Autoalgometry: An Important Tool for Pressure Pain Threshold Evaluation
Abstract
1. Introduction
2. Experimental Section
2.1. Subjects
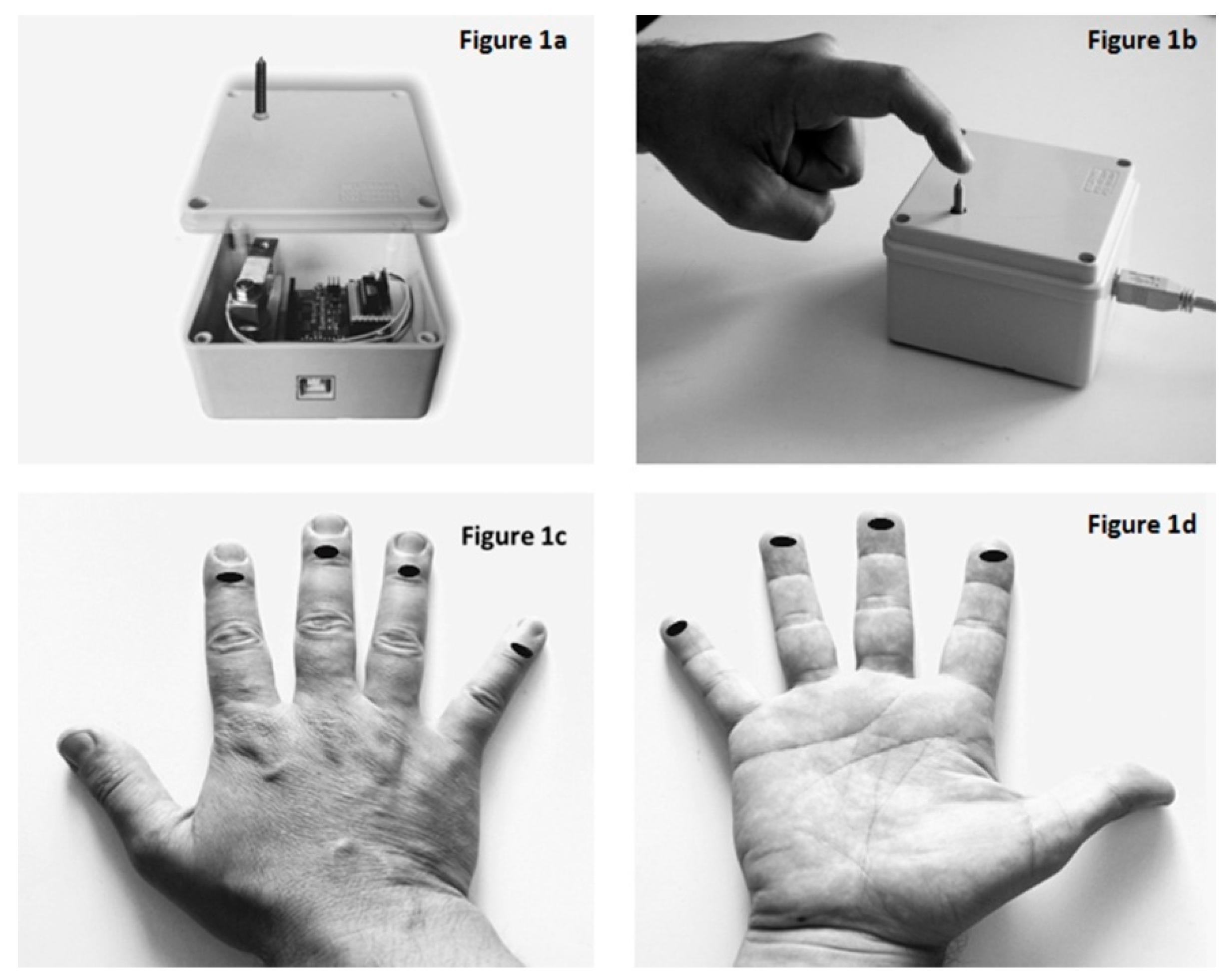
2.2. Autoalgometry
2.3. Statistical Analysis
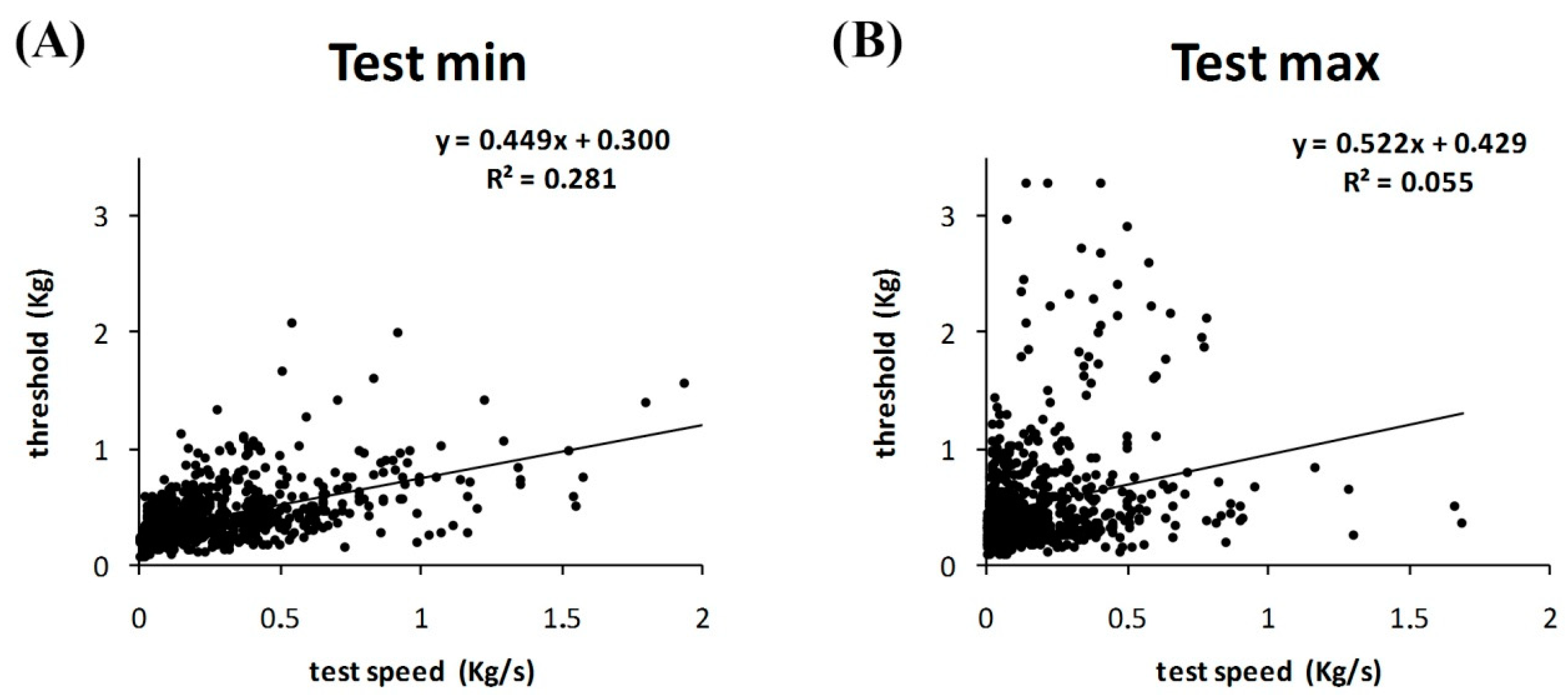
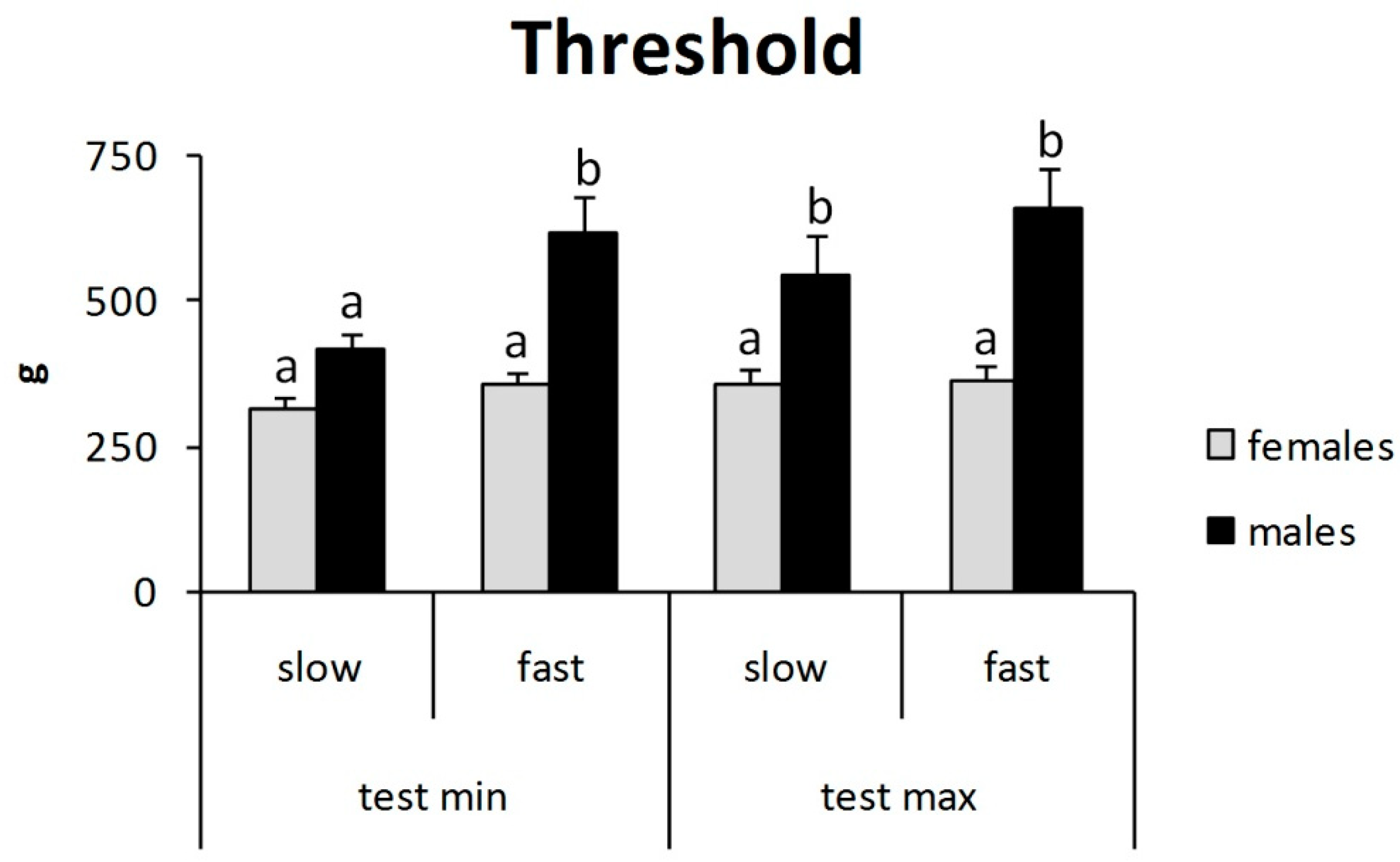
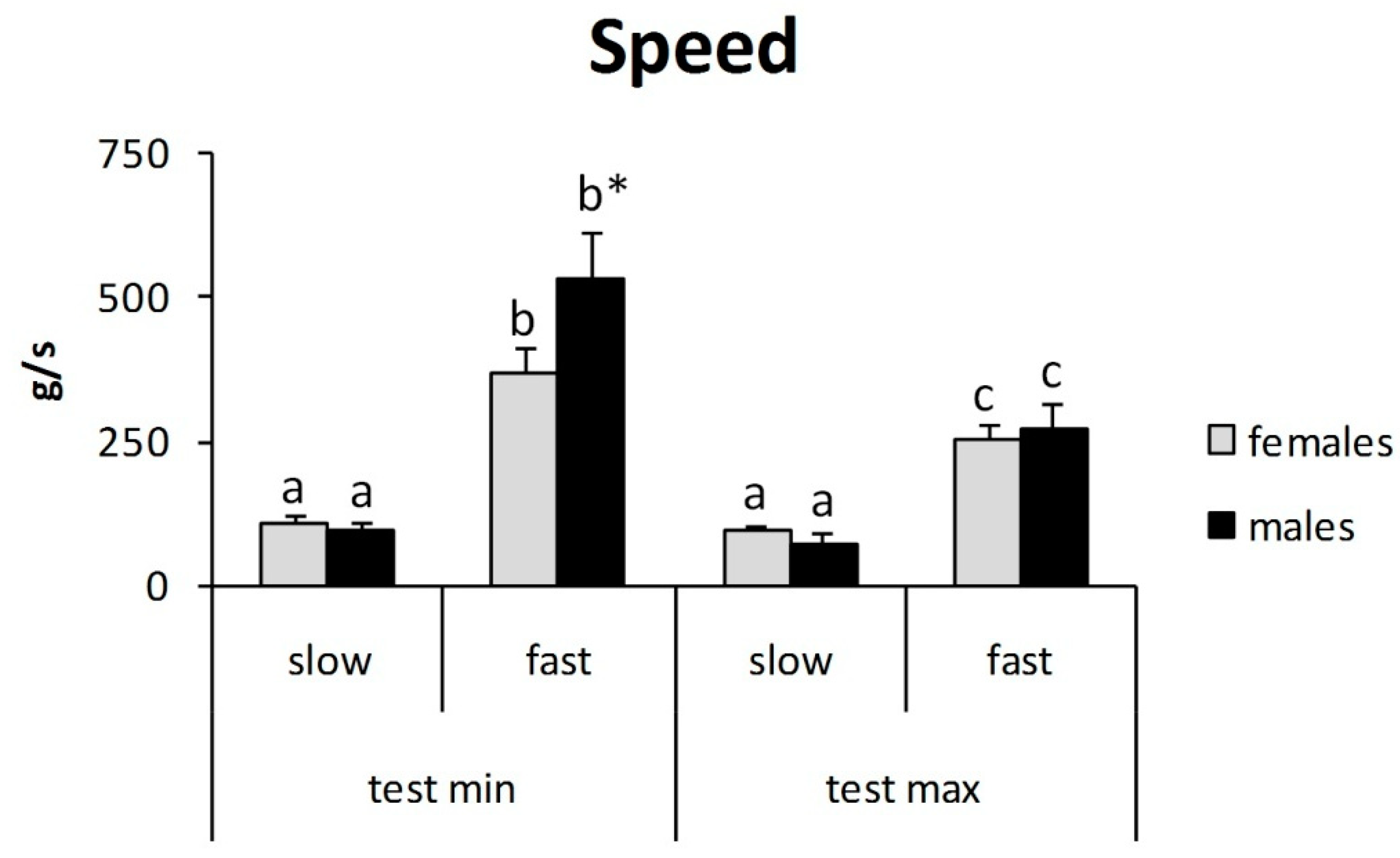
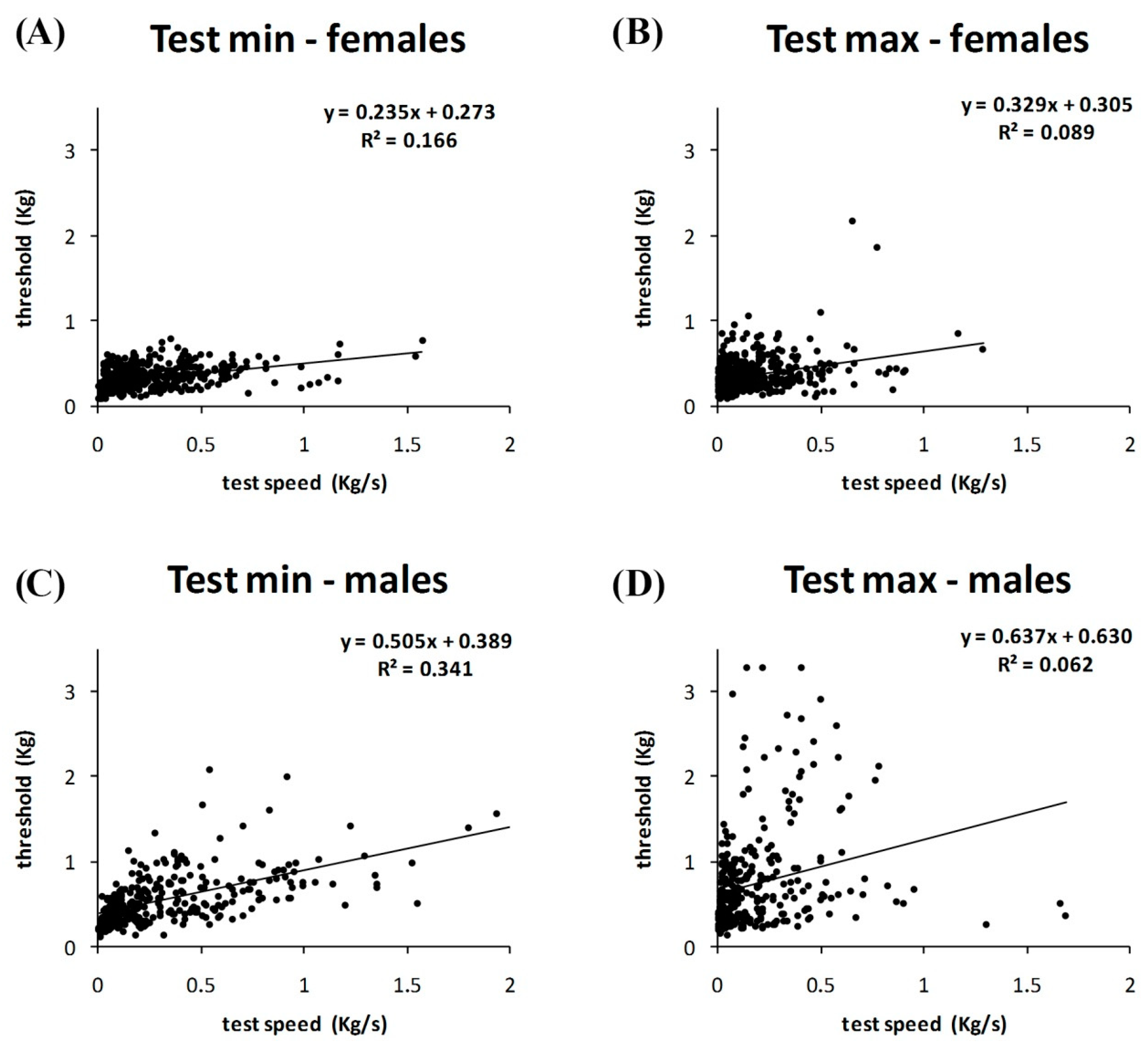
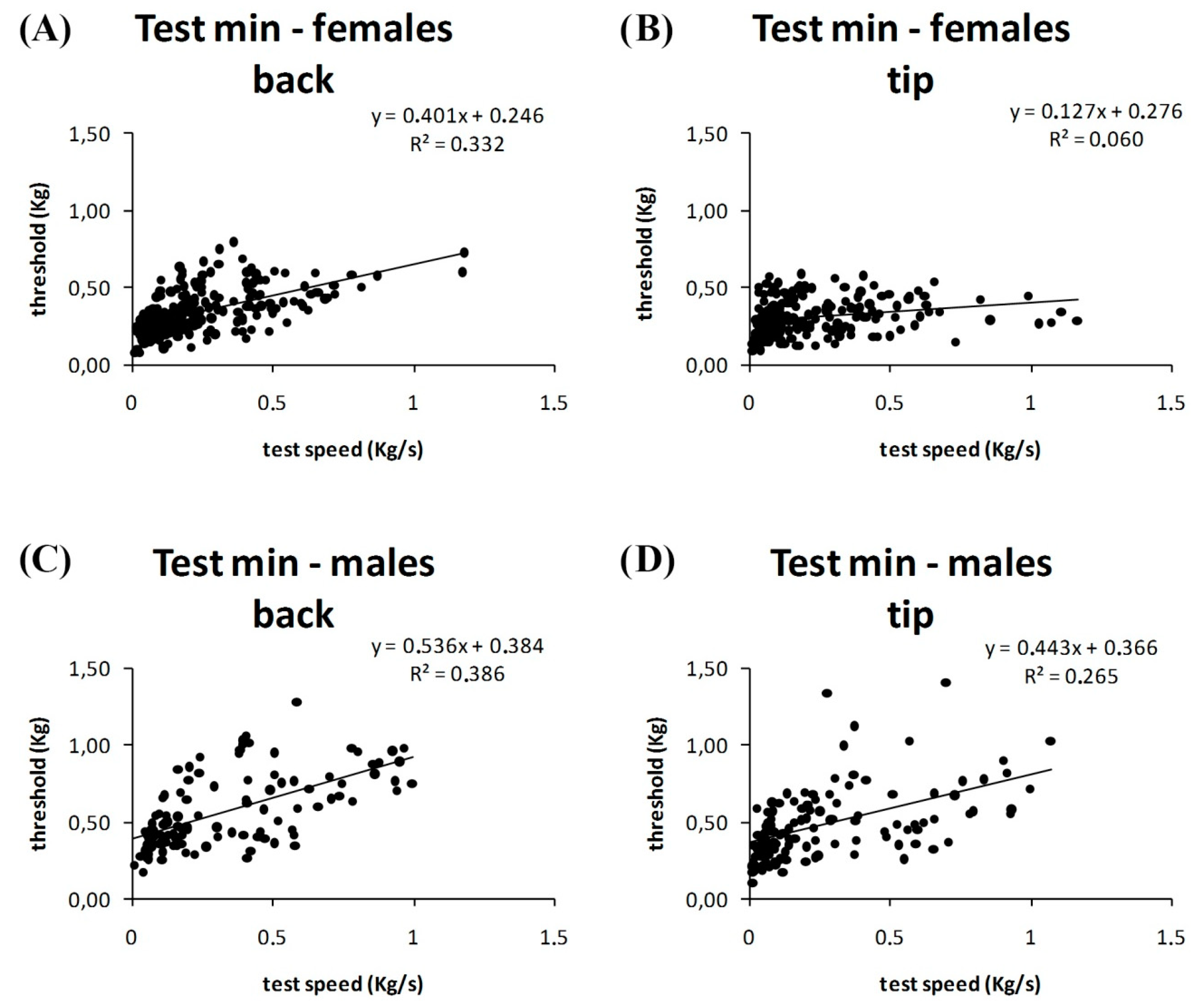
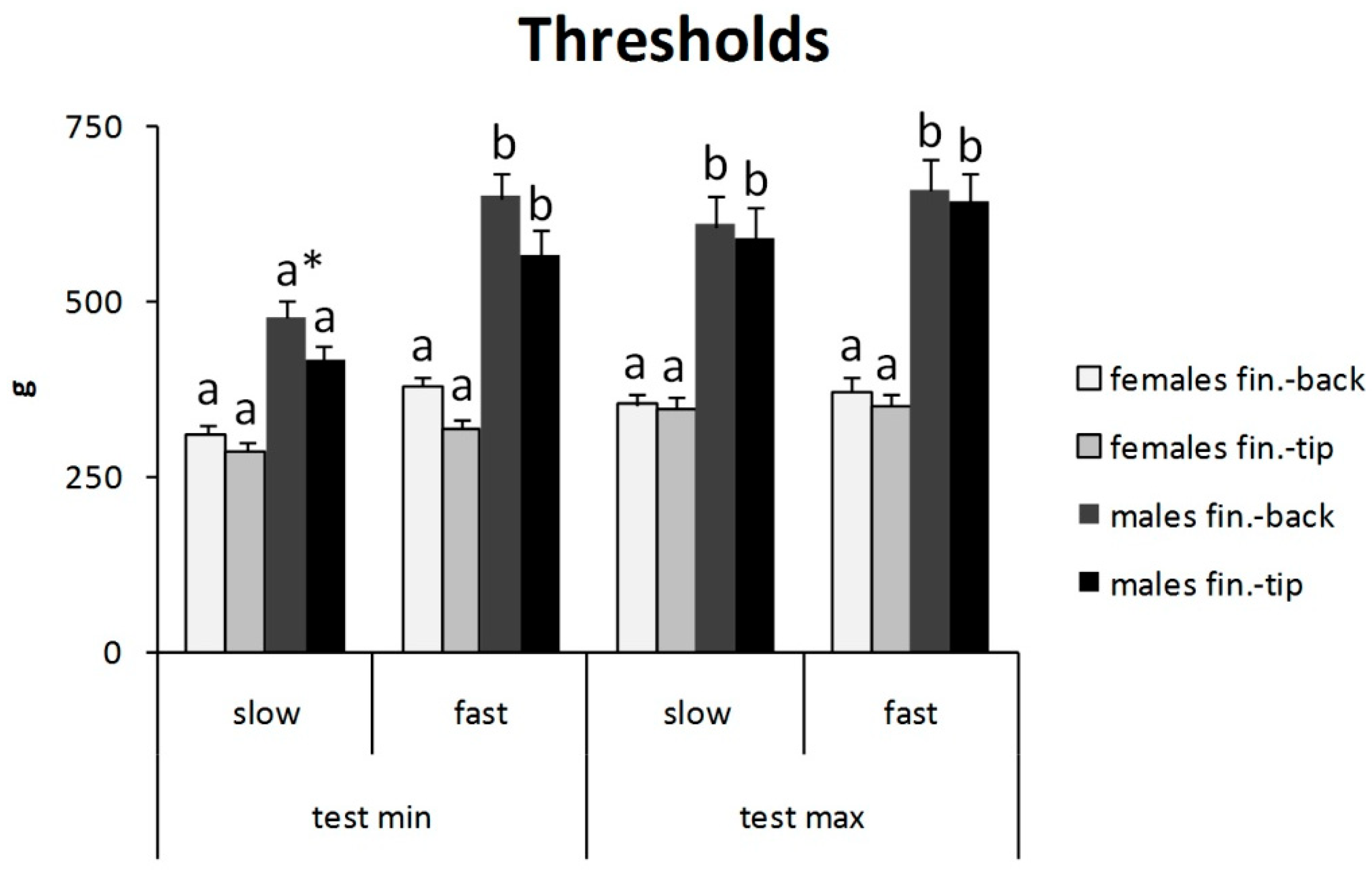
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Weerasinghe, T.W.; Goonetilleke, R.S.; Reischl, U. Pressure thresholds and stiffness on the plantar surface of the human foot. Ergonomics 2017, 60, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Polianskis, R.; Graven-Nielsen, T.; Arendt-Nielsen, L. Spatial and temporal aspects of deep tissue pain assessed by cuff algometry. Pain 2002, 100, 19–26. [Google Scholar] [CrossRef]
- Fischer, A.A. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain 1987, 30, 115–126. [Google Scholar] [CrossRef]
- Kosek, E.; Ekholm, J.; Nordemar, R. A comparison of pressure pain thresholds in different tissues and body regions. Scand. J. Rehabil. Med. 1993, 25, 117–124. [Google Scholar] [PubMed]
- Hogeweg, J.A.; Langereis, M.J.; Bernards, A.T.; Faber, J.A.; Helders, P.J. Algometry. Measuring pain threshold, method and characteristics in healthy subjects. Scand. J. Rehabil. Med. 1992, 24, 99–103. [Google Scholar] [PubMed]
- Gibbs, C.H.; Karpinia, K.; Moorhead, J.E.; Maruniak, J.W.; Heins, P.J. An algometer for intraoral pain tolerance measurements. J. Neurosci. Methods 1999, 88, 135–139. [Google Scholar] [CrossRef]
- Chantelau, E.A. Conventional deep pressure algometry is not suitable for clinical assessment of nociception in painless diabetic neuropathy. Diabet. Foot Ankle 2016, 7, 31922. [Google Scholar] [CrossRef] [PubMed]
- Linde, L.D.; Kumbhare, D.A.; Joshi, M.; Srbely, J.Z. The relationship between rate of algometer application and pain pressure threshold in the assessment of myofascial trigger point sensitivity. Pain Pract. 2018, 18, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Petrini, L.; Matthiesen, S.T.; Arendt-Nielsen, L. The effect of age and gender on pressure pain thresholds and suprathreshold stimuli. Perception 2015, 44, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Andersen, H.Ø.; Olesen, J.; Lindblom, U. Pressure-pain threshold in human temporal region. Evaluation of a new pressure algometer. Pain 1986, 25, 313–323. [Google Scholar] [CrossRef]
- Croze, S.; Duclaux, R. Thermal pain in humans: Influence of the rate of stimulation. Brain Res. 1978, 157, 418–421. [Google Scholar] [CrossRef]
- Treede, R.D.; Meyer, R.A.; Raja, S.N.; Campbell, J.N. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J. Physiol. 1995, 83, 747–758. [Google Scholar] [CrossRef]
- Andersson, S.A.; Ericson, T.; Holmgren, E.; Lindqvist, G. Electro-acupuncture. Effect on pain threshold measured with electrical stimulation of teeth. Brain Res. 1973, 63, 393–396. [Google Scholar] [CrossRef]
- Azerad, J.; Woda, A. Sensation evoked by bipolar intrapulpal stimulation in man. Pain 1977, 4, 145–152. [Google Scholar] [CrossRef]
- Kiso, T.; Nagakura, Y.; Toya, T.; Matsumoto, N.; Tamura, S.; Ito, H.; Okada, M.; Yamaguchi, T. Neurometer measurement of current stimulus threshold in rats. J. Pharmacol. Exp.Ther. 2001, 297, 352–356. [Google Scholar] [PubMed]
- Koga, K.; Furue, H.; Rashid, H.; Takaki, A.; Katafuchi, T.; Yoshimura, M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol. Pain 2005, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Bitetti, I.; Precenzano, F.; Iacono, D.; Messina, G.; Roccella, M.; Parisi, L.; Salerno, M.; Valenzano, A.; Maltese, A.; et al. Non-rapid eye movement sleep parasomnias and migraine: A role of orexinergic projections. Front. Neurol. 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Maldonato, M.N.; Sperandeo, R.; Dell’Orco, S.; Iennaco, D.; Cerroni, F.; Romano, P.; Salerno, M.; Maltese, A.; Roccella, M.; Parisi, L.; et al. Mind, brain and altered states of consciousness. Acta Medica Mediterr. 2018, 34, 357–366. [Google Scholar]
- Magerl, W.; Fuchs, P.N.; Meyer, R.A.; Treede, R.-D. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain 2001, 124, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.G.; Namer, B.; Reeh, P.W.; Fischer, M.J.M. TRPA1 and TRPV1 antagonists do not inhibit human acidosis-induced pain. J. Pain 2017, 18, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, A.; Zagaria, N.; Passavanti, M.B.; Pace, M.C.; Paladini, A.; Aurilio, C.; Tedesco, M.A.; Natale, F.; Calabrò, R.; Monda, M.; et al. New and low-cost auto-algometry for screening hypertension-associated hypoalgesia. Pain Pract. 2009, 9, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Nori, S.L.; Lorusso, L.; Secondulfo, C.; Monda, M.; Viggiano, A. Auricular acupressure can modulate pain threshold. Evid. Based Complement. Alternat. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Valenzano, A.; Moscatelli, F.; Salerno, M.; Sessa, F.; Triggiani, A.I.; Viggiano, A.; Capranica, L.; Marsala, G.; De Luca, V.; et al. Primary motor cortex excitability in karate athletes: A transcranial magnetic stimulation study. Front. Physiol. 2017, 8, 695. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L., III. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Messina, G.; Valenzano, A.; Messina, A.; Salerno, M.; Marsala, G.; Bertozzi, G.; Daniele, A.; Monda, V.; Russo, R. Sports Training and Adaptive Changes. Sport Sci. Health. (in press). [CrossRef]
- Costa, P.B.; Ryan, E.D.; Herda, T.J.; Walter, A.A.; Hoge, K.M.; Cramer, J.T. Acute effects of passive stretching on the electromechanical delay and evoked twitch properties: A gender comparison. J. Appl. Biomech. 2012, 28, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Salerno, M.; Sessa, F.; Bernardini, R.; Valenzano, A.; Marsala, G.; Zammit, C.; Avola, R.; Carotenuto, M.; Messina, G.; et al. Functional changes of orexinergic reaction to psychoactive substances. Mol. Neurobiol. 2018, 55, 6362–6368. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.N.; LaMotte, R.H. Latency to detection of first pain. Brain Res. 1983, 266, 203–208. [Google Scholar] [CrossRef]
- Wilder-Smith, O.H.; Tassonyi, E.; Crul, B.J.; Arendt-Nielsen, L. Quantitative sensory testing and human surgery: Effects of analgesic management on postoperative neuroplasticity. Anesthesiology 2003, 98, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, L.; Salerno, M.; Sessa, F.; Nicolosi, D.; Longhitano, L.; Loreto, C.; Carotenuto, M.; Messina, A.; Monda, V.; Villano, I.; et al. Autoalgometry: An Important Tool for Pressure Pain Threshold Evaluation. J. Clin. Med. 2018, 7, 273. https://doi.org/10.3390/jcm7090273
Lorusso L, Salerno M, Sessa F, Nicolosi D, Longhitano L, Loreto C, Carotenuto M, Messina A, Monda V, Villano I, et al. Autoalgometry: An Important Tool for Pressure Pain Threshold Evaluation. Journal of Clinical Medicine. 2018; 7(9):273. https://doi.org/10.3390/jcm7090273
Chicago/Turabian StyleLorusso, Letizia, Monica Salerno, Francesco Sessa, Daniela Nicolosi, Lucia Longhitano, Carla Loreto, Marco Carotenuto, Antonietta Messina, Vincenzo Monda, Ines Villano, and et al. 2018. "Autoalgometry: An Important Tool for Pressure Pain Threshold Evaluation" Journal of Clinical Medicine 7, no. 9: 273. https://doi.org/10.3390/jcm7090273
APA StyleLorusso, L., Salerno, M., Sessa, F., Nicolosi, D., Longhitano, L., Loreto, C., Carotenuto, M., Messina, A., Monda, V., Villano, I., Cibelli, G., Valenzano, A., Monda, M., Murabito, P., Mollica, M. P., Messina, G., & Viggiano, A. (2018). Autoalgometry: An Important Tool for Pressure Pain Threshold Evaluation. Journal of Clinical Medicine, 7(9), 273. https://doi.org/10.3390/jcm7090273











