Low TIP30 Protein Expression is Associated with a High Risk of Metastasis and Poor Prognosis for Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Extraction from PrognoScan Database and Methodological Assessment
2.2. Cell Culture
2.3. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.4. Western Blot Analysis
2.5. Tissue Microarray
2.6. Immunohistochemistry (IHC) and Patient Survival Analysis
3. Results
3.1. Meta-Analysis Revealed Poor Association between TIP30 mRNA Expression and the Prognosis of Lung Cancer Patients
3.2. The TIP30 Protein Expression Level is Not Determined by the mRNA Level in Human Lung Cancer Cell Lines
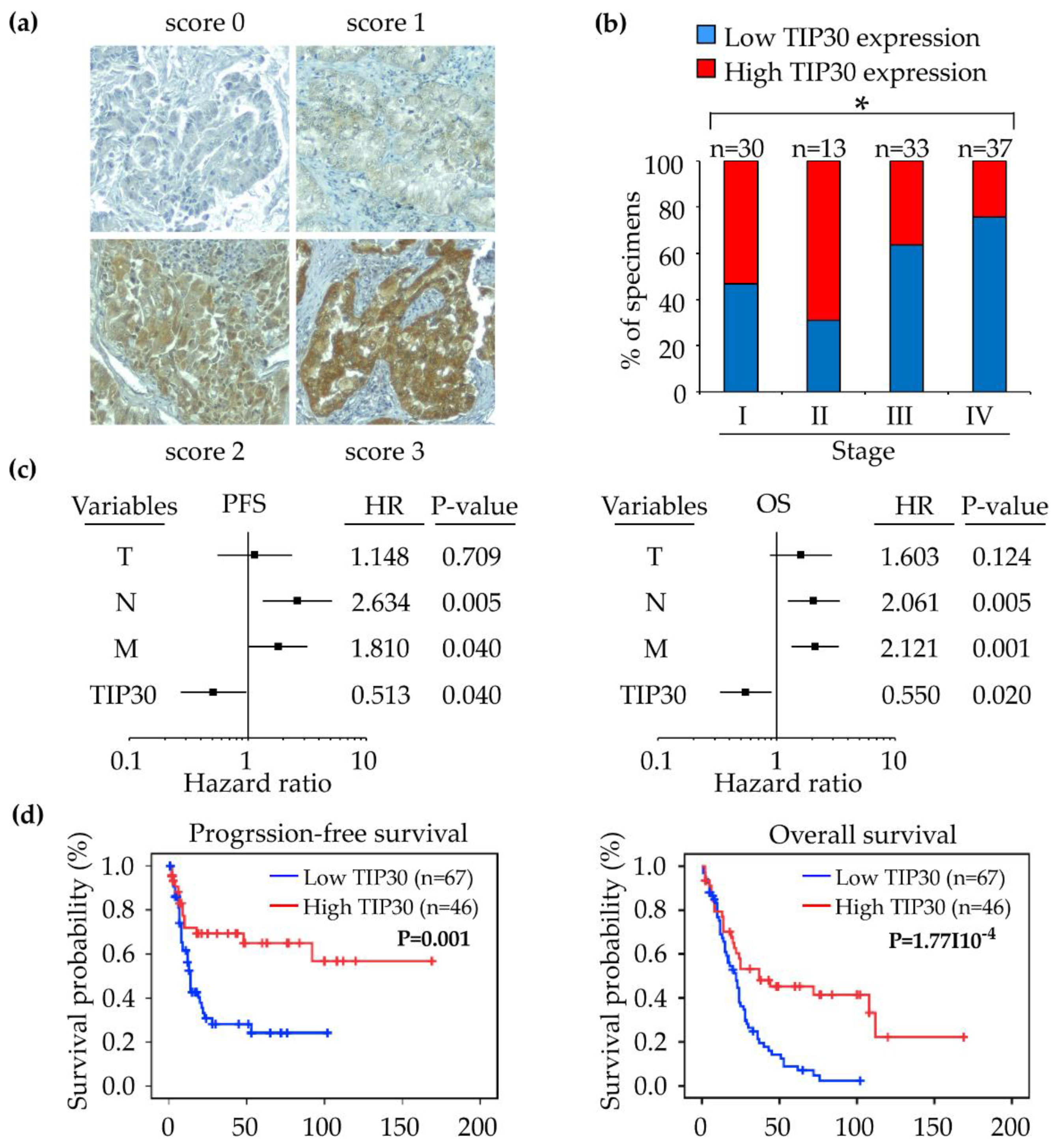
3.3. Down-Regulation of the TIP30 Protein Correlates with Poor Clinical Outcomes in NSCLC Patients
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar] [PubMed]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Koinis, F.; Fallon, M.T.; Fearon, K.C.; Bowden, J.; Solheim, T.S.; Gronberg, B.H.; McMillan, D.C.; Gioulbasanis, I.; Laird, B.J. Prognosis in advanced lung cancer—A prospective study examining key clinicopathological factors. Lung Cancer 2015, 88, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Shuai, S.; Yan, X.; Zhang, J.; Kang, S.; Chen, F.; Luo, R.; Li, A. Tip30 nuclear translocation negatively regulates egf-dependent cyclin d1 transcription in human lung adenocarcinoma. Cancer Lett. 2014, 354, 200–209. [Google Scholar] [CrossRef]
- Zhu, M.; Yin, F.; Fan, X.; Jing, W.; Chen, R.; Liu, L.; Zhang, L.; Liu, Y.; Liang, Y.; Bu, F.; et al. Decreased tip30 promotes snail-mediated epithelial-mesenchymal transition and tumor-initiating properties in hepatocellular carcinoma. Oncogene 2015, 34, 1420–1431. [Google Scholar] [CrossRef]
- Zhu, M.; Yin, F.; Yang, L.; Chen, S.; Chen, R.; Zhou, X.; Jing, W.; Fan, X.; Jia, R.; Wang, H.; et al. Contribution of tip30 to chemoresistance in laryngeal carcinoma. Cell Death Dis 2014, 5, e1468. [Google Scholar] [CrossRef]
- Lee, S.H.; Ju, S.K.; Lee, T.Y.; Huh, S.H.; Han, K.H. Tip30 directly binds p53 tumor suppressor protein in vitro. Mol. Cells 2012, 34, 495–500. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Cao, S.; Chen, X.; Lu, Y.; Jin, H.; Sun, S.; Chen, B.; Liu, J.; Ding, J.; et al. Reduction of tip30 correlates with poor prognosis of gastric cancer patients and its restoration drastically inhibits tumor growth and metastasis. Int. J. Cancer 2009, 124, 713–721. [Google Scholar] [CrossRef]
- Fan, S.S.; Liao, C.S.; Cao, Y.D.; Xiao, P.L.; Deng, T.; Luo, R.C.; Duan, H.X. A low serum tat-interacting protein 30 level is a diagnostic and prognostic biomarker for hepatocellular carcinoma. Oncol. Lett. 2017, 13, 4208–4214. [Google Scholar] [CrossRef]
- Chen, X.; Cao, X.; Dong, W.; Luo, S.; Suo, Z.; Jin, Y. Expression of tip30 tumor suppressor gene is down-regulated in human colorectal carcinoma. Dig. Dis. Sci. 2010, 55, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ni, H.; Ma, Y.; Dong, L.; Dai, J.; Zhao, F.; Yan, X.; Lu, B.; Xu, H.; Guo, Y. Tip30/cc3 expression in breast carcinoma: Relation to metastasis, clinicopathologic parameters, and p53 expression. Hum. Pathol. 2007, 38, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Duan, H.O.; Kirley, S.D.; Lin, S.X.; McDougal, W.S.; Xiao, H.; Wu, C.L. Tip30 is associated with progression and metastasis of prostate cancer. Int. J. Cancer 2008, 123, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, C.; Gao, S.; Chen, F.; Yang, C.; Luo, R.; Xiao, H. Tip30 loss enhances cytoplasmic and nuclear egfr signaling and promotes lung adenocarcinogenesis in mice. Oncogene 2013, 32, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Pecha, J.; Hoshino, I.; Ankrapp, D.; Xiao, H. Tip30 mutant derived from hepatocellular carcinoma specimens promotes growth of hepg2 cells through up-regulation of n-cadherin. Cancer Res. 2007, 67, 3574–3582. [Google Scholar] [CrossRef]
- Ouyang, H.; Gore, J.; Deitz, S.; Korc, M. Microrna-10b enhances pancreatic cancer cell invasion by suppressing tip30 expression and promoting egf and tgf-beta actions. Oncogene 2014, 33, 4664–4674. [Google Scholar] [CrossRef]
- Lu, B.; Ma, Y.; Wu, G.; Tong, X.; Guo, H.; Liang, A.; Cong, W.; Liu, C.; Wang, H.; Wu, M.; et al. Methylation of tip30 promoter is associated with poor prognosis in human hepatocellular carcinoma. Clin. Cancer Res. 2008, 14, 7405–7412. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Wu, W.K. Tip30: A novel tumor-suppressor gene. Oncol. Res. 2014, 22, 339–348. [Google Scholar] [CrossRef]
- Liu, Y.P.; Chen, C.H.; Yen, C.H.; Tung, C.W.; Chen, C.J.; Chen, Y.A.; Huang, M.S. HIV tat-tip30 interaction promotes metastasis by enhancing the nuclear translocation of snail in lung cancer cell lines. Cancer Sci. 2018, 109, 3105–3114. [Google Scholar] [CrossRef]
- Yin, F.; Sharen, G.; Yuan, F.; Peng, Y.; Chen, R.; Zhou, X.; Wei, H.; Li, B.; Jing, W.; Zhao, J. Tip30 regulates lipid metabolism in hepatocellular carcinoma by regulating srebp1 through the akt/mtor signaling pathway. Oncogenesis 2017, 6, e347. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, X.; Yang, L. Metformin enhances the effect of regorafenib and inhibits recurrence and metastasis of hepatic carcinoma after liver resection via regulating expression of hypoxia inducible factors 2alpha (hif-2alpha) and 30 kda hiv tat-interacting protein (tip30). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 2225–2234. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Fu, J.; Zhi, F.; Liu, W.J.; Guo, Y.J.; Zhu, D.D.; Mo, J.G. The effects of interventional therapy on serum htatip2/tip30, b7-h4 and short-term curative effect in primary hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6778–6783. [Google Scholar] [PubMed]
- Chen, H.Y.; Yu, S.L.; Chen, C.H.; Chang, G.C.; Chen, C.Y.; Yuan, A.; Cheng, C.L.; Wang, C.H.; Terng, H.J.; Kao, S.F.; et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N. Engl. J. Med. 2007, 356, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Baty, F.; Facompre, M.; Kaiser, S.; Schumacher, M.; Pless, M.; Bubendorf, L.; Savic, S.; Marrer, E.; Budach, W.; Buess, M.; et al. Gene profiling of clinical routine biopsies and prediction of survival in non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 2010, 181, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Q.; Ding, K.; Strumpf, D.; Weir, B.A.; Meyerson, M.; Pennell, N.; Thomas, R.K.; Naoki, K.; Ladd-Acosta, C.; Liu, N.; et al. Prognostic and predictive gene signature for adjuvant chemotherapy in resected non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 4417–4424. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Takano, A.; Oshita, H.; Akiyama, H.; Tsuchiya, E.; Kohno, N.; Nakamura, Y.; Daigo, Y. Chondrolectin is a novel diagnostic biomarker and a therapeutic target for lung cancer. Clin. Cancer Res. 2011, 17, 7712–7722. [Google Scholar] [CrossRef] [PubMed]
- Botling, J.; Edlund, K.; Lohr, M.; Hellwig, B.; Holmberg, L.; Lambe, M.; Berglund, A.; Ekman, S.; Bergqvist, M.; Ponten, F.; et al. Biomarker discovery in non-small cell lung cancer: Integrating gene expression profiling, meta-analysis, and tissue microarray validation. Clin. Cancer Res. 2013, 19, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Widlak, P.; Pietrowska, M.; Polanska, J.; Marczyk, M.; Ros-Mazurczyk, M.; Dziadziuszko, R.; Jassem, J.; Rzyman, W. Serum mass profile signature as a biomarker of early lung cancer. Lung Cancer 2016, 99, 46–52. [Google Scholar] [CrossRef]
- Tong, X.; Li, K.; Luo, Z.; Lu, B.; Liu, X.; Wang, T.; Pang, M.; Liang, B.; Tan, M.; Wu, M.; et al. Decreased tip30 expression promotes tumor metastasis in lung cancer. Am. J. Pathol. 2009, 174, 1931–1939. [Google Scholar] [CrossRef]
- Okayama, H.; Kohno, T.; Ishii, Y.; Shimada, Y.; Shiraishi, K.; Iwakawa, R.; Furuta, K.; Tsuta, K.; Shibata, T.; Yamamoto, S.; et al. Identification of genes upregulated in alk-positive and egfr/kras/alk-negative lung adenocarcinomas. Cancer Res. 2012, 72, 100–111. [Google Scholar] [CrossRef]
- PrognoScan. Available online: http://dna00.bio.kyutech.ac.jp/PrognoScan/ (accessed on 3 December 2018).
- Cochrane Collaboration. Available online: https://community.cochrane.org/help/tools-and-software/revman-5/revman-5-download (accessed on 3 December 2018).
- Lee, E.S.; Son, D.S.; Kim, S.H.; Lee, J.; Jo, J.; Han, J.; Kim, H.; Lee, H.J.; Choi, H.Y.; Jung, Y.; et al. Prediction of recurrence-free survival in postoperative non-small cell lung cancer patients by using an integrated model of clinical information and gene expression. Clin. Cancer Res. 2008, 14, 7397–7404. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.D.; Yin, X.; Hoadley, K.A.; Liu, Y.; Hayward, M.C.; Cabanski, C.R.; Muldrew, K.; Miller, C.R.; Randell, S.H.; Socinski, M.A.; et al. Lung squamous cell carcinoma mrna expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clin. Cancer Res. 2010, 16, 4864–4875. [Google Scholar] [CrossRef] [PubMed]
- Shedden, K.; Taylor, J.M.; Enkemann, S.A.; Tsao, M.S.; Yeatman, T.J.; Gerald, W.L.; Eschrich, S.; Jurisica, I.; Giordano, T.J.; Misek, D.E.; et al. Gene expression-based survival prediction in lung adenocarcinoma: A multi-site, blinded validation study. Nat. Med. 2008, 14, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Lyons-Weiler, J.; Coello, M.C.; Huang, X.; Gooding, W.E.; Luketich, J.D.; Godfrey, T.E. Prediction of lymph node metastasis by analysis of gene expression profiles in primary lung adenocarcinomas. Clin. Cancer Res. 2005, 11, 4128–4135. [Google Scholar] [CrossRef] [PubMed]
- Tomida, S.; Takeuchi, T.; Shimada, Y.; Arima, C.; Matsuo, K.; Mitsudomi, T.; Yatabe, Y.; Takahashi, T. Relapse-related molecular signature in lung adenocarcinomas identifies patients with dismal prognosis. J. Clin. Oncol. 2009, 27, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Bild, A.H.; Yao, G.; Chang, J.T.; Wang, Q.; Potti, A.; Chasse, D.; Joshi, M.B.; Harpole, D.; Lancaster, J.M.; Berchuck, A.; et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006, 439, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Tomida, S.; Koshikawa, K.; Yatabe, Y.; Harano, T.; Ogura, N.; Mitsudomi, T.; Some, M.; Yanagisawa, K.; Takahashi, T.; Osada, H.; et al. Gene expression-based, individualized outcome prediction for surgically treated lung cancer patients. Oncogene 2004, 23, 5360–5370. [Google Scholar] [CrossRef] [PubMed]
- Raponi, M.; Zhang, Y.; Yu, J.; Chen, G.; Lee, G.; Taylor, J.M.; Macdonald, J.; Thomas, D.; Moskaluk, C.; Wang, Y.; et al. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res. 2006, 66, 7466–7472. [Google Scholar] [CrossRef]
- Dong, W.; Shen, R.; Cheng, S. Reduction of tip30 in esophageal squamous cell carcinoma cells involves promoter methylation and microrna-10b. Biochem. Biophys. Res. Commun. 2014, 453, 772–777. [Google Scholar] [CrossRef]
- Ito, M.; Jiang, C.; Krumm, K.; Zhang, X.; Pecha, J.; Zhao, J.; Guo, Y.; Roeder, R.G.; Xiao, H. Tip30 deficiency increases susceptibility to tumorigenesis. Cancer Res. 2003, 63, 8763–8767. [Google Scholar]
- Zhang, C.; Mori, M.; Gao, S.; Li, A.; Hoshino, I.; Aupperlee, M.D.; Haslam, S.Z.; Xiao, H. Tip30 deletion in mmtv-neu mice leads to enhanced egfr signaling and development of estrogen receptor-positive and progesterone receptor-negative mammary tumors. Cancer Res. 2010, 70, 10224–10233. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.C.; Yang, Z.L. Clinicopathologic significance of minichromosome maintenance protein 2 and tat-interacting protein 30 expression in benign and malignant lesions of the gallbladder. Hum. Pathol. 2011, 42, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jing, W.; Hu, X.; Zhou, X.; Liu, L.; Zhu, M.; Yin, F.; Chen, R.; Zhao, J.; Guo, Y. Decreased tip30 expression predicts poor prognosis in pancreatic cancer patients. Int. J. Cancer 2014, 134, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E. Tip30, a cofactor for HIV-1 tat-activated transcription, is homologous to short-chain dehydrogenases/reductases. Curr. Biol. 1999, 9, R471. [Google Scholar] [CrossRef]
- Xiao, H.; Palhan, V.; Yang, Y.; Roeder, R.G. Tip30 has an intrinsic kinase activity required for up-regulation of a subset of apoptotic genes. EMBO J. 2000, 19, 956–963. [Google Scholar] [CrossRef]
- Baker, M.E.; Yan, L.; Pear, M.R. Three-dimensional model of human tip30, a coactivator for hiv-1 tat-activated transcription, and cc3, a protein associated with metastasis suppression. Cell. Mol. Life Sci. 2000, 57, 851–858. [Google Scholar] [CrossRef]
- King, F.W.; Shtivelman, E. Inhibition of nuclear import by the proapoptotic protein cc3. Mol. Cell. Biol. 2004, 24, 7091–7101. [Google Scholar] [CrossRef]
- Yang, W.; Xiao, L.; Li, C.; Liu, X.; Liu, M.; Shao, Q.; Wang, D.; Huang, A.; He, C. Tip30 inhibits oligodendrocyte precursor cell differentiation via cytoplasmic sequestration of olig1. Glia 2015, 63, 684–698. [Google Scholar] [CrossRef]
- Xu, T.; Jin, Z.; Yuan, Y.; Zheng, H.; Li, C.; Hou, W.; Guo, Q.; Hua, B. Tat-interacting protein 30 (tip30) expression serves as a new biomarker for tumor prognosis: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0168408. [Google Scholar] [CrossRef]
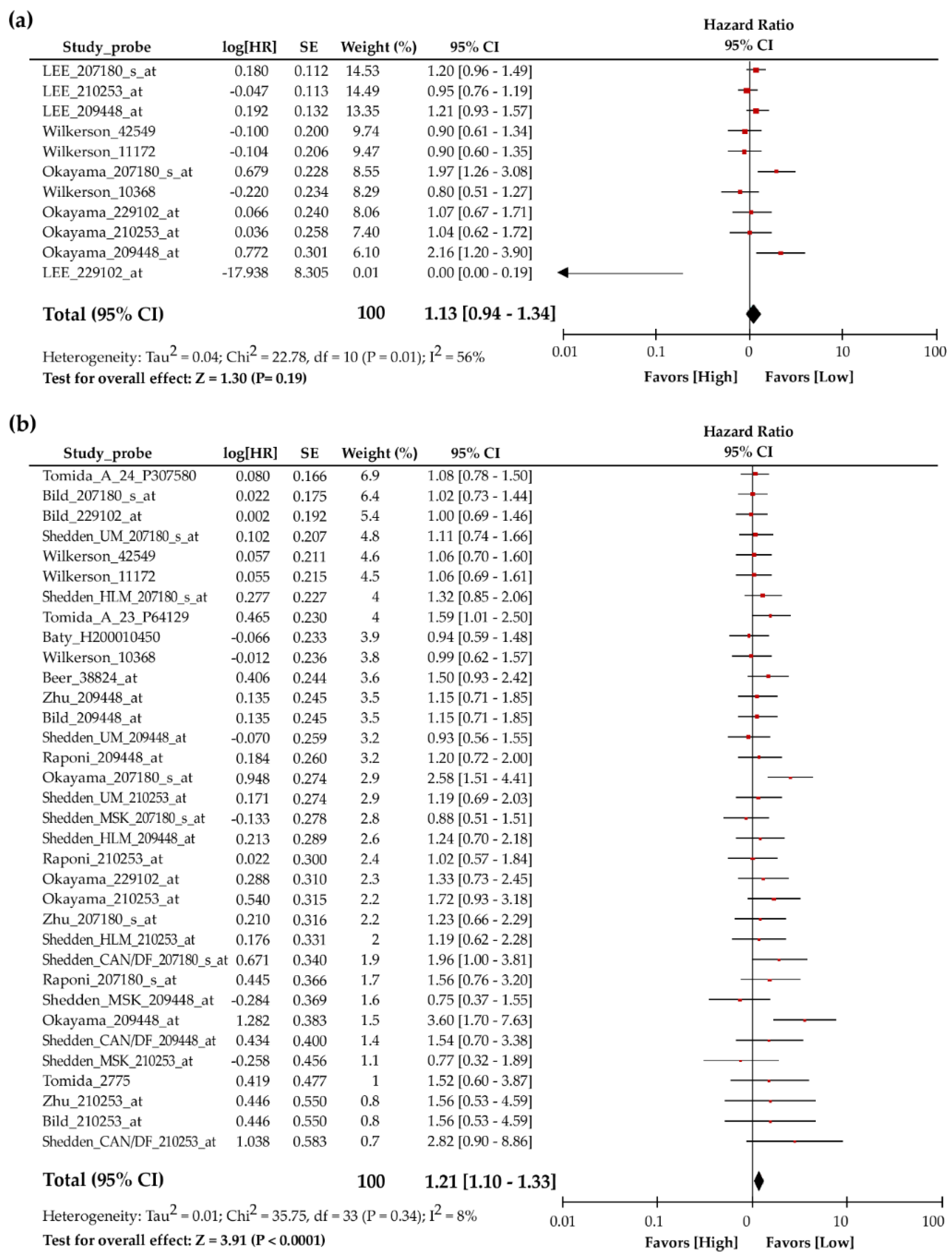

| Dataset | Contributor | Array Type | Probe ID | N | ln(HR) | HR (95% CI-Low CI-Upp) | Ref |
|---|---|---|---|---|---|---|---|
| GSE31210 | Okayama | HG-U133_Plus_2 | 229102_at | 204 | 0.065749 | 1.07 [0.67–1.71] | [30] |
| 209448_at | 204 | 0.771746 | 2.16 [1.20–3.90] | ||||
| 210253_at | 204 | 0.035683 | 1.04 [0.62–1.72] | ||||
| 207180_s_at | 204 | 0.678586 | 1.97 [1.26–3.08] | ||||
| GSE8894 | Lee | HG-U133_Plus_2 | 210253_at | 138 | −0.04662 | 0.95 [0.76–1.19] | [33] |
| 209448_at | 138 | 0.191915 | 1.21 [0.93–1.57] | ||||
| 207180_s_at | 138 | 0.179512 | 1.20 [0.96–1.49] | ||||
| 229102_at | 138 | −17.9383 | 0.00 [0.00–0.19] | ||||
| GSE17710 | Wilkerson | Agilent-UNC-custom-4X44K | 10368 | 56 | −0.22019 | 0.80 [0.51–1.27] | [34] |
| 42549 | 56 | −0.10024 | 0.90 [0.61–1.34] | ||||
| 11172 | 56 | −0.10444 | 0.90 [0.60–1.35] |
| Dataset | Contributor | Array Type | Probe ID | N | ln(HR) | HR (95% CI-Low CI-Upp) | Ref |
|---|---|---|---|---|---|---|---|
| jacob-00182-CANDF | Shedden | HG-U133A | 209448_at | 82 | 0.433604 | 1.54 [0.70–3.38] | [35] |
| 210253_at | 82 | 1.03816 | 2.82 [0.90–8.86] | ||||
| 207180_s_at | 82 | 0.670805 | 1.96 [1.00–3.81] | ||||
| HARVARD-LC | Beer | HG-U95A | 38824_at | 84 | 0.406418 | 1.50 [0.93–2.42] | [36] |
| jacob-00182-HLM | Shedden | HG-U133A | 209448_at | 79 | 0.212509 | 1.24 [0.70–2.18] | [35] |
| 207180_s_at | 79 | 0.277452 | 1.32 [0.85–2.06] | ||||
| 210253_at | 79 | 0.176389 | 1.19 [0.62–2.28] | ||||
| jacob-00182-MSK | Shedden | HG-U133A | 207180_s_at | 104 | −0.13325 | 0.88 [0.51–1.51] | [35] |
| 209448_at | 104 | −0.28405 | 0.75 [0.36–1.55] | ||||
| 210253_at | 104 | −0.25751 | 0.77 [0.32–1.89] | ||||
| GSE13213 | Tomida | G4112F | A_23_P64129 | 117 | 0.464958 | 1.59 [1.01–2.50] | [37] |
| A_24_P307580 | 117 | 0.080112 | 1.08 [0.78–1.50] | ||||
| GSE31210 | Okayama | HG-U133_Plus_2 | 229102_at | 204 | 0.288019 | 1.33 [0.73–2.45] | [30] |
| 209448_at | 204 | 1.28166 | 3.60 [1.70–7.63] | ||||
| 210253_at | 204 | 0.54017 | 1.72 [0.93–3.18] | ||||
| 207180_s_at | 204 | 0.947811 | 2.58 [1.51–4.41] | ||||
| jacob-00182-UM | Shedden | HG-U133A | 209448_at | 178 | −0.06997 | 0.93 [0.56–1.55] | [35] |
| 210253_at | 178 | 0.170614 | 1.19 [0.69–2.03] | ||||
| 207180_s_at | 178 | 0.101831 | 1.11 [0.74–1.66] | ||||
| GSE11117 | Baty | Novachip human 34.5k | H200010450 | 41 | −0.06557 | 0.94 [0.59–1.48] | [24] |
| GSE3141 | Bild | HG-U133_Plus_2 | 209448_at | 111 | 0.072535 | 1.08 [0.71–1.62] | [38] |
| 210253_at | 111 | −0.1588 | 0.85 [0.53–1.38] | ||||
| 207180_s_at | 111 | 0.02155 | 1.02 [0.73–1.44] | ||||
| 229102_at | 111 | 0.001905 | 1.00 [0.69–1.46] | ||||
| GSE14814 | Zhu | HG-U133A | 209448_at | 90 | 0.13546 | 1.15 [0.71–1.85] | [25] |
| 210253_at | 90 | 0.445921 | 1.56 [0.53–4.59] | ||||
| 207180_s_at | 90 | 0.209906 | 1.23 [0.66–2.29] | ||||
| GSE4716-GPL3694 | Tomida | GF200 | 2775 | 50 | 0.41877 | 1.52 [0.60–3.87] | [39] |
| GSE4573 | Raponi | HG-U133A | 207180_s_at | 129 | 0.445355 | 1.56 [0.76–3.20] | [40] |
| 209448_at | 129 | 0.183704 | 1.20 [0.72–2.00] | ||||
| 210253_at | 129 | 0.021825 | 1.02 [0.57–1.84] | ||||
| GSE17710 | Wilkerson | Agilent-UNC-custom-4X44K | 42549 | 56 | 0.056871 | 1.06 [0.70–1.60] | [34] |
| 11172 | 56 | 0.055057 | 1.06 [0.69–1.61] | ||||
| 10368 | 56 | −0.01173 | 0.99 [0.62–1.57] |
| TIP30 Expression | |||
|---|---|---|---|
| Characteristics | Low (0, 1) (n = 67) | High (2, 3) (n = 46) | P-Value |
| Age | 0.962 † | ||
| Years (mean ± SD) | 62.10 ± 10.06 | 62.20 ± 9.85 | |
| Gender | 0.498 ‡ | ||
| Male | 35 | 27 | |
| Female | 32 | 19 | |
| Stage | 0.013 ‡* | ||
| I | 14 | 16 | |
| II | 4 | 9 | |
| III | 21 | 12 | |
| IV | 28 | 9 | |
| Tumor status | 0.929 ‡ | ||
| T1 | 11 | 9 | |
| T2 | 32 | 23 | |
| T3 | 4 | 2 | |
| T4 | 20 | 12 | |
| Lymph node status | 0.059 ‡ | ||
| N0 | 19 | 21 | |
| N1–3 | 48 | 25 | |
| Distal metastasis status | 0.027 ‡* | ||
| M0 | 39 | 36 | |
| M1 | 28 | 10 | |
| Histologic type | 0.016 ‡* | ||
| Adenocarcinoma | 31 | 30 | |
| Squamous cell | 27 | 16 | |
| Large cell | 9 | 0 | |
| Recurrence | 0.002 ‡* | ||
| No | 27 | 32 | |
| Yes | 40 | 14 | |
| Smoking status | 0.093 ‡ | ||
| No | 34 | 16 | |
| Yes | 33 | 30 | |
| Cox univariate analysis (OS) | |||
| Variables | Comparison | HR (95% CI) | P-value |
| T | T1; T2−T4 | 2.099 (1.157−3.807) | 0.015 * |
| N | N0; N1−N3 | 2.803 (1.705−4.608) | <0.001 * |
| M | M0; M1 | 2.952 (1.881−4.632) | <0.001 * |
| TIP30 | Low (0, 1); High (2, 3) | 0.410 (0.252−0.667) | <0.001 * |
| Cox multivariate analysis (OS) | |||
| Variables | Comparison | HR (95% CI) | P-value |
| T | T1; T2−T4 | 1.603 (0.879−2.924) | 0.124 |
| N | N0; N1−N3 | 2.061 (1.240−3.424) | 0.005 * |
| M | M0; M1 | 2.121 (1.338−3.361) | 0.001 * |
| TIP30 | Low (0, 1); High (2, 3) | 0.550 (0.333−0.909) | 0.020 * |
| Cox univariate analysis (PFS) | |||
| Variables | Comparison | HR (95% CI) | P-value |
| T | T1; T2−T4 | 1.608 (0.781−3.313) | 0.198 |
| N | N0; N1−N3 | 3.238 (1.668−6.286) | 0.001 * |
| M | M0; M1 | 2.525 (1.453−4.387) | 0.001 * |
| TIP30 | Low (0, 1); High (2, 3) | 0.384 (0.206−0.715) | 0.003 * |
| Cox multivariate analysis (PFS) | |||
| Variables | Comparison | HR (95% CI) | P-value |
| T | T1; T2−T4 | 1.148 (0.555−2.378) | 0.709 |
| N | N0; N1−N3 | 2.634 (1.340−5.176) | 0.005 * |
| M | M0; M1 | 1.810 (1.028−3.187) | 0.040 * |
| TIP30 | Low (0, 1); High (2, 3) | 0.513 (0.271−0.970) | 0.040 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-J.; Chou, P.-A.; Huang, M.-S.; Liu, Y.-P. Low TIP30 Protein Expression is Associated with a High Risk of Metastasis and Poor Prognosis for Non-Small-Cell Lung Cancer. J. Clin. Med. 2019, 8, 83. https://doi.org/10.3390/jcm8010083
Chen C-J, Chou P-A, Huang M-S, Liu Y-P. Low TIP30 Protein Expression is Associated with a High Risk of Metastasis and Poor Prognosis for Non-Small-Cell Lung Cancer. Journal of Clinical Medicine. 2019; 8(1):83. https://doi.org/10.3390/jcm8010083
Chicago/Turabian StyleChen, Chao-Ju, Po-An Chou, Ming-Shyan Huang, and Yu-Peng Liu. 2019. "Low TIP30 Protein Expression is Associated with a High Risk of Metastasis and Poor Prognosis for Non-Small-Cell Lung Cancer" Journal of Clinical Medicine 8, no. 1: 83. https://doi.org/10.3390/jcm8010083
APA StyleChen, C.-J., Chou, P.-A., Huang, M.-S., & Liu, Y.-P. (2019). Low TIP30 Protein Expression is Associated with a High Risk of Metastasis and Poor Prognosis for Non-Small-Cell Lung Cancer. Journal of Clinical Medicine, 8(1), 83. https://doi.org/10.3390/jcm8010083





