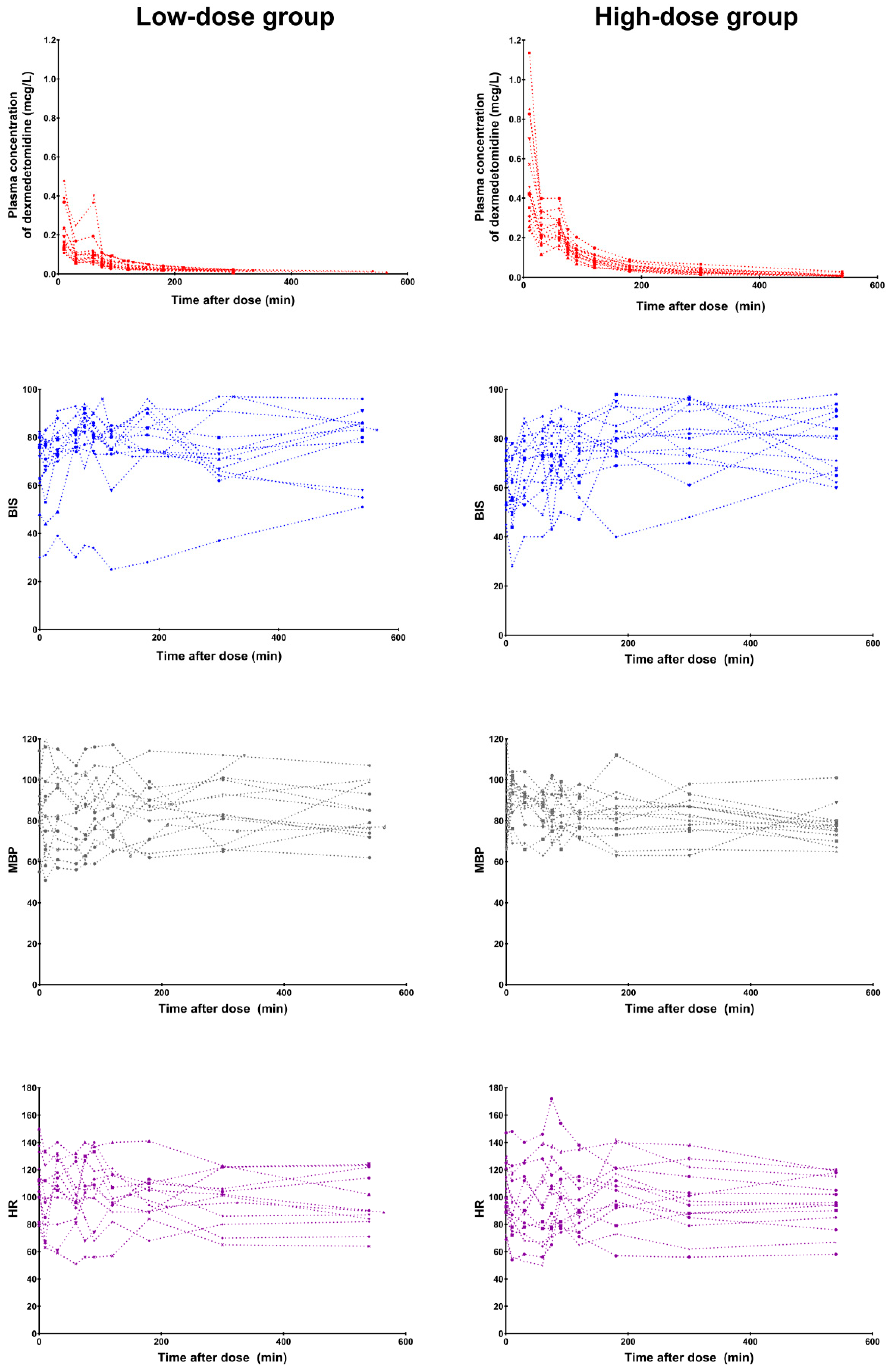
3.2. Efficacy and Safety
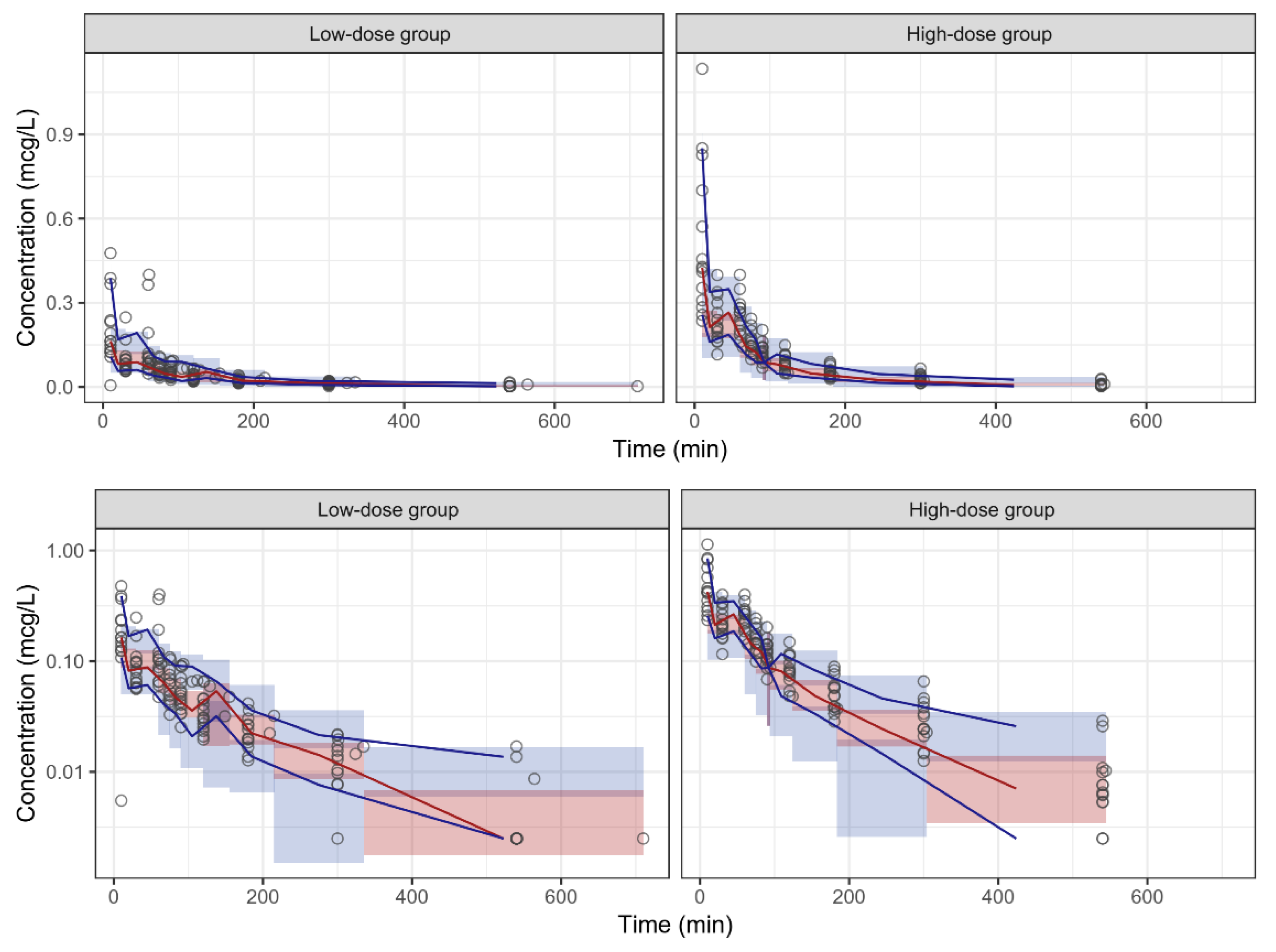
3.3. Pharmacokinetics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- PRECEDEX—Dexmedetomidine Hydrochloride Injection, Solution. Available online: http://labeling.pfizer.com/ShowLabeling.aspx?id=4404 (accessed on 1 May 2014).
- Plambech, M.Z.; Afshari, A. Dexmedetomidine in the pediatric population: A review. Minerva Anestesiol. 2015, 81, 320–332. [Google Scholar] [PubMed]
- Mahmoud, M.; Mason, K.P. Dexmedetomidine: Review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br. J. Anaesth. 2015, 115, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.M.; Rodarte, A.; Foley, J.; Capparelli, E.V. Pharmacokinetics of dexmedetomidine in postsurgical pediatric intensive care unit patients: Preliminary study. Pediatr. Crit. Care Med. 2007, 8, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, C.; Schulman, S.R.; Herrera Castellanos, M.; Cofer, B.E.; Mitra, S.; da Rocha, M.G.; Wisemandle, W.A.; Gramlich, L. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J. Pediatr. 2014, 164, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Gastonguay, M.R.; Nicolson, S.C.; DiLiberto, M.; Ocampo-Pelland, A.; Zuppa, A.F. Dexmedetomidine Pharmacology in Neonates and Infants After Open Heart Surgery. Anesth. Analg. 2016, 122, 1556–1566. [Google Scholar] [CrossRef]
- Vilo, S.; Rautiainen, P.; Kaisti, K.; Aantaa, R.; Scheinin, M.; Manner, T.; Olkkola, K.T. Pharmacokinetics of intravenous dexmedetomidine in children under 11 yr of age. Br. J. Anaesth. 2008, 100, 697–700. [Google Scholar] [CrossRef]
- Liu, H.C.; Lian, Q.Q.; Wu, F.F.; Wang, C.Y.; Sun, W.; Zheng, L.D.; Schuttler, J.; Ihmsen, H. Population Pharmacokinetics of Dexmedetomidine After Short Intravenous Infusion in Chinese Children. Eur. J. Drug Metab. Pharmacokinet 2017, 42, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Potts, A.L.; Anderson, B.J.; Warman, G.R.; Lerman, J.; Diaz, S.M.; Vilo, S. Dexmedetomidine pharmacokinetics in pediatric intensive care--a pooled analysis. Paediatr. Anaesth. 2009, 19, 1119–1129. [Google Scholar] [CrossRef]
- Su, F.; Nicolson, S.C.; Gastonguay, M.R.; Barrett, J.S.; Adamson, P.C.; Kang, D.S.; Godinez, R.I.; Zuppa, A.F. Population pharmacokinetics of dexmedetomidine in infants after open heart surgery. Anesth. Analg. 2010, 110, 1383–1392. [Google Scholar] [CrossRef]
- Wiczling, P.; Bartkowska-Sniatkowska, A.; Szerkus, O.; Siluk, D.; Rosada-Kurasinska, J.; Warzybok, J.; Borsuk, A.; Kaliszan, R.; Grzeskowiak, E.; Bienert, A. The pharmacokinetics of dexmedetomidine during long-term infusion in critically ill pediatric patients. A Bayesian approach with informative priors. J. Pharmacokinet Pharmacodyn. 2016, 43, 315–324. [Google Scholar] [CrossRef]
- Petroz, G.C.; Sikich, N.; James, M.; van Dyk, H.; Shafer, S.L.; Schily, M.; Lerman, J. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology 2006, 105, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.G.; Wu, H.; Laughon, M.; Capparelli, E.; Rowe, S.; Zimmerman, K.O.; Smith, P.B.; Cohen-Wolkowiez, M. Population Pharmacokinetics of Dexmedetomidine in Infants. J. Clin. Pharmacol. 2017, 57, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Perez-Guille, M.G.; Toledo-Lopez, A.; Rivera-Espinosa, L.; Alemon-Medina, R.; Murata, C.; Lares-Asseff, I.; Chavez-Pacheco, J.L.; Gomez-Garduno, J.; Zamora Gutierrez, A.L.; Orozco-Galicia, C.; et al. Population Pharmacokinetics and Pharmacodynamics of Dexmedetomidine in Children Undergoing Ambulatory Surgery. Anesth. Analg. 2018, 127, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.M.; Lee, H.G.; Byon, H.J.; Lee, S.H.; Lee, E.K.; Kim, H.S.; Noh, G.J. Population pharmacokinetic and pharmacodynamic model of propofol externally validated in children. J. Pharmacokinet Pharmacodyn. 2015, 42, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.D.; Suresh, S. Applications of regional anaesthesia in paediatrics. Br. J. Anaesth. 2013, 111, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Su, F.; Shi, H.; Zuppa, A.F. Sensitive and specific liquid chromatography-tandem mass spectrometric method for the quantitation of dexmedetomidine in pediatric plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 852, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Z.; Wu, L.; Tian, Y.; Feng, S.; Chen, Y. Determination of dexmedetomidine in human plasma using high performance liquid chromatography coupled with tandem mass spectrometric detection: Application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2009, 50, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, S.; Ganesh, A.; Robison, A.; Kaye, R.; Watcha, M.F. Validation of the bispectral index monitor for measuring the depth of sedation in children. Anesth. Analg. 2006, 102, 383–388. [Google Scholar] [CrossRef]
- McDermott, N.B.; VanSickle, T.; Motas, D.; Friesen, R.H. Validation of the bispectral index monitor during conscious and deep sedation in children. Anesth. Analg. 2003, 97, 39–43. [Google Scholar] [CrossRef]
- Malviya, S.; Voepel-Lewis, T.; Tait, A.R.; Watcha, M.F.; Sadhasivam, S.; Friesen, R.H. Effect of age and sedative agent on the accuracy of bispectral index in detecting depth of sedation in children. Pediatrics 2007, 120, e461–e470. [Google Scholar] [CrossRef]
- Sciusco, A.; Standing, J.F.; Sheng, Y.; Raimondo, P.; Cinnella, G.; Dambrosio, M. Effect of age on the performance of bispectral and entropy indices during sevoflurane pediatric anesthesia: A pharmacometric study. Paediatr. Anaesth. 2017, 27, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.; Heo, Y.A.; Anderson, B. A pharmacokinetic standard for babies and adults. J. Pharm. Sci. 2013, 102, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Germovsek, E.; Barker, C.I.; Sharland, M.; Standing, J.F. Scaling clearance in paediatric pharmacokinetics: All models are wrong, which are useful? Br. J. Clin. Pharmacol. 2017, 83, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Simplified Calculation of Body-Surface Area. N. Eng. J. Med. 1987, 317, 1098. [CrossRef] [PubMed]
- Hallynck, T.H.; Soep, H.H.; Thomis, J.A.; Boelaert, J.; Daneels, R.; Dettli, L. Should clearance be normalised to body surface or to lean body mass? Br. J. Clin. Pharmacol. 1981, 11, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Traub, S.L.; Johnson, C.E. Comparison of methods of estimating creatinine clearance in children. Am. J. Hosp. Pharm. 1980, 37, 195–201. [Google Scholar] [CrossRef]
- Deurenberg, P.; Weststrate, J.A.; Seidell, J.C. Body mass index as a measure of body fatness: Age- and sex-specific prediction formulas. Br. J. Nutr. 1991, 65, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; Jansen, R.S.; Rosing, H.; Thijssen, B.; Beijnen, J.H.; Schellens, J.H.; Huitema, A.D. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol. Res. Perspect. 2015, 3, e00131. [Google Scholar] [CrossRef]
- Potts, A.L.; Warman, G.R.; Anderson, B.J. Dexmedetomidine disposition in children: A population analysis. Paediatr. Anaesth. 2008, 18, 722–730. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. Tips and traps analyzing pediatric PK data. Paediatr. Anaesth. 2011, 21, 222–237. [Google Scholar] [CrossRef]
- Dutta, S.; Lal, R.; Karol, M.D.; Cohen, T.; Ebert, T. Influence of cardiac output on dexmedetomidine pharmacokinetics. J. Pharm. Sci. 2000, 89, 519–527. [Google Scholar] [CrossRef]
- Li, A.; Yuen, V.M.; Goulay-Dufay, S.; Kwok, P.C. Pharmacokinetics and pharmacodynamics of dexmedetomidine. Drug Dev. Ind. Pharm. 2016, 42, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58, S10–S25. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Ramelet, A.S.; van Dijk, M.; Pokorna, P.; Wielenga, J.; Tume, L.; Tibboel, D.; Ista, E. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: An ESPNIC position statement for healthcare professionals. Intensive Care Med. 2016, 42, 972–986. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Hammer, G.B. Dexmedetomidine: Pediatric pharmacology, clinical uses and safety. Expert Opin. Drug Saf. 2011, 10, 55–66. [Google Scholar] [CrossRef]
- Carroll, C.L.; Krieger, D.; Campbell, M.; Fisher, D.G.; Comeau, L.L.; Zucker, A.R. Use of dexmedetomidine for sedation of children hospitalized in the intensive care unit. J. Hosp. Med. 2008, 3, 142–147. [Google Scholar] [CrossRef]
- Mason, K.P.; Zurakowski, D.; Zgleszewski, S.E.; Robson, C.D.; Carrier, M.; Hickey, P.R.; Dinardo, J.A. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr. Anaesth. 2008, 18, 403–411. [Google Scholar] [CrossRef]
| Low-Dose Group (n = 15) | High-Dose Group (n = 14) | |
|---|---|---|
| Age, years | 8.0 (5.0–10.0) | 7.0 (3.3–10.3) |
| Sex, boys | 6 (40) | 8 (57) |
| Height, cm | 121.9 (108.0–136.0) | 128.0 (104.0–138.8) |
| Weight, kg | 22.0 (19.5–30.5) | 23.0 (16.5–37.8) |
| Body surface area, m2 | 0.9 (0.8–1.0) | 0.9 (0.7–1.2) |
| Lean body mass, kg | 18.2 (14.4–24.4) | 21.5 (12.3–30.2) |
| Ideal body weight, kg | 23.5 (17.8–31.1) | 26.5 (16.4–32.9) |
| Body mass index, kg/m2 | 16.1 (13.8–17.6) | 16.2 (15.1–19.7) |
| Body fat percentage, % | 20 (17–22) | 20 (17–23) |
| Types of operation | ||
| Craniotomy & tumor removal | 12 (79) | 14 (100) |
| Encephaloduroarteriosynangiosis | 1 (7) | 0 (0) |
| Foramen magnum decompression | 1 (7) | 0 (0) |
| Endoscopic transsphenoidal surgery | 1 (7) | 0 (0) |
| Duration of operation, min | 265 (240–300) | 295 (268–321) |
| Laboratory results | ||
| Plasma albumin, g/dL | 4.4 (4.3–4.4) | 4.5 (4.1–4.6) |
| Plasma bilirubin, mg/dL | 0.5 (0.4–0.6) | 0.5 (0.3–0.5) |
| Plasma creatinine, mg/dL | 0.41 (0.32–0.45) | 0.45 (0.32–0.52) |
| Parameters | Estimate | RSE (%) a | Bootstrap Median (95% Confidence Interval) b |
|---|---|---|---|
| Structural model | |||
| Allometric clearance of central compartment c (CLpop, L/h) | 81.0 | 5.5 | 81.1 (72.9–90.9) |
| Allometric volume of central compartment c (V1pop, L) | 64.2 | 12.6 | 63.7 (50.6–81.0) |
| Allometric clearance of peripheral compartment c (Qpop, L/h) | 116.4 | 13.1 | 119.2 (90.6–156.0) |
| Allometric volume of peripheral compartment c (V2pop, L) | 167 | 12.5 | 167 (132–217) |
| Inter-individual variability | |||
| CL (CV%) | 27.1 | 26.6 | 26.4 (18.5–34.2) |
| V1 (CV%) | 60.0 | 27.6 | 57.4 (41.8–75.0) |
| Q (CV%) | 46.7 | 55.0 | 44.7 (12.7–65.7) |
| V2 (CV%) | 60.7 | 36.3 | 59.5 (39.0–81.9) |
| Residual error | |||
| Additive error (µg/L) | 0.0227 | 81.5 | 0.0245 (0.0179–0.0515) |
| Proportional error (%) | 42.7 | 14.2 | 42.0 (33.4–47.6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, I.-K.; Yi, S.; Lim, H.-S.; Lee, J.-H.; Kim, E.-H.; Cho, J.-Y.; Kim, M.-C.; Kim, J.-T.; Kim, H.-S. A Population Pharmacokinetic Model of Intravenous Dexmedetomidine for Mechanically Ventilated Children after Neurosurgery. J. Clin. Med. 2019, 8, 1563. https://doi.org/10.3390/jcm8101563
Song I-K, Yi S, Lim H-S, Lee J-H, Kim E-H, Cho J-Y, Kim M-C, Kim J-T, Kim H-S. A Population Pharmacokinetic Model of Intravenous Dexmedetomidine for Mechanically Ventilated Children after Neurosurgery. Journal of Clinical Medicine. 2019; 8(10):1563. https://doi.org/10.3390/jcm8101563
Chicago/Turabian StyleSong, In-Kyung, SoJeong Yi, Hyeong-Seok Lim, Ji-Hyun Lee, Eun-Hee Kim, Joo-Youn Cho, Min-Chang Kim, Jin-Tae Kim, and Hee-Soo Kim. 2019. "A Population Pharmacokinetic Model of Intravenous Dexmedetomidine for Mechanically Ventilated Children after Neurosurgery" Journal of Clinical Medicine 8, no. 10: 1563. https://doi.org/10.3390/jcm8101563
APA StyleSong, I.-K., Yi, S., Lim, H.-S., Lee, J.-H., Kim, E.-H., Cho, J.-Y., Kim, M.-C., Kim, J.-T., & Kim, H.-S. (2019). A Population Pharmacokinetic Model of Intravenous Dexmedetomidine for Mechanically Ventilated Children after Neurosurgery. Journal of Clinical Medicine, 8(10), 1563. https://doi.org/10.3390/jcm8101563






