Correction of Hyponatremia May Be a Treatment Stratification Biomarker: A Two-Stage Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verbalis, J.G.; Goldsmith, S.R.; Greenberg, A.; Korzelius, C.; Schrier, R.W.; Sterns, R.H.; Thompson, C.J. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am. J. Med. 2013, 126, S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Jaber, B.L.; Madias, N.E. Incidence and prevalence of hyponatremia. Am. J. Med. 2006, 119, S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Rondon-Berrios, H.; Agaba, E.I.; Tzamaloukas, A.H. Hyponatremia: Pathophysiology, classification, manifestations and management. Int. Urol. Nephrol. 2014, 46, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Buffington, M.A.; Abreo, K. Hyponatremia: A Review. J. Intensive Care Med. 2016, 31, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Urso, C.; Brucculeri, S.; Caimi, G. Employment of vasopressin receptor antagonists in management of hyponatraemia and volume overload in some clinical conditions. J. Clin. Pharm. Ther. 2015, 40, 376–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghiade, M.; Rossi, J.S.; Cotts, W.; Shin, D.D.; Hellkamp, A.S.; Piña, I.L.; Fonarow, G.C.; DeMarco, T.; Pauly, D.F.; Rogers, J.; et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch. Intern. Med. 2007, 167, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R.; Gheorghiade, M. Vasopressin antagonism in heart failure. J. Am. Coll. Cardiol. 2005, 46, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Guevara, M. Hyponatremia in cirrhosis: Pathogenesis, clinical significance, and management. Hepatology 2008, 48, 1002–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, G.L. Vaptans for the treatment of hyponatremia. Nat. Rev. Endocrinol. 2011, 7, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.P.; Bell, R.; Buckman, S.; Burckart, G.J.; Eichler, H.G.; Fang, K.C.; Goodsaid, F.M.; Jusko, W.J.; Lesko, L.L.; Meibohm, B.; et al. Translational biomarkers: From preclinical to clinical a report of 2009 AAPS/ACCP Biomarker Workshop. AAPS J. 2011, 13, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (IOM). Guidelines, standards, oversight, and incentives needed for biomarker development. In The Promises and Challenges of Improving Detection and Treatment: Cancer Biomarkers; Nass, S.J., Moses, H.L., Eds.; The National Academies Press: Washington, DC, USA, 2007; pp. 73–113. [Google Scholar]
- Annane, D.; Decaux, G.; Smith, N.; Conivaptan Study Group. Efficacy and safety of oral conivaptan, a vasopressin-receptor antagonist, evaluated in a randomized, controlled trial in patients with euvolemic or hypervolemic hyponatremia. Am. J. Med. Sci. 2009, 337, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G.; Zeltser, D.; Smith, N.; Barve, A.; Andoh, M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: Subgroup analysis of a randomized, controlled study. Clin. Endocrinol. 2008, 69, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zeltser, D.; Rosansky, S.; van Rensburg, H.; Verbalis, J.G.; Smith, N.; Conivaptan Study Group. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am. J. Nephrol. 2007, 27, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Ghali, J.K.; Koren, M.J.; Taylor, J.R.; Brooks-Asplund, E.; Fan, K.; Long, W.A.; Smith, N. Efficacy and safety of oral conivaptan: A V1A/V2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia. J. Clin. Endocrinol. Metab. 2006, 91, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Decaux, G.; Josiassen, R.C.; Yagil, Y.; Kopyt, N.; Thacker, H.P.; Mannelli, M.; Bichet, D.G.; Orlandi, C.; HARMONY Study Group. Oral lixivaptan effectively increases serum sodium concentrations in outpatients with euvolemic hyponatremia. Kidney Int. 2012, 82, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Hensen, J.; Gross, P.A.; Bichet, D.G.; Josiassen, R.C.; Chafekar, D.S.; Orlandi, C.; LIBRA Study Group. Lixivaptan safely and effectively corrects serum sodium concentrations in hospitalized patients with euvolemic hyponatremia. Kidney Int. 2012, 82, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Gerbes, A.L.; Gülberg, V.; Ginès, P.; Decaux, G.; Gross, P.; Gandjini, H.; Djian, J.; VPA Study Group. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: A randomized double-blind multicenter trial. Gastroenterology 2003, 124, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.; Decaux, G.; Gerbes, A.; Djian, J. A0621 Treatment of hyponatremia (HYPO) with VPA-985. American Society of Nephrology 32nd Annual Meeting and the 1999 Renal Week. November 1–8, 1999, Miami Beach, Florida, USA. Abstracts. J. Am. Soc. Nephrol. 1999, 10, 1A–867A. [Google Scholar]
- Wong, F.; Blei, A.T.; Blendis, L.M.; Thuluvath, P.J. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: A multicenter, randomized, placebo-controlled trial. Hepatology 2003, 37, 182–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronson, D.; Verbalis, J.G.; Mueller, M.; Krum, H.; DILIPO Investigators. Short- and long-term treatment of dilutional hyponatraemia with satavaptan, a selective arginine vasopressin V2-receptor antagonist: The DILIPO study. Eur. J. Heart Fail. 2011, 13, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Soupart, A.; Gross, P.; Legros, J.J.; Alföldi, S.; Annane, D.; Heshmati, H.M.; Decaux, G. Successful long-term treatment of hyponatremia in syndrome of inappropriate antidiuretic hormone secretion with satavaptan (SR121463B), an orally active nonpeptide vasopressin V2-receptor antagonist. Clin. J. Am. Soc. Nephrol. 2006, 1, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Wong, F.; Watson, H.; Milutinovic, S.; del Arbol, L.R.; Olteanu, D.; HypoCAT Study Investigators. Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: A randomized trial. Hepatology 2008, 48, 204–213. [Google Scholar]
- Verbalis, J.G.; Adler, S.; Schrier, R.W.; Berl, T.; Zhao, Q.; Czerwiec, F.S.; SALT Investigators. Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur. J. Endocrinol. 2011, 164, 725–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrier, R.W.; Gross, P.; Gheorghiade, M.; Berl, T.; Verbalis, J.G.; Czerwiec, F.S.; Orlandi, C.; SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N. Engl. J. Med. 2006, 355, 2099–2112. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.; Bayram, M.; Udelson, J.E.; Lloyd-Jones, D.; Adams, K.F.; Oconnor, C.M.; Stough, W.G.; Ouyang, J.; Shin, D.D.; Orlandi, C.; et al. Improvement in hyponatremia during hospitalization for worsening heart failure is associated with improved outcomes: Insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) trial. Acute Card. Care 2007, 9, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Gattis, W.A.; O’Connor, C.M.; Adams, K.F., Jr.; Elkayam, U.; Barbagelata, A.; Ghali, J.K.; Benza, R.L.; McGrew, F.A.; Klapholz, M.; et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004, 291, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, P.J.; Burnett, J.; Gheorghiade, M.; Grinfeld, L.; Konstam, M.A.; Kostic, D.; Krasa, H.B.; Maggioni, A.; Ouyang, J.; Swedberg, K.; et al. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J. Card. Fail. 2013, 19, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Konstam, M.A.; Burnett, J.C., Jr.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST Clinical Status Trials. JAMA 2007, 297, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Gheorghiade, M.; Burnett, J.C., Jr.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Gottlieb, S.S.; Udelson, J.E.; Konstam, M.A.; Czerwiec, F.; Ouyang, J.; Orlandi, C.; Tolvaptan Investigators. Vasopressin v(2) receptor blockade with tolvaptan versus fluid restriction in the treatment of hyponatremia. Am. J. Cardiol. 2006, 97, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, J.J.; Tong, N.W.; Guo, X.H.; Qiu, M.C.; Yang, G.Y.; Liu, Z.M.; Ma, J.H.; Zhang, Z.W.; Gu, F. Randomized, double blinded, placebo-controlled trial to evaluate the efficacy and safety of tolvaptan in Chinese patients with hyponatremia caused by SIADH. J. Clin. Pharmacol. 2014, 54, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bai, H.; Zhu, W.L.; Tolvaptan Therapy in Hyponatremia with Heart Failure Collaborative Group. The efficacy and safety of tolvaptan on treating heart failure patients with hyponatremia. Zhonghua Xin Xue Guan Bing Za Zhi 2011, 39, 936–940. [Google Scholar] [PubMed]
- Li, L.; Bai, H.; Zhu, W.L. The efficacy and safety of tolvaptan on treating congestive heart failure patients with hyponatremia. Heart 2011, 97, A127. [Google Scholar] [CrossRef]
- Salahudeen, A.K.; Ali, N.; George, M.; Lahoti, A.; Palla, S. Tolvaptan in hospitalized cancer patients with hyponatremia: A double-blind, randomized, placebo-controlled clinical trial on efficacy and safety. Cancer 2014, 120, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, E.; Doss, C.R.; George, M.; Jena, A.; Rajaram, M.; Ramaraj, B.; Anjaneyan, K.; Kanagesh, B. Effect of tolvaptan on acute heart failure with hyponatremia—A randomized, double blind, controlled clinical trial. Indian Heart J. 2016, 68, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, D.; Goodman, C.; Dickinson, A.; Gage, H.; McLaughlin, J.; Manthorpe, J.; Ashaye, K.; Iliffe, S. A protocol for a systematic review of research on managing behavioural and psychological symptoms in dementia for community-dwelling older people: Evidence mapping and syntheses. Syst. Rev. 2013, 2, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Altman, D.G.; Sterne, J.A. Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Centre for Reviews and Dissemination. Systematic Reviews: Centre for Reviews and Dissemination’s (CRD) Guidance for Undertaking Reviews in Health Care. Available online: www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed on 17 July 2018).
- Merlin, T.; Farah, C.; Schubert, C.; Mitchell, A.; Hiller, J.E.; Ryan, P. Assessing personalized medicines in Australia: A national framework for reviewing codependent technologies. Med. Decis. Mak. 2013, 33, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Product Type 4—Codependent Technologies. Guidelines for Preparing a Submission to the Pharmaceutical Benefits Advisory Committee (Version 5.0). Available online: https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf (accessed on 17 July 2018).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Terrin, N.; Schmid, C.H.; Lau, J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J. Clin. Epidemiol. 2005, 58, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.G.; Higgins, J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Assessment of Co-Dependent Technologies. Procedural Guidance for the Systematic Evaluation of Biomarker Tests. Decision Support Document Nr. 77; Kisser, A., Zechmeister-Koss, I., Eds.; Ludwig Boltzmann Institute for Health Technology Assessment: Vienna, Austria, 2014; pp. 37–39.
- Hang, X.; Zhao, M.; Du, W.; Zu, D.; Sun, Y.; Xiang, R.; Yang, J. Efficacy and Safety of Vasopressin Receptor Antagonists for Euvolemic or Hypervolemic Hyponatremia: A Meta-Analysis. Medicine 2016, 95, e3310. [Google Scholar] [CrossRef]
- Jaber, B.L.; Almarzouqi, L.; Borgi, L.; Seabra, V.F.; Balk, E.M.; Madias, N.E. Short-term efficacy and safety of vasopressin receptor antagonists for treatment of hyponatremia. Am. J. Med. 2011, 124, 977.e1–977.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiong, B.; Cai, L. Effects of Tolvaptan in patients with acute heart failure: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Alskaf, E.; Tridente, A.; Al-Mohammad, A. Tolvaptan for Heart Failure, Systematic Review and Meta-Analysis of Trials. J. Cardiovasc. Pharmacol. 2016, 68, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Huang, Y.; Tan, J.; Yao, Y.; Wang, C.; Qian, J.; Rong, S.; Deng, S.; Cao, Y.; Zou, Y.; et al. The short-term and long-term effects of tolvaptan in patients with heart failure: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2015, 20, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Yang, J.; Yang, J.; Fan, Z.X. Arginine vasopressin antagonist tolvaptan in the treatment of heart failure: A meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2015, 8, 22117–22128. [Google Scholar] [PubMed]
- Nistor, I.; Bararu, I.; Apavaloaie, M.C.; Voroneanu, L.; Donciu, M.D.; Kanbay, M.; Nagler, E.V.; Covic, A. Vasopressin receptor antagonists for the treatment of heart failure: A systematic review and meta-analysis of randomized controlled trials. Int. Urol. Nephrol. 2015, 47, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xie, F.; Lu, J.; Ni, Q.; Shi, C.; Tang, C.; Yang, J. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2015, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Jepsen, P.; Wong, F.; Ginès, P.; Córdoba, J.; Vilstrup, H. Satavaptan treatment for ascites in patients with cirrhosis: A meta-analysis of effect on hepatic encephalopathy development. Metab. Brain Dis. 2013, 28, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.; Gluud, L.L.; Kimer, N.; Krag, A. Meta-analysis: The safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment. Pharmacol. Ther. 2012, 36, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). Biomarkers: An Overview of the Opportunities and Challenges. Policy Issues for the Development and Use of Biomarkers in Health. Available online: https://www.oecd.org/health/biotech/49023036.pdf (accessed on 17 July 2018).
- Kirkham, J.J.; Altman, D.G.; Williamson, P.R. Bias due to changes in specified outcomes during the systematic review process. PLoS ONE 2010, 5, e9810. [Google Scholar] [CrossRef] [PubMed]
- Moher, D. The problem of duplicate systematic reviews. BMJ 2013, 347, f5040. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Hernandez-Boussard, T.; Ioannidis, J.P. Overlapping meta-analyses on the same topic: Survey of published studies. BMJ 2013, 347, f4501. [Google Scholar] [CrossRef] [PubMed]
- Wortman, P.M. Judging research quality. In The Handbook of Research Synthesis; Cooper, H., Hedges, L.V., Eds.; Russell Sage Foundation: New York, NY, USA, 1994; pp. 97–110. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, S.G. Why sources of heterogeneity in meta-analysis should be investigated. BMJ 1994, 309, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M. Treating individuals 2. Subgroup analysis in randomised controlled trials: Importance, indications, and interpretation. Lancet 2005, 365, 176–186. [Google Scholar] [CrossRef]
- O’Neil, M.; Berkman, N.; Hartling, L.; Chang, S.; Anderson, J.; Motu’apuaka, M.; Guise, J.M.; McDonagh, M.S. Observational evidence and strength of evidence domains: Case examples. Syst. Rev. 2014, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Gómez, F.; Asensio-González, M.; González-López, A.; Álvarez, F.J. Effects of Intensive Control of Glycemia on Clinical Kidney Outcomes in Type 2 Diabetes Patients Compared with Standard Control: A Meta-Analysis. Front. Pharmacol. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
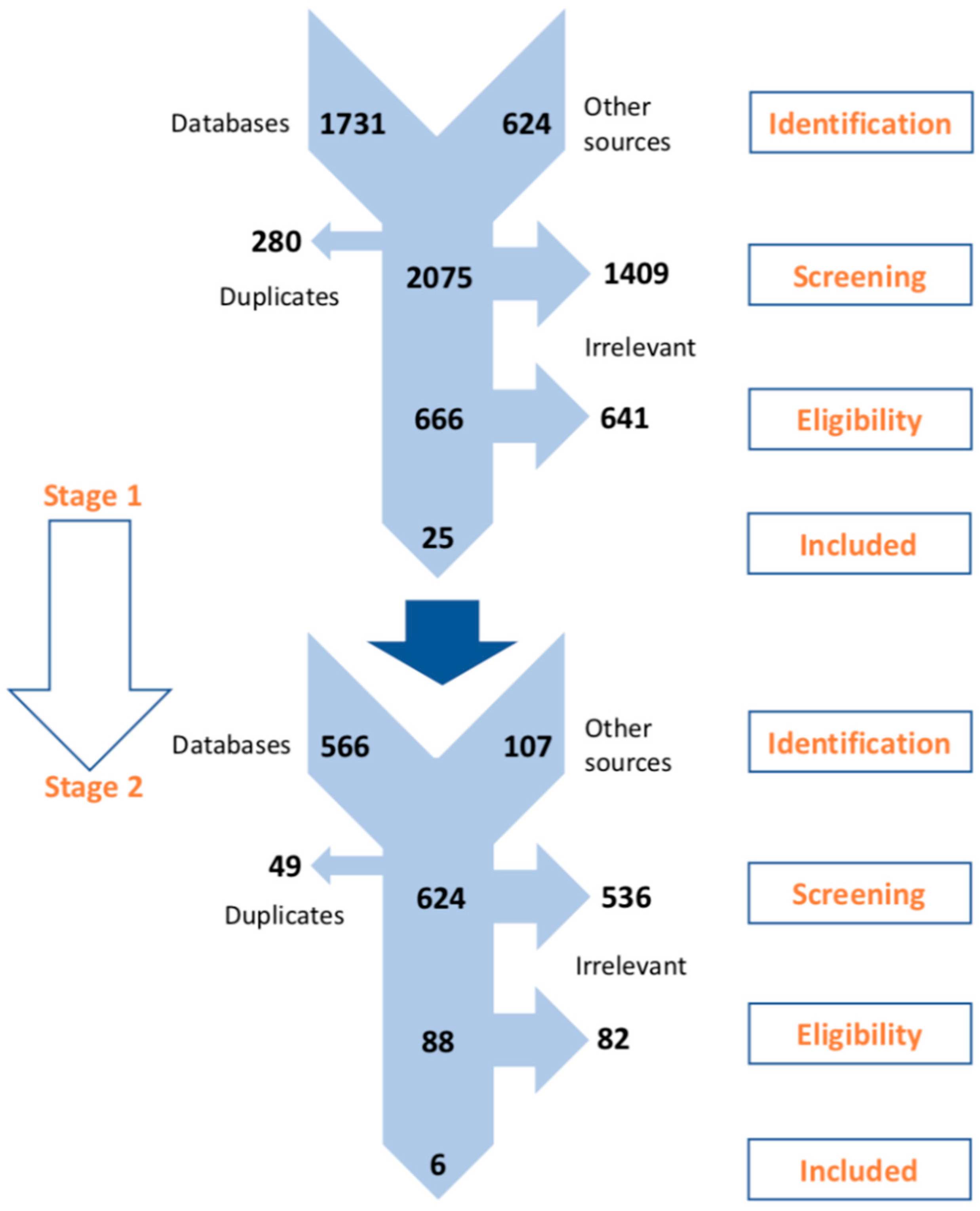
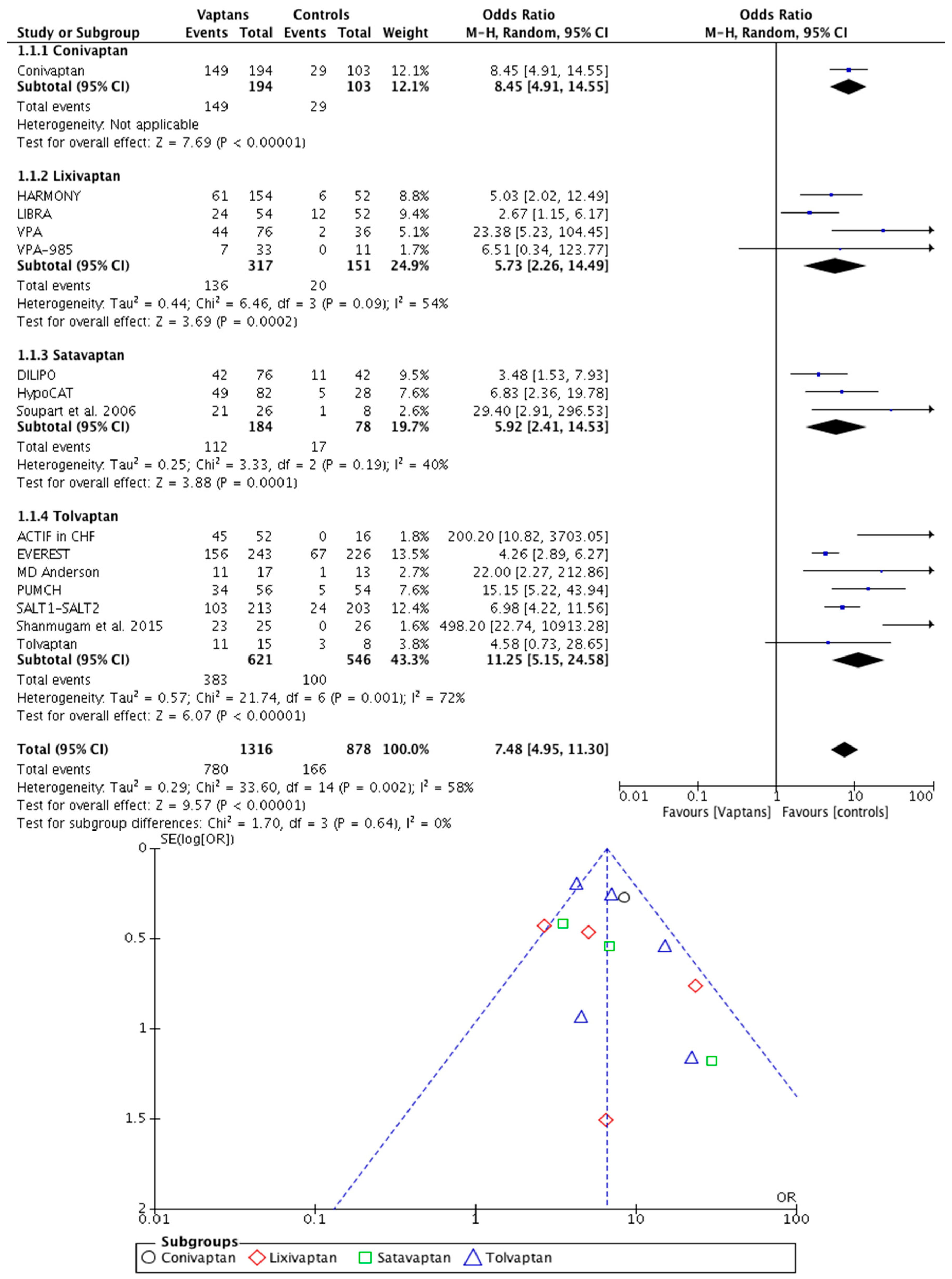
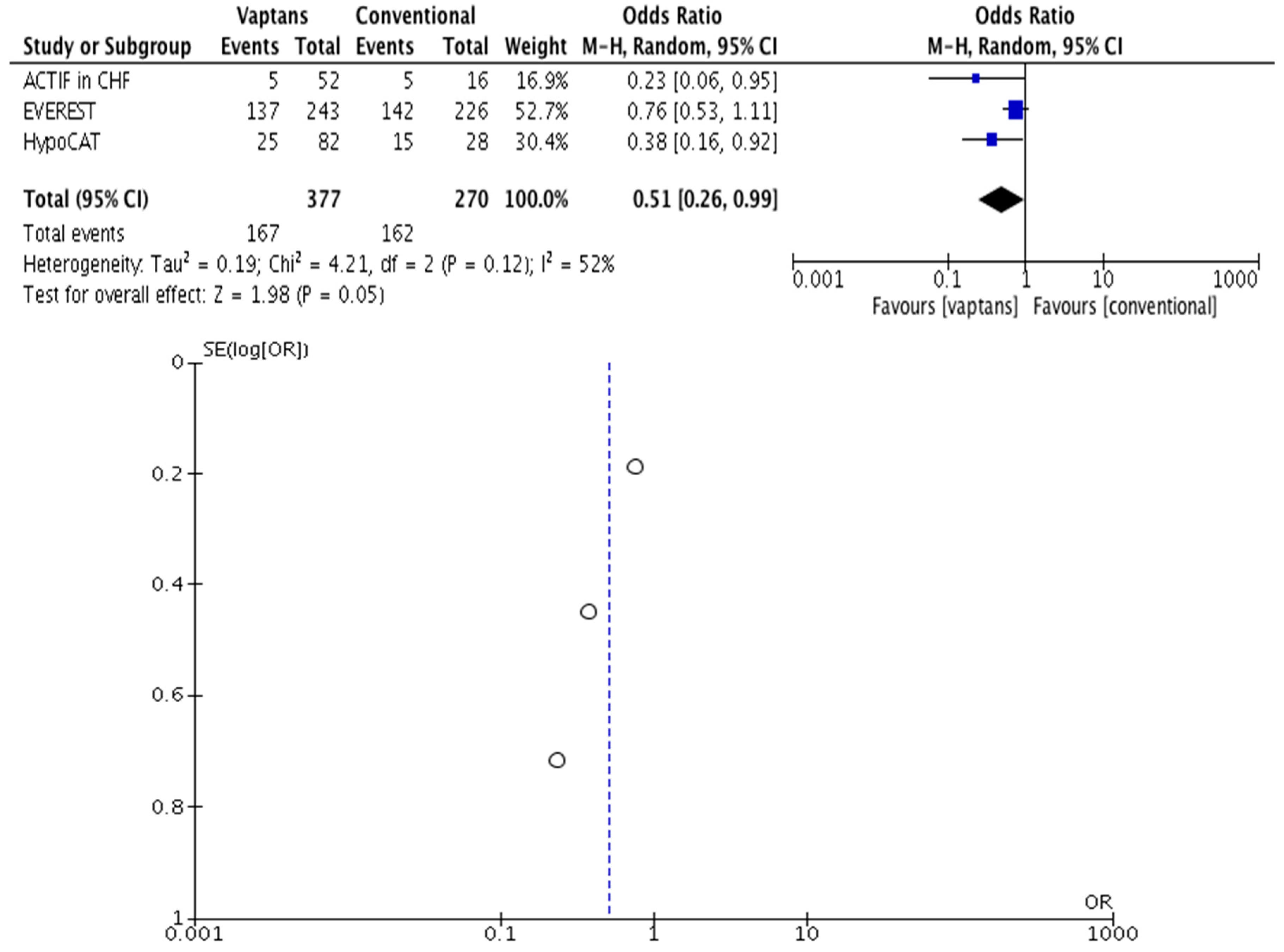
| Systematic Mapping (Stage 1) | In-Depth Meta-Analysis (Stage 2) | |
|---|---|---|
| Review Question | Do vaptans have an influence on hyponatremia €? | Is there an association between correction of hyponatremia $ under vaptans and improvement of clinical outcomes in both worsening HF and cirrhotic ascites? |
| Participants/ Population | Patients with hypervolemic/euvolemic hyponatremia of diverse causes | Patients with worsening HF or with cirrhotic ascites, having hyponatremia. |
| Intervention(s)/ Exposures(s) | Vaptans | Correction of hyponatremia and improvement of the following clinical outcomes: rehospitalization and/or death in patients with worsening HF and ascites worsening in liver cirrhosis patients with ascites. |
| Comparators | Placebo/standard care | No correction of hyponatremia and no clinical improvement of worsening HF or cirrhotic ascites. |
| Trials Details | Design | Follow-Up | Participants/Population Characteristics | Interventions (n) | Comparators (n) | Outcomes | Co-Interventions |
|---|---|---|---|---|---|---|---|
| Conivaptan Global [12,13,14,15] | RCT | 7–9 days | Males (%)/≥65 year (%): 52.31/68.48. [Na+]serum < 130 mEq/lt. Causes (%): SIADH (51.44), HF/COPD (32.30), cancer (11.11), postsurgical (4.47). | Conivaptan 40 mg/day (98) or 80 mg/day (96), IV/PO. | Placebo (103) | Day-5 [Na+]serum. Efficacy outcomes. £ | Fluid restriction to <2.0 L/24 h. Dietary and medication restrictions. |
| HARMONY NCT00876798 Global [16] | RCT | 24 weeks | Males (%)/≥65 year (%): 49.70/51.42. [Na+]serum < 135 mEq/lt Causes (%): SIADH (98.00), cancer (2.00). | Lixivaptan 25 mg plus dose titration (154). | Placebo (52) | Day-7 [Na+]serum. | Fluid restriction (investigator’s discretion). |
| LIBRA NCT00660959 Global [17] | RCT | 30 days | Males (%)/≥65 year (%): 53.05/54.51. [Na+]serum < 130 mEq/L Causes (%): SIADH (92.50), cancer (7.50). | Lixivaptan 50 mg plus dose titration (54). | Placebo (52) | Day-7 [Na+]serum. | Fluid restriction (investigator’s discretion). |
| VPA Europe [18,19] | RCT | 7 days | Males (%): 76.79. [Na+]serum < 130 mEq/lt Causes (%): liver cirrhosis (55.29), SIADH (29.53), HF (13.46). | Lixivaptan 100 mg/day (36) or 200 mg/day (40). | Placebo (36) | Day-7 [Na+]serum. | Fluid restriction to <1.0 L/24 h. |
| VPA-985 Europe [20] | RCT | 9 days | Males (%): 70.52. [Na+]serum < 130 mEq/lt Causes (%): liver cirrhosis (75.00), HF (13.60), SIADH (11.40). | Lixivaptan 25 mg/day (12), 125 mg/day (11), or 250 mg/day (10) | Placebo (11) | Day-7 [Na+]serum. | Diuretics Fluid restriction to <1.5 L/24 h. Dietary restrictions. |
| DILIPO# NCT00274326 Global [21] | RCT | 48 weeks | Males (%)/≥65 year (%): 57.03/38.42. [Na+]serum < 135 mEq/lt Causes (%): HF (76.44), SIADH (17.17), postsurgical (4.35). | Satavaptan 25 mg/day (35) or 50 mg/day (41). | Placebo (42) | Day-2 [Na+]serum. Efficacy outcomes. £ | Fluid restriction to <1.5 L/24 h. |
| Soupart et al., 2006 Europe [22] | RCT | 12 months | Males (%)/≥65 year (%): 57.03/38.41. [Na+]serum < 135 mEq/lt Causes (%): SIADH (85.67), cancer (14.33). | Satavaptan 25 mg/day (14) or 50 mg/day (12). | Placebo (9) | Day-5 [Na+]serum. Efficacy outcomes. £ | Fluid restriction to <1.5 L/24 h. |
| HypoCAT& NCT00501722 Europe [23] | RCT STR ¥ | 14 days | Males (%): 70.05. [Na+]serum < 130 mEq/lt Cause: ascites in liver cirrhosis. | Satavaptan 5 mg/day (28), 12.5 mg/day (26) or 25 mg/day (28). | Placebo (28) | Day-5 [Na+]serum in association to clinical outcomes at Day-30. | Fluid restriction to <1.5 L/24 h. |
| SALT1–SALT2† NCT00072683 NCT00201994 Global [24,25] | RCT | 37 days | Males (%): 58.33. [Na+]serum < 135 mEq/lt Causes (%): SIADH (42.70), HF (30.75), liver cirrhosis (26.55). | Tolvaptan 15 mg/day (225) | Placebo (223) | Day-4 and Day-30 [Na+]serum. Efficacy outcomes. £ | Medication restrictions. |
| ACTIF in CHF□ Global [26,27] | RCT STR ¥ | 60 days | Males (%): 69.10. [Na+]serum < 135 mEq/lt Cause: HF. | Tolvaptan 30 mg/day (15), 60 mg/day (22), or 90 mg/day (15). | Placebo (16) | Day-3 [Na+]serum in association to clinical outcomes at Day-60. | HF therapy. |
| EVEREST□ NCT00071331 Global [28,29,30] | RCT STR ¥ | 60 days | Males (%): 76.48. [Na+]serum < 135 mEq/lt Cause: HF. | Tolvaptan 30 mg/day (243) | Placebo (232) | Day-3 [Na+]serum in association to clinical outcomes at Day-60. | HF therapy. |
| Tolvaptan USA [31] | RCT | 65 days | Males (%): 57.00. [Na+]serum < 135 mEq/lt Causes (%): HF (50.00), SIADH (36.00), liver cirrhosis (14.00). | Tolvaptan 10 mg/day plus dose titration (17). | Placebo (11) | Day-5 [Na+]serum. Efficacy outcomes. £ | Fluid restriction to <1.2 L/24 h. |
| PUMCH‡ NCT00664014 China [32,33,34] | RCT | 7 days | Males (%): 51.11. [Na+]serum < 135 mEq/lt Causes (%): HF (59.90), SIADH (40.10). | Tolvaptan 15 mg/day plus dose titration (56). | Placebo (54) | Day-4 and Day-7 [Na+]serum. Efficacy outcomes. £ | Fluid restriction (investigator’s discretion). |
| MD Anderson Cancer Center NCT01199198 USA [35] | RCT | 14 days | Males (%): 53.57. [Na+]serum < 130 mEq/lt Causes (%): cancer (89.00), SIADH (11.00). | Tolvaptan 15 mg/day plus dose titration (17). | Placebo (13) | Day-14 [Na+]serum. | DiureticsFluid restriction to <1.5 L/24 h. |
| Shanmugam et al., 2015 CTRI/2013/05/003643 [36] India | RCT | 30 days | Males (%): 70.70. [Na+]serum < 135 mEq/lt Cause: HF. | Tolvaptan 15 mg/day (25) | Placebo (26) | Day-5 [Na+]serum. | None |
| Information Requests | Comments |
|---|---|
| Section 1 : Context | |
| Details about the Biomarker, the Test and the Medicine | |
| 1 (O) Current reimbursement arrangements. | Changes in [Na+]serum would permit evaluation of treatment effect or response to vaptans in patients with worsening HF or cirrhotic ascites. Testing is widely available and affordable. |
| 2 (T) Test sponsor. | Three methods (flame photometry, indirect and direct potentiometry) and many sponsors are currently available to measure sodium levels in serum. |
| 3 (M) Medicine sponsor. | Otsuka: Samsca® (tolvaptan). |
| 4 (O) Biomarker. | Correction of hyponatremia: normalization or increase of [Na+]serum of at least 3–5 mEq/L after 2–5 days of treatment with vaptans. |
| 5 (T) Proposed test. | Determination of serum sodium. |
| 6 (O) Medical condition or problem being managed. | Worsening HF and cirrhotic ascites. |
| 7 (O) Clinical management pathways. | Decision-making in the management of patients with worsening HF or cirrhotic ascites under treatment with vaptans. |
| Rationale for the Codependency | |
| 8 (O) Definition of the biomarker. | Treatment stratification biomarker. |
| 9 (O) Biological rationale for targeting that biomarker(s). | Correction of hyponatremia could be associated with favorable clinical outcomes in patients with worsening HF and cirrhotic ascites. |
| 10 (O) Other biomarker(s) to assess treatment effect of the medicine. | NA |
| 11 (O) Prevalence of the condition being targeted in the population that is likely to receive the test. | The conditions are very prevalent. |
| Proposed Impact of Codependent Technologies on Current Clinical Practice | |
| 12 (T) Consistency of the test results over time. | Clinical outcomes in both worsening HF and cirrhotic ascites improved under the effect of vaptans if correction of hyponatremia was achieved. |
| 13 (T) Use of the proposed test with other treatments and/or for other purposes. | NA |
| 14 (T) Use of the test in the clinical management pathway. | The test is most likely to be an additional test for managing patients. |
| 15 (T) Provision of the test. | The test is in routine use worldwide. |
| 16 (T) Specimen or sample collection. | Blood serum |
| 17 (T) Use of the test for monitoring purposes (if relevant) | For identifying good and poor responders to vaptans. |
| 18 (O) Availability of other tests for the biomarker. | None |
| Section 2: Clinical Evaluation | |
| Direct Evidence Approach | |
| Section 2a: Evidence of Prognostic Effect of the Biomarker | |
| 19 (O) Prognostic effect of the biomarker. | Not assessed. |
| Section 2d: Clinical Evaluation of the Codependent Technologies (Combined) | |
| 20 (O) Selection of the direct evidence. | Direct evidence, albeit of a lower level, is provided by retrospective biomarker-stratified RCTs. |
| 21 (O) Quality of the direct evidence. | Adequate quality. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Gómez, F.; Monge-Donaire, D.; Ochoa-Sangrador, C.; Bustamante-Munguira, J.; Alamartine, E.; Álvarez, F.J. Correction of Hyponatremia May Be a Treatment Stratification Biomarker: A Two-Stage Systematic Review and Meta-Analysis. J. Clin. Med. 2018, 7, 262. https://doi.org/10.3390/jcm7090262
Herrera-Gómez F, Monge-Donaire D, Ochoa-Sangrador C, Bustamante-Munguira J, Alamartine E, Álvarez FJ. Correction of Hyponatremia May Be a Treatment Stratification Biomarker: A Two-Stage Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2018; 7(9):262. https://doi.org/10.3390/jcm7090262
Chicago/Turabian StyleHerrera-Gómez, Francisco, Diana Monge-Donaire, Carlos Ochoa-Sangrador, Juan Bustamante-Munguira, Eric Alamartine, and F. Javier Álvarez. 2018. "Correction of Hyponatremia May Be a Treatment Stratification Biomarker: A Two-Stage Systematic Review and Meta-Analysis" Journal of Clinical Medicine 7, no. 9: 262. https://doi.org/10.3390/jcm7090262






