Non-Invasive Assessment Method Using Thoracic-Abdominal Profile Image Acquisition and Mathematical Modeling with Bezier Curves
Abstract
1. Introduction
1.1. Hepatic Steatosis: Investigation Methods
1.1.1. Non-Invasive Methods
1.1.2. Measurement of Body Fat and Fat Distribution
1.1.3. Waist Circumference (WC) Is a Simple and Inexpensive Tool for Assessing Body Fat Distribution
1.2. Bezier Curves
1.2.1. Theoretical Consideration
1.2.2. Bezier Curves, Mathematical Formulation
- 1.
- The ends of the curve are tangent to the segmentsrespectively;
- 2.
- A Bézier curve can be divided into two Bézier curves in any of its points;
- 3.
- Bézier curve is entirely included in the convex cover of its control points;
- 4.
- A quadratic Bézier curve is a parabola segment;
- 5.
- By translating or rotating a Bézier curve, another Bézier curve is obtained.
2. Experimental Section
2.1. Ethical Statement
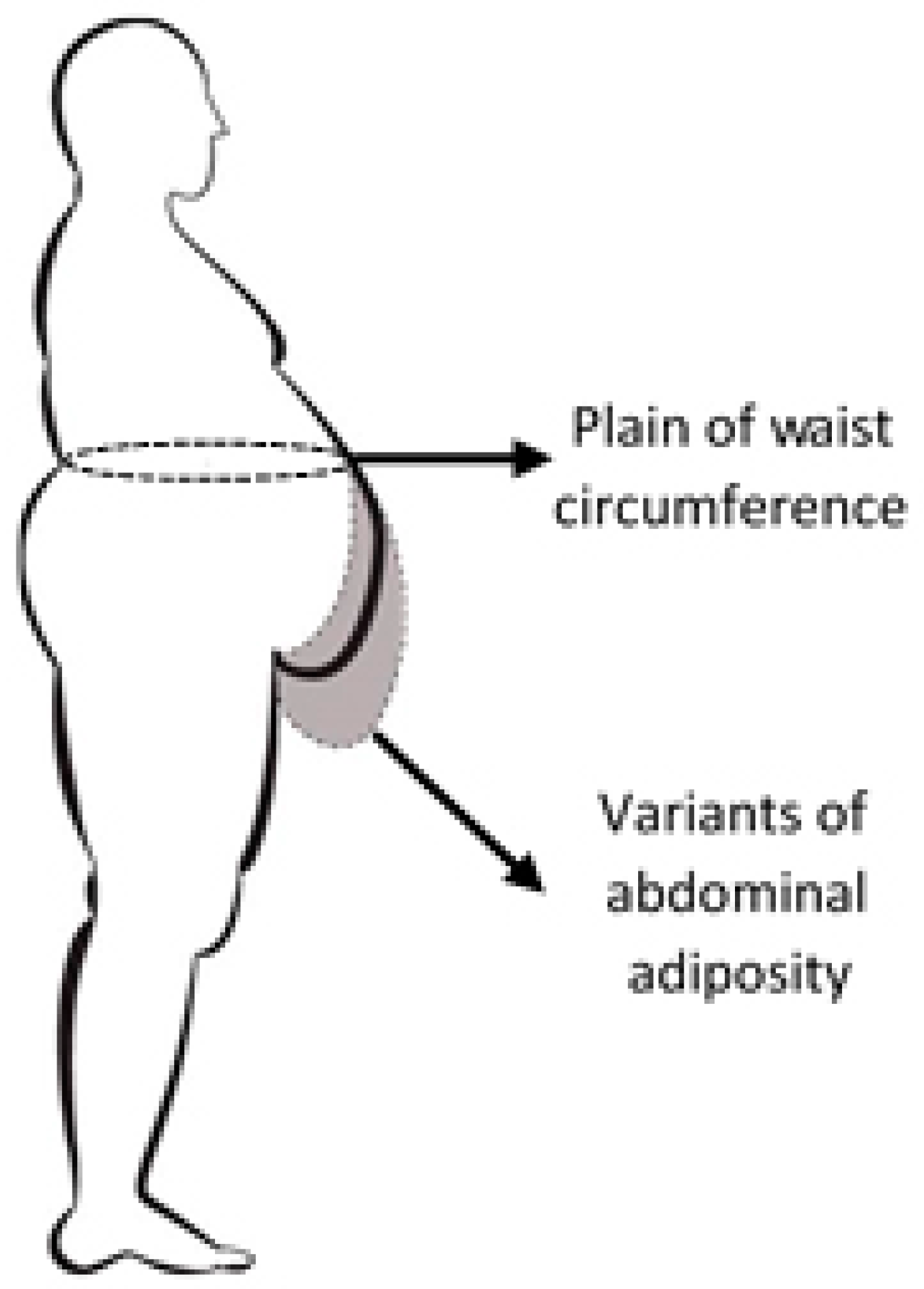
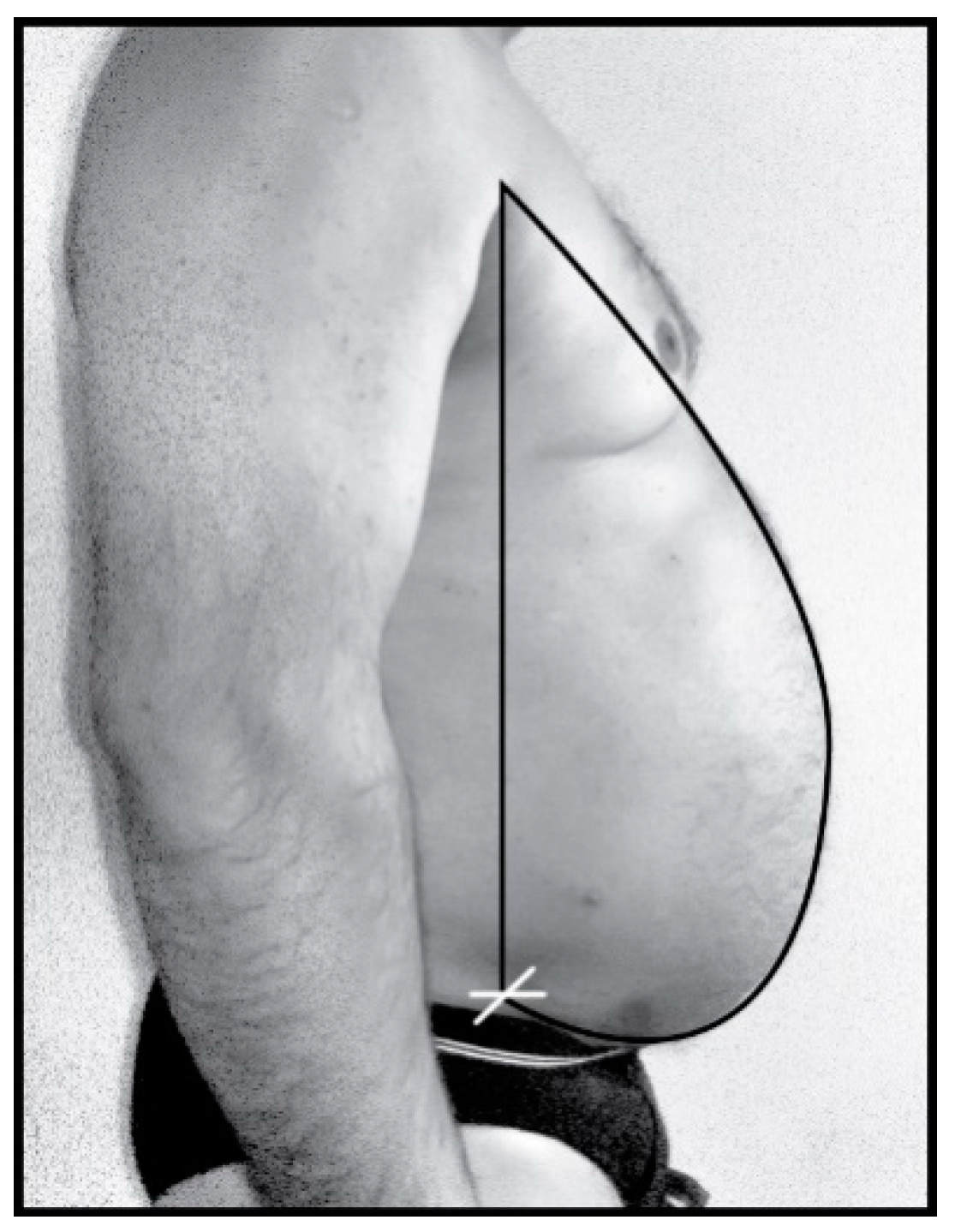
2.2. Experimental Method of Designing Thoracic-Abdominal Profile
2.3. Research Mathematical Model Based on Bezier Curves
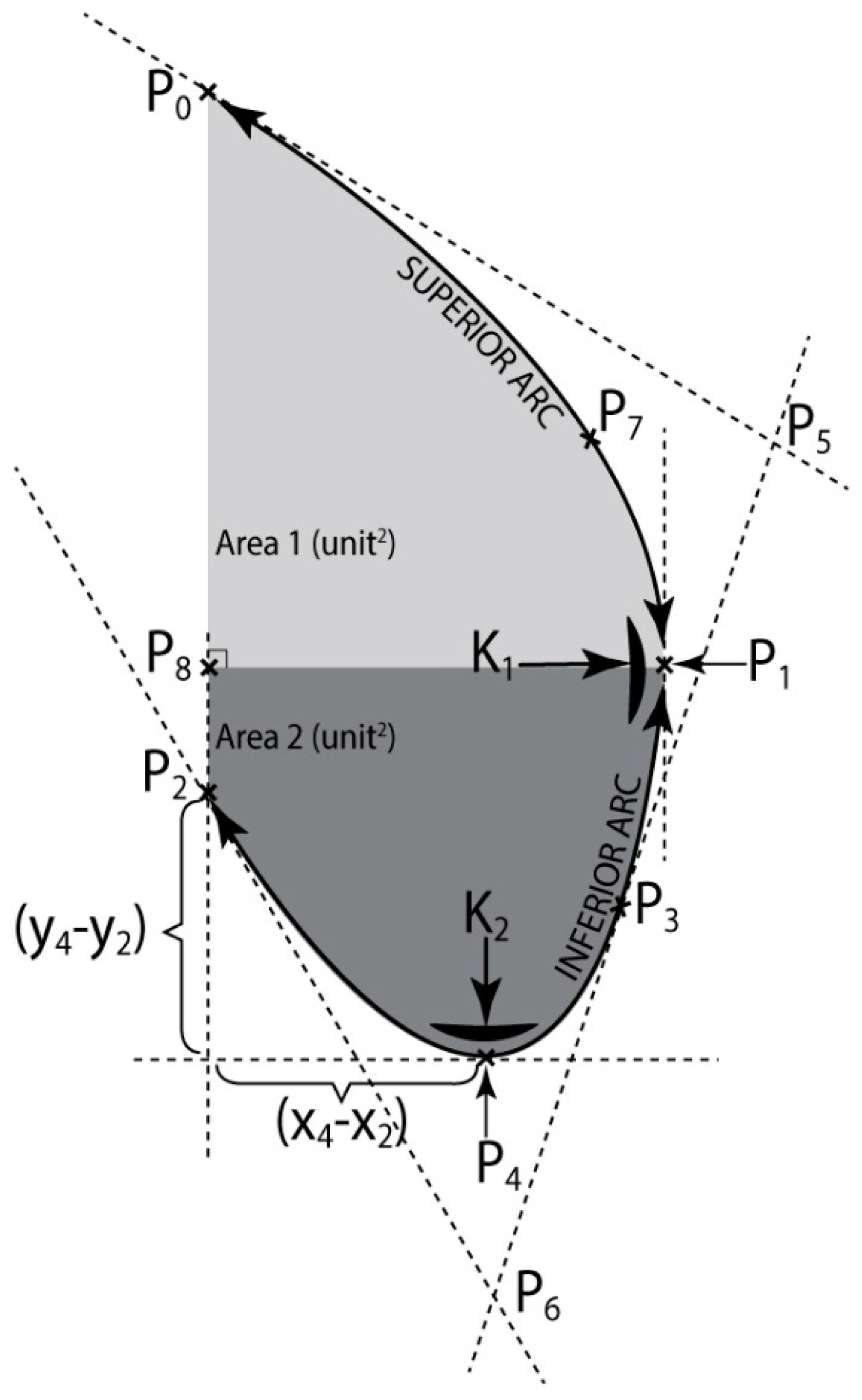
2.3.1. Calculus of Thoracic-Abdominal Curve Parameters
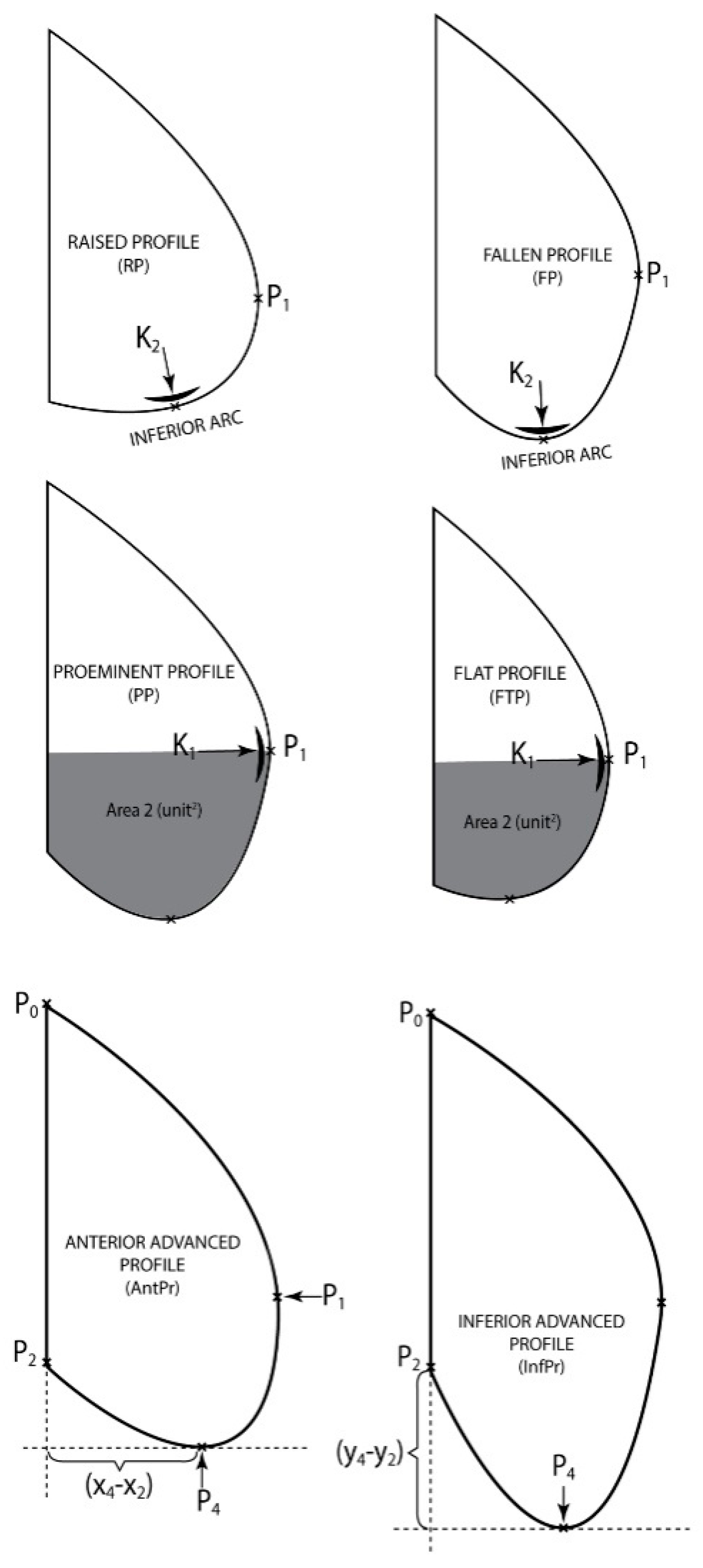
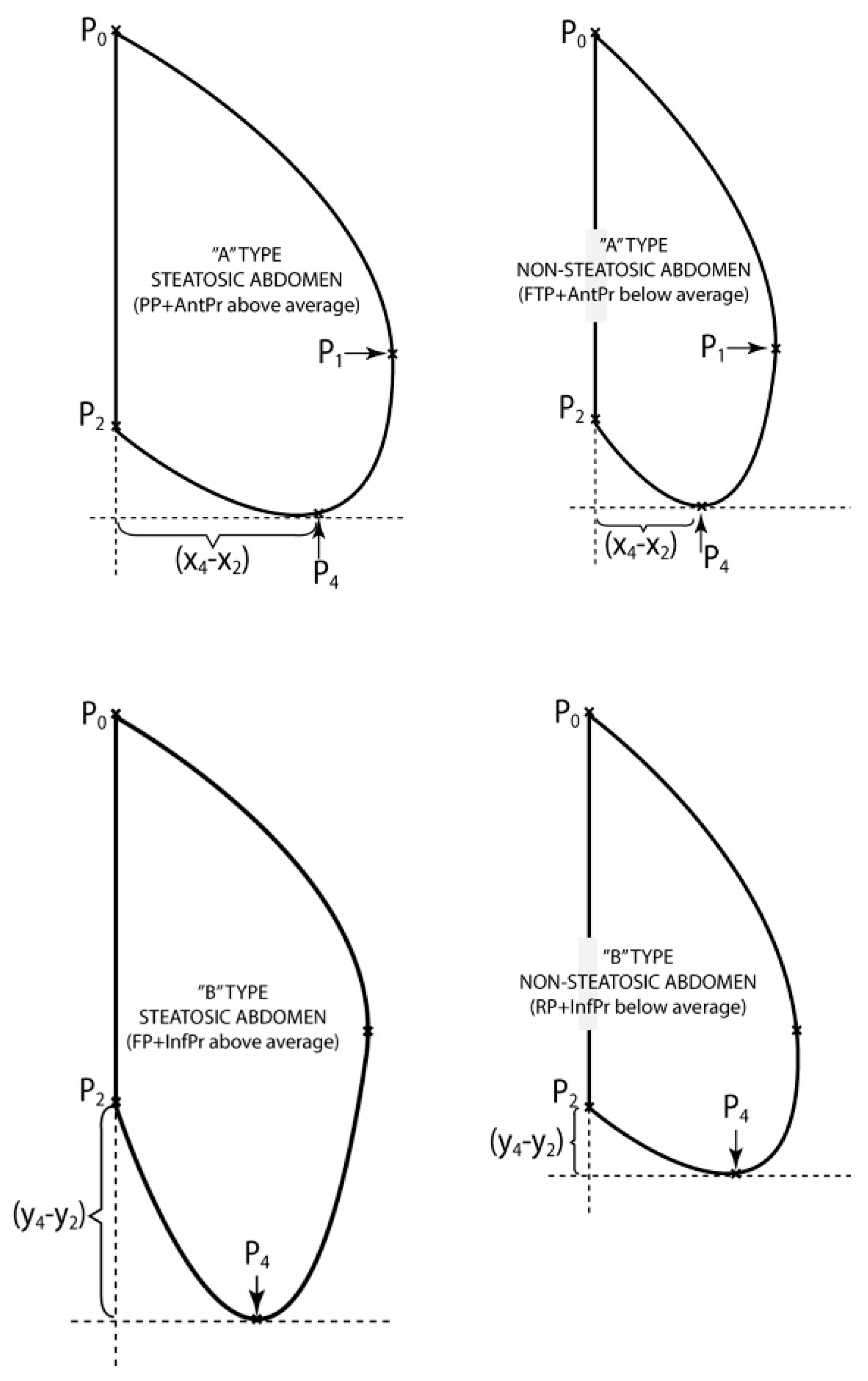
2.3.2. Classification of Abdominal Curves
- I
- The second classification has been made according to K1, in P1 and the area limited by the arc (P1P3P4P2) and the segments [P2P8] [P3P1], Area2, giving a clue on the size of the bottom part of the patient’s belly.The thoracic—abdominal profile curve is characterized by the fact that arcs (P7P1) and (P1P3), respectively are very far away from the vicinity of point P1, meaning that arc (P7P3) is almost the same with the segment [P7P3], that is the belly is straight at the front;The thoracic-abdominal profile curve is characterized by the fact that arcs (P7P1) and (P1P3), respectively are closer to the vicinity of point P1, meaning the belly is “pursed” towards the front part.
- II
- The third classification was done according to parameters (Anterior advancement) and (Inferior advancement)
2.4. Data Processing and Statistical Analysis
2.4.1. Data Processing
2.4.2. Statistical Analysis of the Obtained Data
3. Results
Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- AlShaalan, R.; Aljiffry, M.; Al-Busafi, S.; Metrakos, P.; Hassanain, M. Nonalcoholic fatty liver disease: Noninvasive methods of diagnosing hepatic steatosis. Saudi J. Gastroenterol. 2015, 21, 64–70. [Google Scholar] [PubMed]
- Wong, V.W.S.; Wong, G.L.H.; Choi, P.C.L.; Chan, A.W.H.; Li, M.K.P.; Chan, H.Y.; Chim, A.M.L.; Yu, J.; Sung, J.J.Y.; Chan, H.L.Y. Disease progression of non-alcoholic fatty liver disease: A prospective study with paired liver biopsies at 3 years. Gut 2010, 59, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.A.; Abu-Rashed, A.H. Liver biopsy remains the gold standard for evaluation of chronic hepatitis and fibrosis. J. Gastrointest. Liver Dis. 2007, 16, 425–426. [Google Scholar]
- Karlas, T.; Petroff, D.; Garnov, N.; Böhm, S.; Tenckhoff, H.; Wittekind, C.; Wiese, M.; Schiefke, I.; Linder, N.; Schaudinn, A.; Busse, H. Non-Invasive Assessment of Hepatic Steatosis in Patients with NAFLD Using Controlled Attenuation Parameter and 1H-MR Spectroscopy. PLoS ONE 2014, 9, e91987. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Chen, J.J.; Chen, J.; Li, L.; Lin, Z.Q.; Wang, W.J.; Xu, J.R. Nonalcoholic fatty liver disease: Quantitative assessment of liver fat content by computed tomography, magnetic resonance imaging and proton magnetic resonance spectroscopy. J. Dig. Dis. 2009, 10, 315–320. [Google Scholar] [CrossRef]
- Myers, R.P.; Pollett, A.; Kirsch, R.; Pomier-Layrargues, G.; Beaton, M.; Levstik, M.; Duarte-Rojo, A.; Wong, D.; Crotty, P.; Elkashab, M. Controlled Attenuation Parameter (CAP): A noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012, 32, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Al-Busafi, S.A.; Ghali, P.; Wong, P.; Novales-Diaz, J.A.; Deschênes, M. The utility of Xenon-133 liver scan in the diagnosis and management of nonalcoholic fatty liver disease. Can. J. Gastroenterol. 2012, 26, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Khov, N.; Sharma, A.; Riley, T.R. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 6821–6825. [Google Scholar] [CrossRef]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Joseph, R.; Lopez, R.; McCullough, A.J. Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. J. Hepatol. 2009, 51, 1061–1067. [Google Scholar] [CrossRef]
- Logue, E.; Smucker, W.D.; Bourguet, C.C. Identification of obesityWaistlines or weight? Nutrition, Exercise, and Obesity Research Group. J. Fam. Pract. 1995, 41, 357–363. [Google Scholar]
- The Royal Colleges of Physicians, Psychiatrists and General Practitioners. Available online: https://www.rcplondon.ac.uk/publications/alcohol-and-heart-perspective-0 (accessed on 24 August, 2018).
- Koteish, A.; Diehl, A.M. Animal models of steatosis. Semin. Liver Dis. 2001, 21, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Lotan, R.; Shlomai, A.; Webb, M.; Harrari, G.; Buch, A.; Kaluski, D.N.; Halpern, Z.; Oren, R. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J. Hepatol. 2012, 56, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Keach, J.C.; Batts, K.P.; Lindor, K.D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999, 30, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Frantzides, C.T.; Carlson, M.A.; Moore, R.E.; Zografakis, J.G.; Madan, A.K.; Puumala, S.; Keshavarzian, A. Effect of body mass index on nonalcoholic fatty liver disease in patients undergoing minimally invasive bariatric surgery. J. Gastrointest. Surg. 2004, 8, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.J.; Lee, K.E.; Kim, D.J.; Kim, S.K.; Ahn, C.W.; Lim, S.K.; Kim, K.R.; Lee, H.C.; Huh, K.B.; et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch. Intern. Med. 2004, 164, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.A.; Chuang, W.Y.; Hsu, K.; Pham, H.T. Design of a Bézier-profile horn for high displacement amplification. Ultrasonics 2011, 51, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.E.; Dyer, A.G.; Greentree, A.D.; Spring, G.; Wilksch, P.A. Linearisation of RGB Camera Responses for Quantitative Image Analysis of Two Techniques. PLoS ONE 2013, 8, e79534. [Google Scholar] [CrossRef]
- Murthy, A.; Bartocci, E.; Fenton, F.H.; Glimm, J.; Gray, R.A.; Cherry, E.M.; Smolka, S.A.; Grosu, R. Curvature Analysis of Cardiac Excitation Wavefronts. IEEE/ACM Trans. Comput. Biol. Bioinform. 2013, 10, 323–336. [Google Scholar] [CrossRef]
- Rogers, D.F.; Adams, J.A. Mathematical Elements for Computer Graphics, 2nd ed.; McGRAW-Hill: New York, NY, USA, 1990. [Google Scholar]
- Stupariu, M.S. Geometrie Computaţională, Note De Curs; University of Bucharest: Bucharest, Romania, 2013; pp. 18–27. [Google Scholar]
- Mullineux, G. CAD: Computational Concepts and Methods; MacMillan Publishing Company: New York, NY, USA, 1986; pp. 64–66. [Google Scholar]
- Shang, Y. Deffuant model with general opinion distributions: First impression and critical confidence bound. Complexity 2013, 19, 38–49. [Google Scholar] [CrossRef]
- Available online: https://intranet.unitbv.ro/Portals/0/etica%20si%20integritate%20academica_2018.pdf?ver=2018-11-29-090226-940 (accessed on 29 November 2018).
- Douglas, C.W. Clinical Methods: The History, Physical, and Laboratory Examinations. (Chapter 94 Evaluation of the Size, Shape, and Consistency of the Liver), 3rd ed.; Butterworths: Boston, MI, USA, 1990. [Google Scholar]
- Radmard, A.R.; Abrishami, A.; Gerami Seresht, M.; Yoonessi, A.; Dadgostar, M.; Hashemi Taheri, A.; Poustchi, H.; Merat, S.; Malekzadeh, R. Association between Abdominal Adiposity and Fatty Liver: A Non-Invasive Assessment in General Population by Magnetic Resonance Imaging. Available online: http://dx.doi.org/10.1594/ecr2014/C-1383 (accessed on 29 November 2018).
- Gelber, R.P.; Gaziano, J.M.; Orav, E.J.; Manson, J.E.; Buring, J.E.; Kurth, T. Measures of obesity and cardiovascular risk among men and women. J. Am. Coll. Cardiol. 2008, 52, 605–615. [Google Scholar] [CrossRef]
- Waist Circumference and Waist-Hip Rati. Report of a WHO Expert Consultation. Available online: https://www.who.int/nutrition/publications/obesity/WHO_report_waistcircumference_and_waisthip_ratio/en/ (accessed on 29 November 2018).
- WHO. Manual of Diagnostic Ultrasound. Available online: http://apps.who.int/iris/bitstream/handle/10665/43881/9789241547451_eng.pdf (accessed on 29 November 2018).
- Loomis, A.K.; Kabadi, S.; Preiss, D.; Hyde, C.; Bonato, V.; St. Louis, M.; Desai, J.; Gill, J.M.; Welsh, P.; Waterworth, D.; et al. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J. Clin. Endocrinol. Metab. 2016, 101, 945–952. [Google Scholar] [CrossRef] [PubMed]
| Group Measurements | Measurements | Abbreviations | Parameters of Interest |
|---|---|---|---|
| Geometric parameters | Superior arc length | SupArc | |
| Inferior arc length | InfArc | ✓ | |
| Total arc length | TotalArc | ||
| Superior curvature | K2 | ✓ | |
| Osculating circle radius up | R.osc.up | ||
| Inferior curvature | K1 | ✓ | |
| Osculating circle radius down | R.osc.down | ||
| Anterior push up | ✓ | ||
| Inferior push up | ✓ | ||
| Total area | |||
| Area above waist circumference | Area1 | ||
| Area below waist circumference | Area2 | ✓ | |
| Volume of area 2 | Vol. InfArc | ✓ | |
| Anthropological parameters | Height | ||
| Weight | |||
| Body mass index | BMI | ✓ | |
| Waist circumference | WC | ✓ | |
| Profile type (raised, fallen, flat, prominent, anterior advanced and inferior advanced) | RP, FP, FTP, PP, AntPr, InfPr | ✓ | |
| Ultrasound parameters | Anterior diameter of the right hepatic lobe | RHD | ✓ |
| Anterior diameter of the left hepatic lobe | LHD | ✓ | |
| Sum of hepatic lobes diameters | SHD | ✓ |
| Correlations | ||||
|---|---|---|---|---|
| RHD | LHD | SHD | ||
| WC | Pearson correlation (r) | 0,.253 * | 0.369 ** | 0.355 ** |
| Significance (p) | 0.011 | <0.001 | <0.001 | |
| BMI | Pearson correlation (r) | 0.159 | 0.197 * | 0.209 * |
| Significance (p) | 0.113 | 0.049 | 0.037 | |
| K2 | Pearson correlation (r) | 0.087 | −0.115 | 0.020 |
| Significance (p) | 0.390 | 0.253 | 0.845 | |
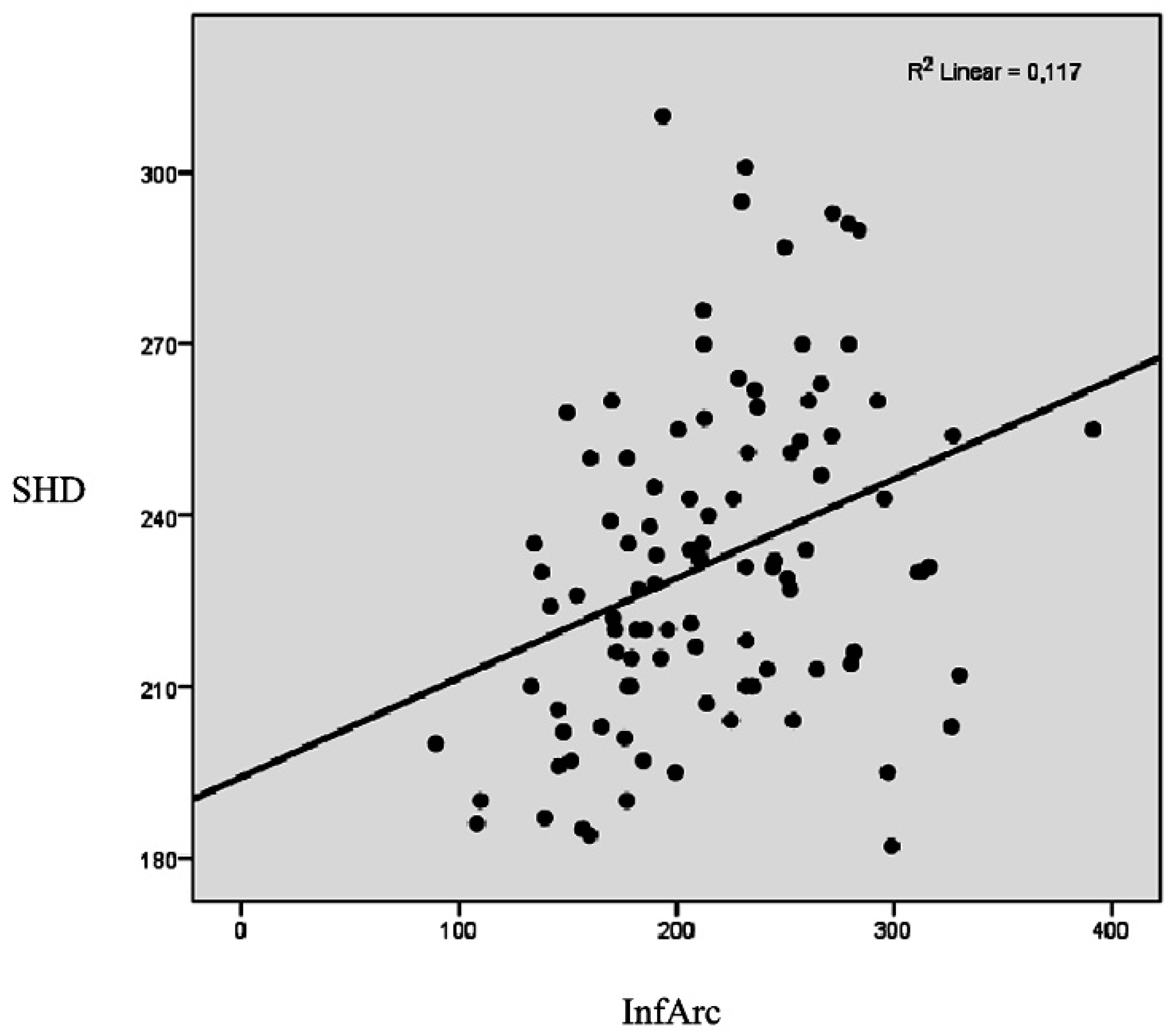
| InfArc | Pearson correlation (r) | 0.286 ** | 0.276 ** | 0.342 ** |
| Significance (p) | 0.004 | 0.005 | <0.001 | |
| Area2 | Pearson correlation (r) | 0.267 ** | 0.303 ** | 0.338 ** |
| Significance (p) | 0.007 | 0.002 | 0.001 | |
| Vol. InfArc | Pearson correlation (r) | 0.215 * | 0.195 | 0.252 * |
| Significance (p) | 0.031 | 0.051 | 0.011 | |
| Partial Correlations | |||
|---|---|---|---|
| SHD | |||
| Control Variables (Sex, Height, Weight) | InfArc | Correlation | 0.283 |
| p-value | 0.005 | ||
| Area2 | Correlation | 0.230 | |
| p-value | 0.024 | ||
| Vol. InfArc | Correlation | 0.121 | |
| p-value | 0.239 | ||
| t-Test for Equality of Means | |||
|---|---|---|---|
| p-Value | Mean Difference | ||
| SHD | Raised profile (RP) | 0.028 | 13.6 |
| Fallen profile (FP) | 0.373 | −6.7 | |
| Flat profile (FTP) | 0.237 | 7.1 | |
| Prominent profile (PP) | 0.024 | −18.1 | |
| t-Test for Equality of Means | |||
|---|---|---|---|
| p-Value | Mean Difference | ||
| Type A steatosis profile vs. Type A non-steatosis profile (PP + AntPr over average vs. FTP + AntPr below average) | RHD | 0.119 | −11.0 |
| LHD | 0.167 | −5.4 | |
| SHD | 0.071 | −16.4 | |
| Type B steatosis profile vs. Type B non-steatosis profile (FP + InfPr over average vs. RP + InfPr below average) | RHD | 0.025 | −20.6 |
| LHD | 0.905 | 0.7 | |
| SHD | 0.102 | −20.0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purcaru, M.A.P.; Repanovici, A.; Nedeloiu, T. Non-Invasive Assessment Method Using Thoracic-Abdominal Profile Image Acquisition and Mathematical Modeling with Bezier Curves. J. Clin. Med. 2019, 8, 65. https://doi.org/10.3390/jcm8010065
Purcaru MAP, Repanovici A, Nedeloiu T. Non-Invasive Assessment Method Using Thoracic-Abdominal Profile Image Acquisition and Mathematical Modeling with Bezier Curves. Journal of Clinical Medicine. 2019; 8(1):65. https://doi.org/10.3390/jcm8010065
Chicago/Turabian StylePurcaru, Monica Ana Paraschiva, Angela Repanovici, and Tiberiu Nedeloiu. 2019. "Non-Invasive Assessment Method Using Thoracic-Abdominal Profile Image Acquisition and Mathematical Modeling with Bezier Curves" Journal of Clinical Medicine 8, no. 1: 65. https://doi.org/10.3390/jcm8010065
APA StylePurcaru, M. A. P., Repanovici, A., & Nedeloiu, T. (2019). Non-Invasive Assessment Method Using Thoracic-Abdominal Profile Image Acquisition and Mathematical Modeling with Bezier Curves. Journal of Clinical Medicine, 8(1), 65. https://doi.org/10.3390/jcm8010065






