The Conditions Under Which Piracetam Is Used and the Factors That Can Improve National Institute of Health Stroke Scale Score in Ischemic Stroke Patients and the Importance of Previously Unnoticed Factors From a Hospital-Based Observational Study in Taiwan
Abstract
:1. Introduction
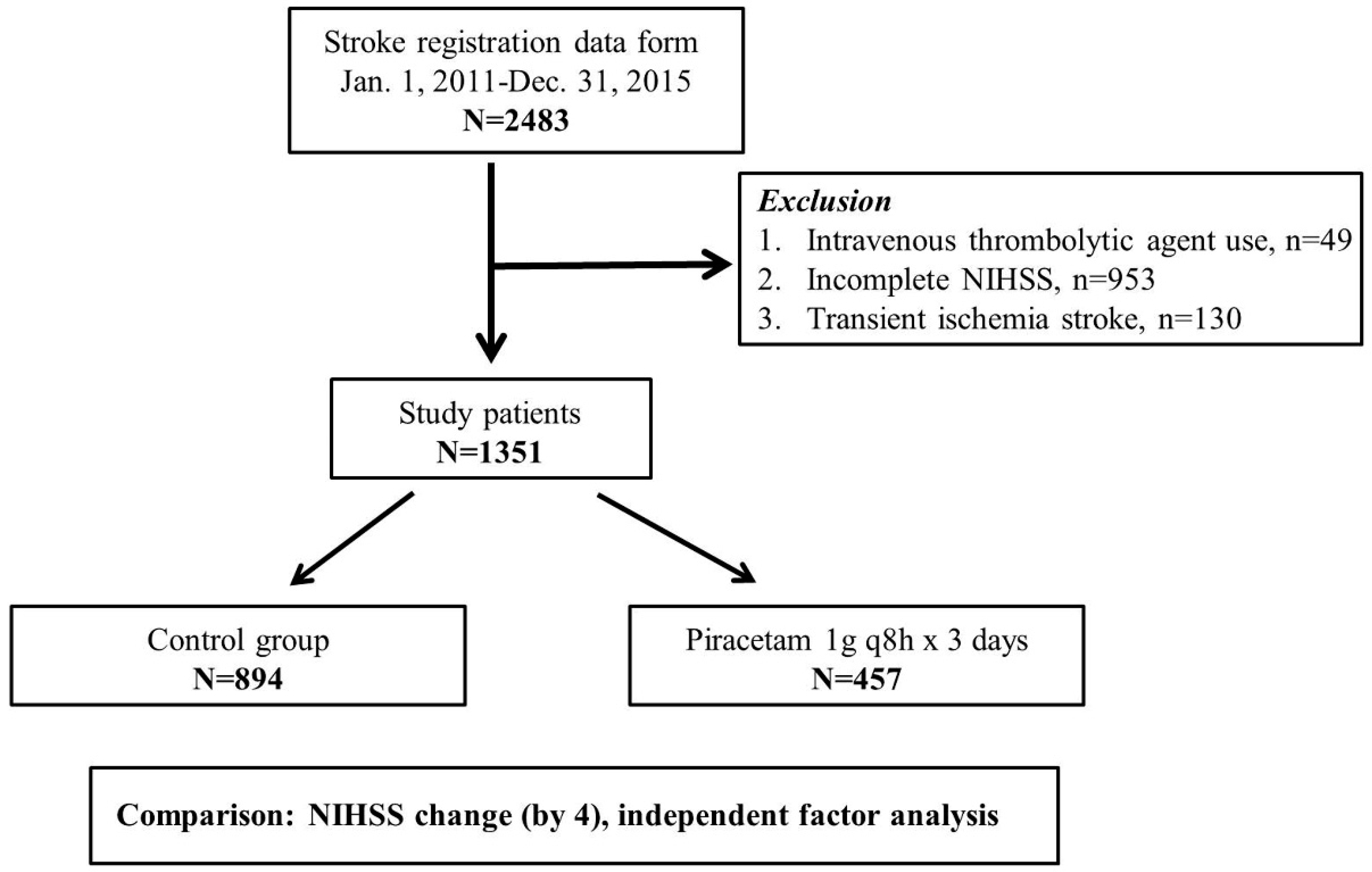
2. Materials and Methods
2.1. Source of Data and Study Cohort
- α = 0.05, Zα/2 = 1.96; β = 0.2, Zβ = 0.842
- p1: NIHSS improved rate of piracetam treatment; p2: NIHSS improved rate of no piracetam treatment; = (p1 + p2)/2
2.2. Definition of Epidemiological and Comorbidity Variables
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Balance of Piracetam and no Piracetam Subgroups between Baseline Epidemiological and Comorbidity Characteristics
3.2. The Factors Associated with Ischemic Stroke Based on Univariate Analysis
3.3. The Factors Associated with Ischemic Stroke Based on Multivariate Analysis
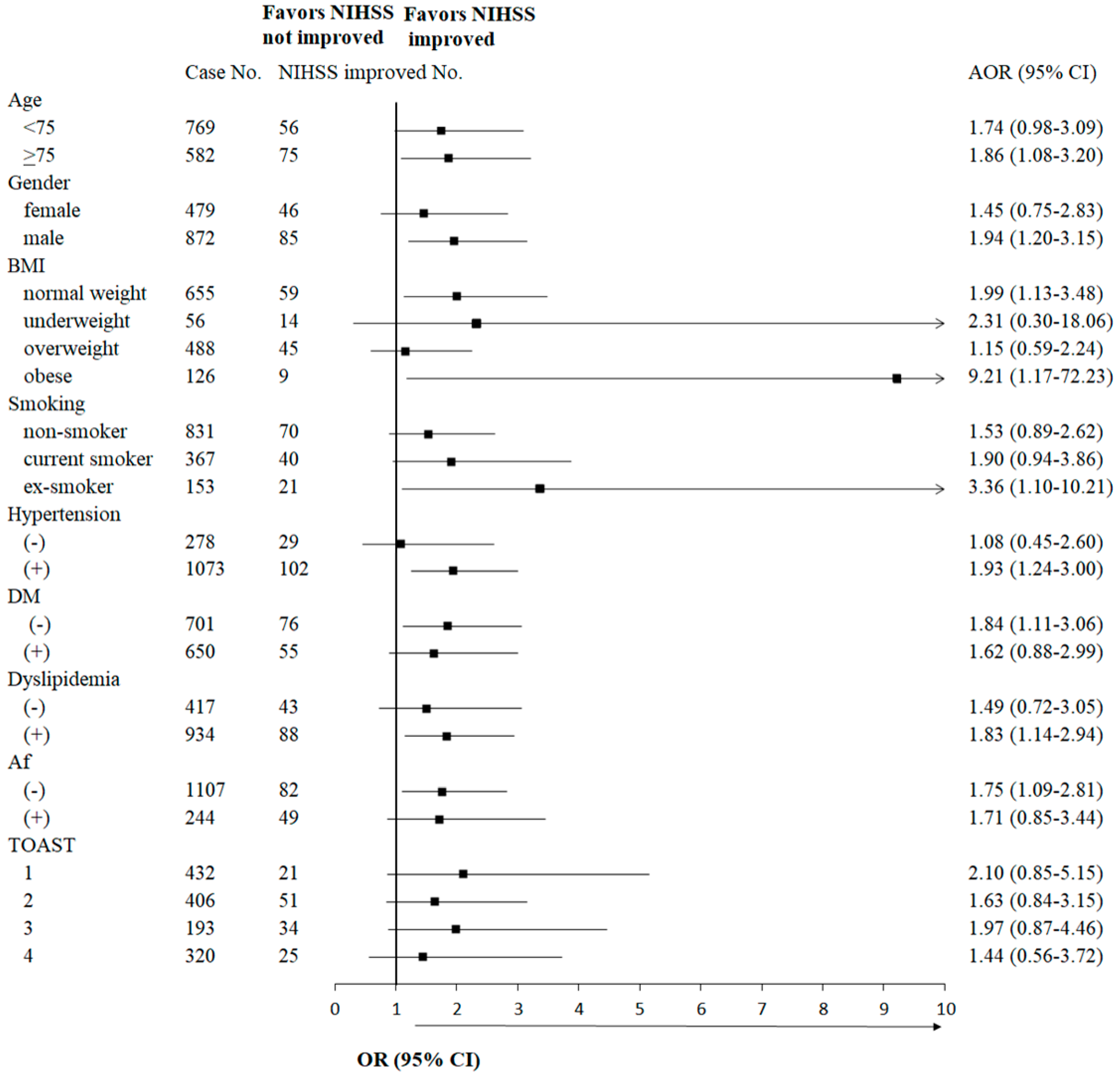
3.4. Sensitivity Analysis for Possible Interaction Test Among Piracetam and Other Covariates, and the Adjusted Ratio Difference in Favor of NIHSS Improvement for These Variables
3.5. Subgroup Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tuttolomondo, A.; Pecoraro, R.; Arnao, V.; Maugeri, R.; Iacopino, D.G.; Pinto, A. Developing drug strategies for the neuroprotective treatment of acute ischemic stroke. Expert Rev. Neurother. 2015, 46, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Derdeyn, C.P.; Biller, J.; Coffey, C.S.; Hoh, B.L.; Jauch, E.C.; Johnston, K.C.; Johnston, S.C.; Khalessi, A.A.; Kidwell, C.S.; et al. 2015 American Heart ssociation/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart ssociation/American Stroke Association. Stroke 2015, 46, 3020–3035. [Google Scholar] [PubMed]
- Winnicka, K.; Tomasiak, M.; Bielawska, A. Piracetam—An old drug with novel properties? Acta Pol. Pharm. 2005, 62, 405–409. [Google Scholar] [PubMed]
- Malykh, A.G.; Sadaie, M.R. Piracetam and piracetam-like drugs: From basic science to novel clinical applications to CNS disorders. Drugs 2010, 70, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Gupta, S.; Biswas, J.; Joshi, N.; Singh, A.; Gupta, P.; Tiwari, S.; Sivarama, R.K.; Chaturvedi, S.; Wahajuddin, M.; et al. New therapeutic activity of metabolic enhancer piracetam in treatment of neurodegenerative disease: Participation of caspase independent death factors, oxidative stress, inflammatory responses and apoptosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2078–2096. [Google Scholar] [CrossRef] [PubMed]
- Kum, N.Y.; Yilmaz, Y.F.; Gurgen, S.G.; Kum, R.O.; Ozcan, M.; Unal, A. Effects of parenteral papaverine and piracetam administration on cochlea following acoustic trauma. Noise Health 2018, 20, 47–52. [Google Scholar] [PubMed]
- Verma, D.K.; Joshi, N.; Raju, K.S.; Wahajuddin, M.; Singh, R.K.; Singh, S. Metabolic enhancer piracetam attenuates rotenone induced oxidative stress: A study in different rat brain regions. Acta Neurobiol. Exp. 2015, 75, 399–411. [Google Scholar]
- Guidelines for intensive care unit admission, discharge, and triage Task Force of the American College American College of Critical Care Medicine, Society of Critical Care Medicine. Crit. Care Med. 1999, 27, 633–638. [CrossRef]
- Bruno, A.; Saha, C.; Williams, L.S. Using Change in the National Institutes of Health Stroke Scaleto Measure Treatment Effect in Acute Stroke Trials. Stroke 2006, 37, 920–921. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Marler, J.R. NINDS clinical trials in stroke: Lessons learned and future directions. Stroke 2007, 38, 3302–3307. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 15 May 2016).
- Chiang, C.E.; Wang, T.D.; Li, Y.H.; Lin, T.H.; Chien, K.L.; Yeh, H.I.; Shyu, K.G.; Tsai, W.C.; Chao, T.H.; Hwang, J.J.; et al. 2010 guidelines of the Taiwan Society of Cardiology for the management of hypertension. J. Formos. Med. Assoc. 2010, 109, 740–773. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.E.; Boehme, A.K.; Kumar, A.D.; Gillette, M.A.; Albright, K.C.; Martin-Schild, S. What change in the National Institutes of Health Stroke Scale should define neurologic deterioration in acute ischemic stroke? J. Stroke Cerebrovasc. Dis. 2013, 22, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Leira, E.C.; Hansen, M.D.; Adams, H.P., Jr. Early recovery after cerebral ischemia risk of subsequent neurological deterioration. Ann. Neurol. 2003, 54, 439–444. [Google Scholar] [CrossRef]
- Birkeland, K.I.; Jørgensen, M.E.; Carstensen, B.; Persson, F.; Gulseth, H.L.; Thuresson, M.; Fenici, P.; Nathanson, D.; Nyström, T.; Eriksson, J.W.; et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): A multinational observational analysis. Lancet Diabetes Endocrinol. 2017, 5, 709–717. [Google Scholar] [CrossRef]
- Ray, W.A.; Chung, C.P.; Murray, K.T.; Hall, K.; Stein, C.M. Prescription of Long-Acting Opioids and Mortality in Patients with Chronic Noncancer Pain. JAMA 2016, 315, 2415–2423. [Google Scholar] [CrossRef]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates Publishers: Hillsdale, NJ, USA, 1988. [Google Scholar]
- McGraw, K.O.; Wong, S.P. A common language effect size statistic. Psychol. Bull. 1992, 111, 361–365. [Google Scholar] [CrossRef]
- De Deyn, P.P.; Reuck, J.D.; Deberdt, W.; Vlietinck, R.; Orgogozo, J.M. Treatment of acute ischemic stroke with piracetam. Members of the Piracetam in Acute Stroke Study (PASS) Group. Stroke 1997, 28, 2347–2352. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Celani, M.G.; Cantisani, T.A.; Righetti, E. Piracetam for acute ischaemic stroke. Cochrane Database Syst. Rev. 2012, 9, CD000419. [Google Scholar] [CrossRef] [PubMed]
- Tortiglione, A.; Minale, M.; Pignataro, G.; Amoroso, S.; DiRenzo, G.; Annunziato, L. The 2-oxopyrrolidinacetamide piracetam reduces infarct brain volume induced by permanent middle cerebral artery occlusion in male rats. Neuropharmacology 2002, 43, 427–433. [Google Scholar] [CrossRef]
- Wheble, P.C.; Sena, E.S.; Macleod, M.R. A systematic review and meta-analysis of the efficacy of piracetam and piracetam-like compounds in experimental stroke. Cerebrovasc. Dis. 2008, 25, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, P.; Dash, D.; Krishnamurthy, S. Pharmacokinetic Study of Piracetam in Focal Cerebral Ischemic Rats. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Celani, M.G.; Cantisani, T.A.; Righetti, E. Piracetam in acute stroke: A systematic review. J. Neurol. 2000, 247, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Bick, R.L. In-vivo platelet inhibition by piracetam. Lancet 1979, 2, 752–753. [Google Scholar] [CrossRef]
- Evers, S.; Grotemeyer, K.H. Piracetam and platelets—A review of laboratory and clinical data. Pharmacopsychiatry 1999, 32 (Suppl. 1), 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Thiel, A.; Karbe, H.; Heiss, W.D. Piracetam improves activated blood flow and facilitates rehabilitation of poststroke aphasic patients. Stroke 2000, 31, 2112–2116. [Google Scholar] [CrossRef]
- Peuvot, J.; Schanck, A.; Deleers, M.; Brasseur, R. Piracetam-induced changes to membrane physical properties. A combined approach by 31P nuclear magnetic resonance and conformational analysis. Biochem. Pharmacol. 1995, 50, 1129–1134. [Google Scholar] [CrossRef]
- Tanizaki, Y.; Kiyohara, Y.; Kato, I.; Iwamoto, H.; Nakayama, K.; Shinohara, N.; Arima, H.; Tanaka, K.; Ibayashi, S.; Fujishima, M. Incidence and risk factors for subtypes of cerebral infarction in a general population: The Hisayama study. Stroke 2000, 31, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Woodward, M.; Kengne, A.P. Predicting a post-thrombolysis intracerebral hemorrhage: A systematic review. J. Thromb. Haemost. 2013, 11, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Bluhmki, E.; Chamorro, A.; Davalos, A.; Machnig, T.; Sauce, C.; Wahlgren, N.; Wardlaw, J.; Hacke, W. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): Additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009, 8, 1095–1102. [Google Scholar] [CrossRef]
| Characteristics | Piracetam n = 457 (%) | No Piracetam n = 894 (%) | p Value | Standardized Difference |
|---|---|---|---|---|
| Age (years) | ||||
| mean ± SD | 72.0 ± 12.2 | 70.5 ± 12.5 | 0.031 | 0.130 |
| Age (years) | ||||
| <75 | 246 (53.8%) | 523 (58.5%) | 0.101 | 0.095 |
| ≥75 | 211 (46.2%) | 371 (41.5%) | ||
| Gender | ||||
| male | 295 (64.6%) | 577 (64.5%) | 0.960 | 0.002 |
| female | 162 (35.4%) | 317 (35.5%) | ||
| BMI | ||||
| underweight | 17 (3.8%) | 39 (4.5%) | 0.564 | 0.035 |
| normal weight | 243 (52.0%) | 421 (48.1%) | 0.078 | |
| overweight | 160 (35.6%f) | 328 (37.5%) | 0.039 | |
| obese | 39 (8.7%) | 87 (9.9%) | 0.041 | |
| Smoking | ||||
| non-smoker | 272 (59.5%) | 559 (62.5%) | 0.179 | 0.061 |
| current smoker | 138 (30.2%) | 229 (25.6%) | 0.103 | |
| ex-smoker | 47 (10.3%) | 106 (11.9%) | 0.051 | |
| Hypertension | ||||
| negative | 88 (19.3%) | 190 (21.3%) | 0.390 | 0.050 |
| positive | 369 (80.7%) | 704 (78.7%) | ||
| Diabetes mellitus | ||||
| negative | 252 (55.1%) | 449 (50.2%) | 0.087 | 0.094 |
| positive | 205 (44.9%) | 445 (49.8%) | ||
| Dyslipidemia | ||||
| negative | 127 (27.8%) | 290 (32.4%) | 0.080 | 0.100 |
| positive | 330 (72.2%) | 604 (67.6%) | ||
| Atrial fibrillation | ||||
| negative | 370 (81.0%) | 737 (82.4%) | 0.505 | 0.036 |
| positive | 87 (19.0%) | 157 (17.6%) | ||
| TOAST | ||||
| small-vessel occlusion | 135 (29.5%) | 297 (33.2%) | 0.558 | 0.080 |
| large-artery atherosclerosis | 140 (30.6%) | 266 (29.8%) | 0.017 | |
| cardioembolism | 70 (15.3%) | 123 (13.8%) | 0.043 | |
| others | 112 (24.5%) | 208 (23.3%) | 0.028 | |
| Variables | Group A–NIHSS Improved (>4) n = 131 (%) | Group B–NIHSS Not Improved (≤4) n = 1220 (%) | Univariate Analysis OR (95% CI) | p Value | Multivariate Analysis OR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| <75 | 56 (42.7%) | 713 (58.4%) | 1.00 | <0.001 | 1.00 | 0.053 |
| ≥75 | 75 (57.3%) | 507 (41.6%) | 1.88 (1.31–2.71) | 1.52 (1.00–2.33) | ||
| Gender | ||||||
| male | 46 (35.1%) | 443 (35.5%) | 1.00 | 0.932 | 1.00 | 0.437 |
| female | 85 (64.9%) | 787 (64.5%) | 1.02 (0.70–1.48) | 0.82 (0.50–1.35) | ||
| BMI | ||||||
| underweight | 59 (46.5%) | 596 (49.7%) | 1.00 | 0.002 | 1.00 | 0.026 |
| normal weight | 14 (11.0%) | 42 (3.6%) | 3.37 (1.74–6.52) | <0.001 | 3.05 (1.47–6.30) | 0.003 |
| overweight | 45 (35.4%) | 443 (37.0%) | 1.03 (0.68–1.54) | 0.901 | 1.11 (0.72–1.70) | 0.644 |
| obese | 9 (7.1%) | 117 (9.8%) | 0.78 (0.38–1.61) | 0.498 | 0.93 (0.44–2.00) | 0.858 |
| Smoking | ||||||
| non-smoker | 70 (53.4%) | 761 (62.4%) | 1.00 | 0.083 | 1.00 | 0.017 |
| current smoker | 40 (30.5%) | 327 (26.8%) | 1.33 (0.88–2.00) | 0.172 | 1.81 (1.07–3.06) | 0.026 |
| ex-smoker | 21 (16.0%) | 132 (10.8%) | 1.73 (1.03–2.91) | 0.039 | 2.29 (1.22–4.28) | 0.009 |
| Piracetam | ||||||
| negative | 72 (55.0%) | 822 (67.4%) | 1.00 | 0.005 | 1.00 | 0.005 |
| positive | 59 (45.0%) | 398 (32.6%) | 1.69 (1.18–2.44) | 1.73 (1.18–2.55) | ||
| Hypertension | ||||||
| negative | 29 (22.1%) | 249 (20.4%) | 1.00 | 0.642 | 1.00 | 0.465 |
| positive | 102 (77.9%) | 971 (79.6%) | 0.90 (0.58–1.39) | 0.84 (0.52–1.35) | ||
| Diabetes mellitus | ||||||
| negative | 76 (58.0%) | 625 (51.2%) | 1.00 | 0.141 | 1.00 | 0.468 |
| positive | 55 (42.0%) | 595 (48.8%) | 0.76 (0.53–1.10) | 0.86 (0.58–1.29) | ||
| Dyslipidemia | ||||||
| negative | 43 (32.8%) | 374 (30.7%) | 1.00 | 0.610 | 1.00 | 0634 |
| positive | 88 (67.2%) | 846 (69.3%) | 0.91 (0.62–1.33) | 1.11 (0.37–1.69) | ||
| Atrial fibrillation | ||||||
| negative | 82 (62.6%) | 1025 (84.0%) | 1.00 | <0.001 | 1.00 | <0.001 |
| positive | 49 (37.4%) | 195 (16.0%) | 3.14 (2.14–4.62) | 3.92 (2.31–8.24) | ||
| TOAST | ||||||
| small-vessel occlusion | 21 (16.0%) | 411 (33.7%) | 1.00 | <0.001 | 1.00 | <0.001 |
| large-artery atherosclerosis | 51 (38.9%) | 355 (29.1%) | 2.81 (1.66–4.77) | <0.001 | 2.68 (1.57–4.60) | <0.001 |
| cardioembolism | 34 (26.0%) | 159 (13.0%) | 4.19 (2.36–7.43) | <0.001 | 0.87 (0.38–1.96) | 0.933 |
| others | 25 (19.1%) | 295 (24.2%) | 1.66 (0.91–3.02) | 0.098 | 1.06 (0.56–2.02) | 0.640 |
| Variables | NIHSS Improved (n = 131) | NIHSS NotImproved (n = 1220) | Rate (Proportion) | 95% CI Lower-Upper Rate | SE | p Value * | Adjusted Ratio Difference of NIHSS Improvement (95% CI) † |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| <75 | 56 | 713 | 0.073 | 0.054–0.091 | 0.009 | 0.061 | - |
| ≥75 | 75 | 507 | 0.129 | 0.102–0.156 | 0.014 | 0.025 | |
| Gender | |||||||
| male | 46 | 433 | 0.096 | 0.070–0.122 | 0.013 | 0.273 | - |
| female | 85 | 787 | 0.097 | 0.078–0.117 | 0.010 | 0.007 | |
| BMI | |||||||
| normal weight | 59 | 596 | 0.090 | 0.068–0.112 | 0.011 | 0.016 | reference |
| underweight | 14 | 42 | 0.250 | 0.137–0.363 | 0.058 | 0.425 | 20.3% (4.7%–52.2%) |
| overweight | 45 | 443 | 0.092 | 0.067–0.118 | 0.013 | 0.688 | - |
| obese | 9 | 117 | 0.071 | 0.026–0.116 | 0.023 | 0.035 | - |
| Smoking | |||||||
| non-smoker | 70 | 761 | 0.084 | 0.065–0.103 | 0.010 | 0.123 | reference |
| current smoker | 40 | 327 | 0.109 | 0.077–0.141 | 0.016 | 0.074 | 7.5% (0.6%–18.9%) |
| ex-smoker | 21 | 132 | 0.137 | 0.083–0.192 | 0.028 | 0.033 | 11.9% (2.0%–30.2%) |
| Piracetam | |||||||
| negative | 72 | 822 | 0.081 | 0.063–0.098 | 0.009 | - | reference |
| positive | 59 | 398 | 0.129 | 0.098–0.160 | 0.016 | 6.4% (2.2%–13.6%) | |
| Hypertension | |||||||
| negative | 29 | 249 | 0.104 | 0.070–0.140 | 0.018 | 0.866 | - |
| positive | 102 | 971 | 0.095 | 0.078–0.113 | 0.009 | 0.004 | |
| Diabetes mellitus | |||||||
| negative | 76 | 625 | 0.108 | 0.085–0.131 | 0.012 | 0.018 | - |
| positive | 55 | 595 | 0.085 | 0.063–0.106 | 0.011 | 0.124 | |
| Dyslipidemia | |||||||
| negative | 43 | 374 | 0.103 | 0.074–0.132 | 0.015 | 0.280 | - |
| positive | 88 | 846 | 0.094 | 0.075–0.113 | 0.010 | 0.013 | |
| Atrial fibrillation | |||||||
| negative | 82 | 1025 | 0.074 | 0.059–0.090 | 0.008 | 0.020 | reference |
| positive | 49 | 195 | 0.201 | 0.151–0.251 | 0.026 | 0.131 | 23.4% (2.5%–57.9%) |
| TOAST | |||||||
| Small-vessel occlusion | 21 | 411 | 0.049 | 0.028–0.069 | 0.010 | 0.106 | reference |
| large-artery atherosclerosis | 51 | 355 | 0.126 | 0.093–0.158 | 0.016 | 0.145 | 8.6% (2.9%–18.4%) |
| cardioembolism | 34 | 159 | 0.176 | 0.122–0.230 | 0.027 | 0.106 | - |
| others | 25 | 295 | 0.078 | 0.049–0.108 | 0.015 | 0.453 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-Y.; Liu, J.-W.; Wang, Y.-H.; Huang, J.-Y.; Chen, S.-C.; Yang, S.-F.; Wang, P.-H. The Conditions Under Which Piracetam Is Used and the Factors That Can Improve National Institute of Health Stroke Scale Score in Ischemic Stroke Patients and the Importance of Previously Unnoticed Factors From a Hospital-Based Observational Study in Taiwan. J. Clin. Med. 2019, 8, 122. https://doi.org/10.3390/jcm8010122
Chen S-Y, Liu J-W, Wang Y-H, Huang J-Y, Chen S-C, Yang S-F, Wang P-H. The Conditions Under Which Piracetam Is Used and the Factors That Can Improve National Institute of Health Stroke Scale Score in Ischemic Stroke Patients and the Importance of Previously Unnoticed Factors From a Hospital-Based Observational Study in Taiwan. Journal of Clinical Medicine. 2019; 8(1):122. https://doi.org/10.3390/jcm8010122
Chicago/Turabian StyleChen, Shu-Yi, Jai-Wen Liu, Yu-Hsun Wang, Jing-Yang Huang, Shiuan-Chih Chen, Shun-Fa Yang, and Po-Hui Wang. 2019. "The Conditions Under Which Piracetam Is Used and the Factors That Can Improve National Institute of Health Stroke Scale Score in Ischemic Stroke Patients and the Importance of Previously Unnoticed Factors From a Hospital-Based Observational Study in Taiwan" Journal of Clinical Medicine 8, no. 1: 122. https://doi.org/10.3390/jcm8010122
APA StyleChen, S.-Y., Liu, J.-W., Wang, Y.-H., Huang, J.-Y., Chen, S.-C., Yang, S.-F., & Wang, P.-H. (2019). The Conditions Under Which Piracetam Is Used and the Factors That Can Improve National Institute of Health Stroke Scale Score in Ischemic Stroke Patients and the Importance of Previously Unnoticed Factors From a Hospital-Based Observational Study in Taiwan. Journal of Clinical Medicine, 8(1), 122. https://doi.org/10.3390/jcm8010122






