Clinicopathologic Features and Treatment Outcomes in Patients with Stage I, High-Risk Histology or High-Grade Endometrial Cancer after Primary Staging Surgery: A Taiwanese Gynecologic Oncology Group Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Statistical Analysis
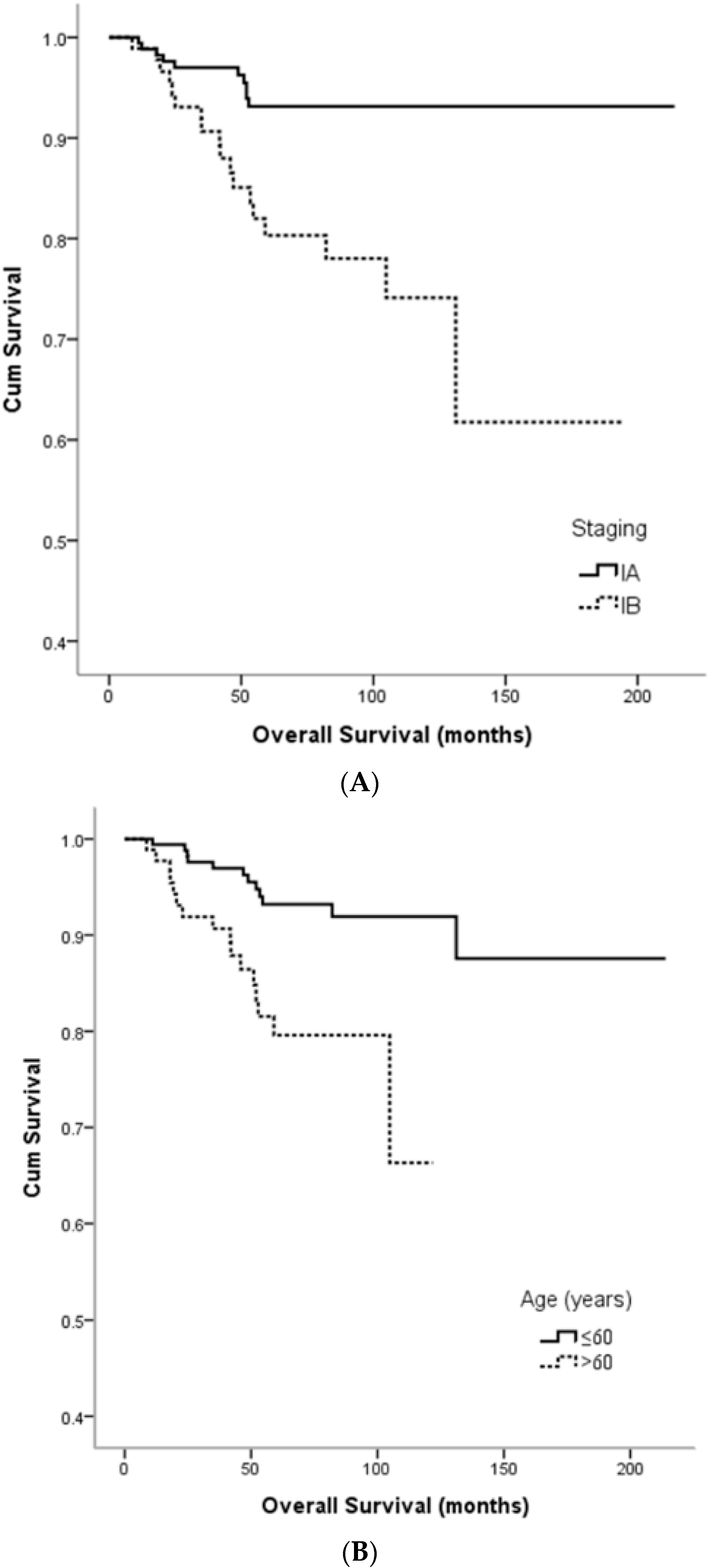
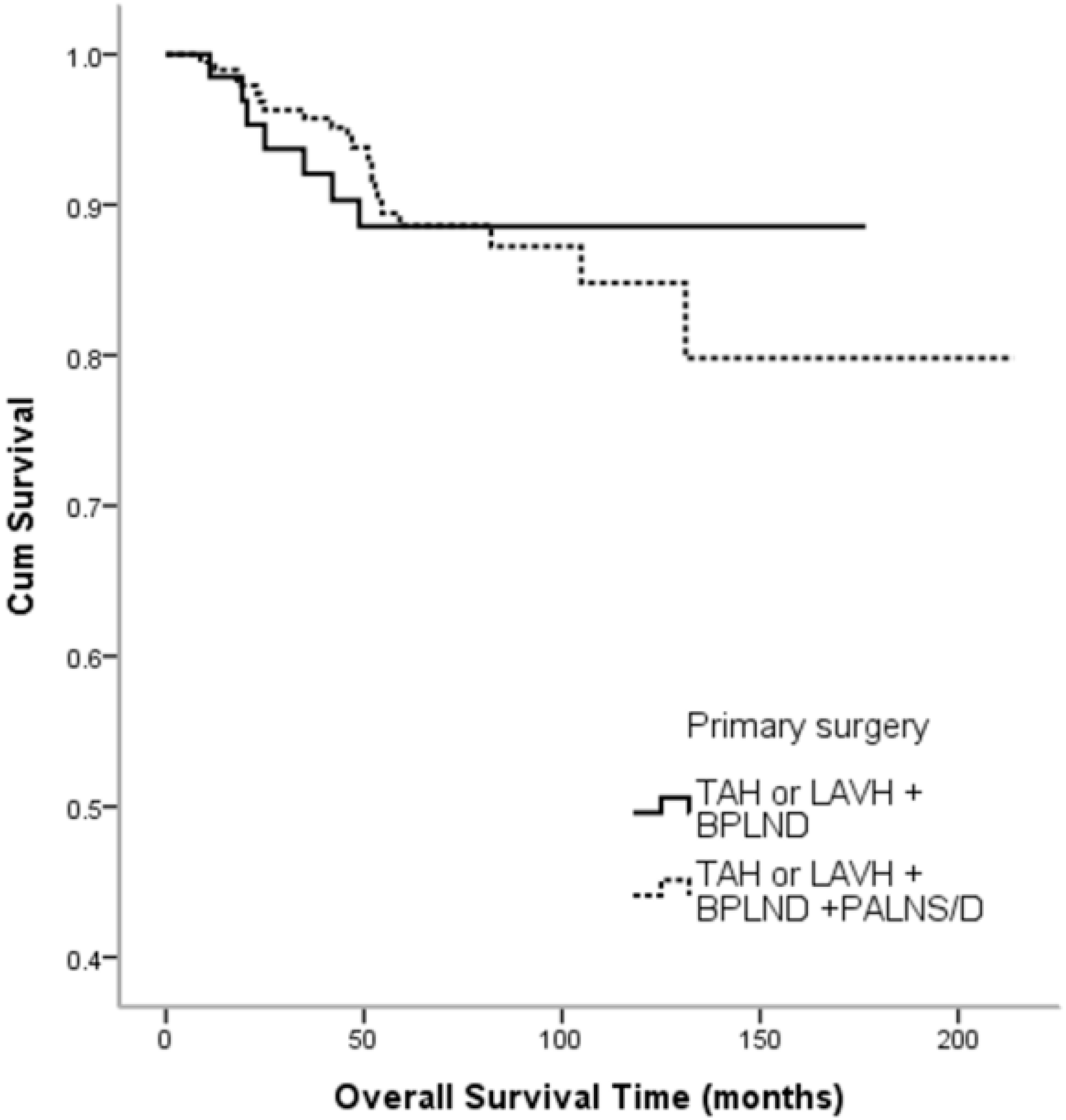
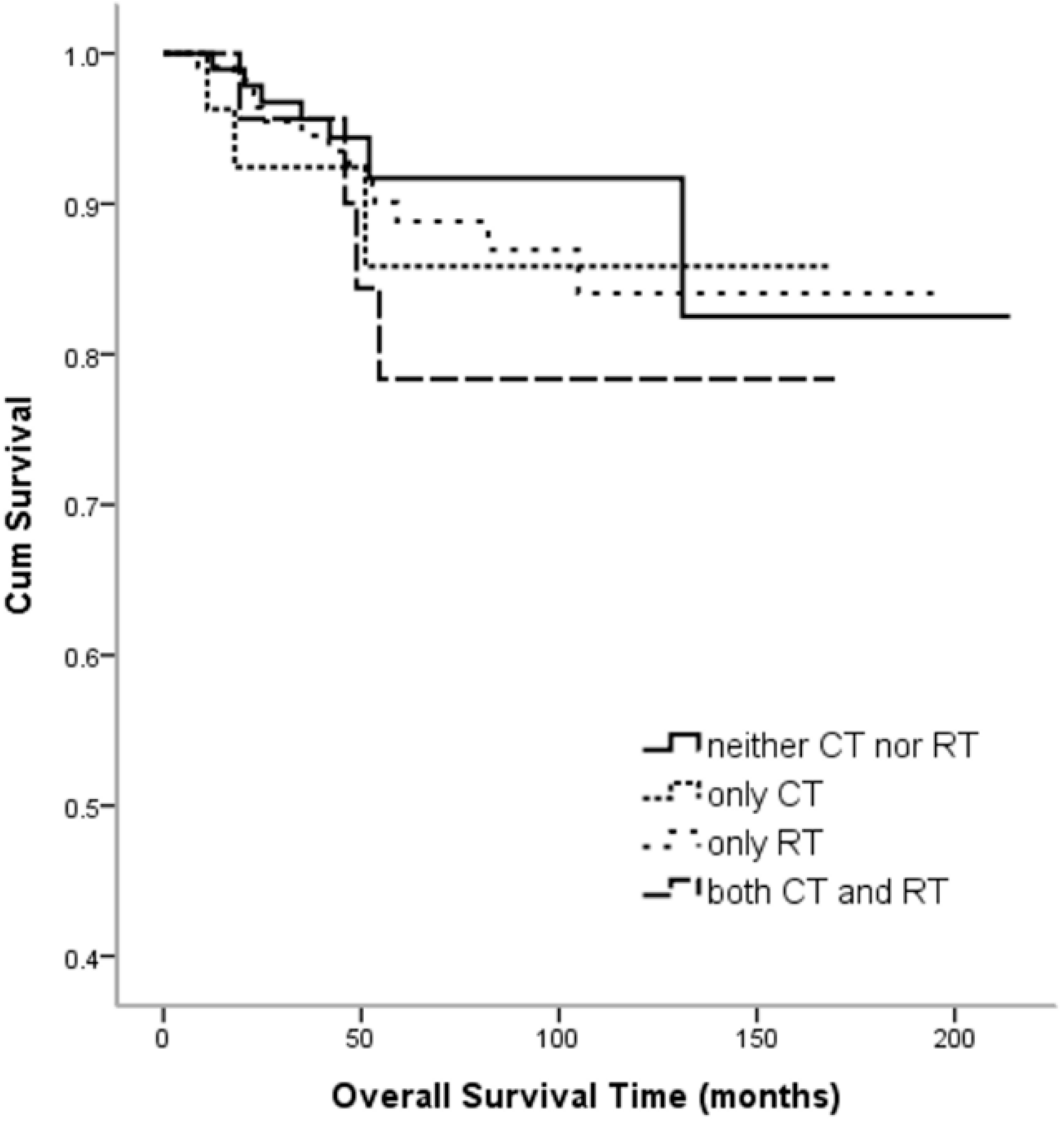
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Creutzberg, C.L.; van Putten, W.L.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van de Steen-Banasik, E.; et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC study group. Post operative radiation therapy in endometrial carcinoma. Lancet 2000, 355, 1404–1411. [Google Scholar] [CrossRef]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A gynecologic oncology group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Semaan, A.; Ali-Fehmi, R.; Munkarah, A.R.; Bandyopadhyay, S.; Morris, R.T.; Rizk, S.; Mert, I.; Ruterbusch, J.J.; Cote, M.L. Clinical/Pathologic features and patient outcome in early onset endometrial carcinoma: A population based analysis and an institutional perspective from the Detroit metropolitan area, Michigan. Gynecol. Oncol. 2012, 124, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Tuomi, T.; Pasanen, A.; Leminen, A.; Butzow, R.; Loukovaara, M. Prediction of site-specific tumor relapses in patients with stage I-II endometrioid endometrial cancer. Int. J. Gynecol. Cancer 2017, 27, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Lesnick, T.G.; Podratz, K.C. Surgical stage I endometrial cancer: Predictors of distant failure and death. Gynecol. Oncol. 2002, 87, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Nout, R.A.; Smit, V.T.; Putter, H.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Kroese, M.C.; et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010, 375, 816–823. [Google Scholar] [CrossRef]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C. ESMO Guidelines Working Group. Endometrial cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 6, 33–38. [Google Scholar]
- Morrow, C.P.; Bundy, B.N.; Kurman, R.J.; Creasman, W.T.; Heller, P.; Homesley, H.D.; Graham, J.E. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A gynecologic oncology group study. Gynecol. Oncol. 1991, 40, 55–65. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; van Putten, W.L.; Warlam-Rodenhuis, C.C.; van den Bergh, A.C.; de Winter, K.A.; Koper, P.C.; Lybeert, M.L.; Slot, A.; Lutgens, L.C.; Stenfert Kroese, M.C.; et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: The postoperative radiation therapy in endometrial carcinoma trial. J. Clin. Oncol. 2004, 22, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Kim, H.S.; Lee, M.; Chung, H.H.; Song, Y.S. Prognostic factors for tumor recurrence in endometrioid endometrial cancer stages IA and IB. Medicine (Baltimore) 2017, 96, e6976. [Google Scholar] [CrossRef] [PubMed]
- PNCCN Guidelines for Uterine Neoplasm (Version 1. 2018). Available online: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 23 June 2018).
- Latif, N.A.; Haggerty, A.; Jean, S.; Lin, L.; Ko, E. Adjuvant therapy in early-stage endometrial cancer: A systematic review of the evidence, guidelines and clinical practice in the U.S. Oncologist 2014, 19, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Eggink, F.A.; Mom, C.H.; Boll, D.; Ezendam, N.P.M.; Kruitwagen, R.; Pijnenborg, J.M.A.; van der Aa, M.A.; Nijman, H.W. Compliance with adjuvant treatment guidelines in endometrial cancer: Room for improvement in high risk patients. Gynecol. Oncol. 2017, 146, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.; Swart, A.M.; Orton, J.; Kitchener, H.; Whelan, T.; Lukka, H.; Eisenhauer, E.; Bacon, M.; Tu, D.; Parmar, M.K.; et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review and meta-analysis. Lancet 2009, 373, 137–146. [Google Scholar] [PubMed]
- Bendifallah, S.; Canlorbe, G.; Collinet, P.; Arsene, E.; Huguet, F.; Coutant, C.; Hudry, D.; Graesslin, O.; Raimond, E.; Touboul, C.; et al. Just how accurate are the major risk stratification systems for early-stage endometrial cancer. Br. J. Cancer 2015, 112, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Creutzberg, C.L.; Nout, R.A.; Lybeert, M.L.; Warlam-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; Pras, E.; van de Poll-Franse, L.V.; van Putten, W.L.; et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Aalders, J.; Abeler, V.; Kolstad, P.; Onsrud, M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients. Obstet. Gynecol. 1980, 56, 419–427. [Google Scholar] [PubMed]
- Creutzberg, C.L.; Nout, R.A. The role of radiotherapy in endometrial cancer: Current evidence and trends. Curr. Oncol. Rep. 2011, 13, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Hogberg, T.; Signorelli, M.; de Oliveira, C.F.; Fossati, R.; Lissoni, A.A.; Sorbe, B.; Andersson, H.; Grenman, S.; Lundgren, C.; Rosenberg, P.; et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer—Results from two randomised studies. Eur. J. Cancer 2010, 46, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Boothe, D.; Williams, N.; Odei, B.; Poppe, M.M.; Werner, T.L.; Suneja, G.; Gaffney, D.K. The addition of adjuvant chemotherapy to radiation in early-stage high-risk endometrial cancer: Survival outcomes and patterns of care. Int. J. Gynecol. Cancer 2017, 27, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.; Filiaci, V.; McMeekin, D.; Yashar, C.M.; Mannel, R.; Salani, R.; DiSilvestro, P.; Burke, J.; Rutherford, T.; Spirtos, N.; et al. A phase 3 trial of pelvic radiation therapy versus vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: A gynecology oncology group study. Int. J. Radiat. Oncol. 2017, 99, 1313. [Google Scholar] [CrossRef]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef]
- Yi, L.; Zhang, H.; Zou, J.; Luo, P.; Zhang, J. Adjuvant chemoradiotherapy versus radiotherapy alone in high-risk endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2018, 149, 612–619. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.J.; Bell, D.W. The genomics and genetics of endometrial cancer. Adv. Genom. Genet. 2012, 2012, 33–47. [Google Scholar]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [PubMed]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jurgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef] [PubMed]
- Piulats, J.M.; Guerra, E.; Gil-Martin, M.; Roman-Canal, B.; Gatius, S.; Sanz-Pamplona, R.; Velasco, A.; Vidal, A.; Matias-Guiu, X. Molecular approaches for classifying endometrial carcinoma. Gynecol. Oncol. 2017, 145, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Haruma, T.; Nagasaka, T.; Nakamura, K.; Haraga, J.; Nyuya, A.; Nishida, T.; Goel, A.; Masuyama, H.; Hiramatsu, Y. Clinical impact of endometrial cancer stratified by genetic mutational profiles, POLE mutation and microsatellite instability. PLoS ONE 2018, 13, e0195655. [Google Scholar] [CrossRef] [PubMed]
- MacKay, H.J.; Levine, D.A.; Bae-Jump, V.L.; Bell, D.W.; McAlpine, J.N.; Santin, A.; Fleming, G.F.; Mutch, D.G.; Nephew, K.P.; Wentzensen, N.; et al. Moving forward with actionable therapeutic targets and opportunities in endometrial cancer: NCI clinical trials planning meeting report on identifying key genes and molecular pathways for targeted endometrial cancer trials. Oncotarget 2017, 8, 84579–84594. [Google Scholar] [CrossRef] [PubMed]
- Maida, Y.; Takakura, M.; Nishiuchi, T.; Yoshimoto, T.; Kyo, S. Exosomal transfer of functional small RNAs mediates cancer-stroma communication in human endometrium. Cancer Med. 2016, 5, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Ahmad, A.; Zubair, H.; Miree, O.; Singh, S.; Rocconi, R.P.; Scalici, J.; Singh, A.P. MicroRNAs in gynecological cancers: Small molecules with big implications. Cancer Lett. 2017, 407, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, S.; Cermakian, N. The tumor circadian clock: A new target for cancer therapy? Future Oncol. 2017, 13, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Takenoshita, S.; Akaike, M.; Kunisaki, C.; Fujii, S.; Nozaki, A.; Numata, K.; Shiozawa, M.; Rino, Y.; Tanaka, K.; et al. Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol. Rep. 2011, 25, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Chen, D.; Yang, K.; Zhao, Q.; Zhao, D.; Lv, X.; Ao, Y. The circadian clock gene PER2 plays an important role in tumor suppression through regulating tumor-associated genes in human oral squamous cell carcinoma. Oncol. Rep. 2017, 38, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.W.; Chen, D.; Sun, S.P.; Liu, Z.J.; Liu, F.; Xian, S.Z.; Wu, P.S.; Kong, G.Q. Overexpression of PER3 reverses paclitaxel resistance of prostate cancer cells by inhibiting the notch pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2572–2579. [Google Scholar] [PubMed]
- Gatti, G.; Lucini, V.; Dugnani, S.; Calastretti, A.; Spadoni, G.; Bedini, A.; Rivara, S.; Mor, M.; Canti, G.; Scaglione, F.; et al. Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget 2017, 8, 68338–68353. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.C.; Yeh, K.T.; Tang, K.P.; Chen, J.C.; Chang, J.G. Promoter methylation in circadian genes of endometrial cancers detected by methylation-specific PCR. Mol. Carcinog. 2006, 45, 732–740. [Google Scholar] [CrossRef] [PubMed]
| Variables | n | (%) |
|---|---|---|
| FIGO Stage | ||
| IA | 175 | (65.5) |
| IB | 92 | (34.5) |
| Median age (year) (range) | 57 (31–83) | |
| ≤60 | 175 | (65.5) |
| >60 | 92 | (34.5) |
| Median follow-up (m) (range) | 67.9 (4–201) | |
| Primary surgery | ||
| TAH (LAVH) + BPLND | 70 | (26.2) |
| TAH (LAVH) + BPLND + PALNS/D | 197 | (73.8) |
| BSO | ||
| Yes | 263 | (98.5) |
| No | 4 | (1.5) |
| Histology | ||
| Endometrioid | 203 | (76.0) |
| Papillary serous | 37 | (13.9) |
| Clear cell carcinoma | 27 | (10.1) |
| Tumor size | ||
| <2 cm | 80 | (30.7) |
| ≥2 cm | 181 | (69.3) |
| LVSI | ||
| Positive | 65 | (24.6) |
| Negative | 199 | (75.4) |
| Postoperative treatment | ||
| Observation | 102 | (38.2) |
| R/T | 114 | (42.7) |
| C/T | 28 | (10.5) |
| R/T + C/T | 23 | (8.6) |
| Recurrent site | ||
| Local | 9 | (24.3) |
| Distant | 24 | (64.9) |
| Local + Distant | 4 | (10.8) |
| Prognostic Factor | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Stage (IB) | 2.939 | 1.448–5.967 | 0.003 | 3.305 | 1.488–7.338 | 0.003 |
| Tumor size (≥2 cm) | 1.174 | 0.537–2.567 | 0.687 | 0.843 | 0.356–1.996 | 0.698 |
| LVSI (+) | 1.024 | 0.454–2.307 | 0.955 | 0.772 | 0.321–1.854 | 0.562 |
| Age (>60) | 2.582 | 1.277–5.220 | 0.008 | 2.342 | 1.128–4.863 | 0.022 |
| Surgery with PALNS/D | 1.123 | 0.502–2.514 | 0.778 | 1.190 | 0.510–2.778 | 0.688 |
| Postoperative Treatment | Recurrence | |||||
|---|---|---|---|---|---|---|
| No | (%) | Yes | (%) | Total | (%) | |
| Neither CT nor RT | 91 | 89.2% | 11 | 10.8% | 102 | 100.0% |
| Only CT | 23 | 82.1% | 5 | 17.9% | 28 | 100.0% |
| Only RT | 98 | 86.0% | 16 | 14.0% | 114 | 100.0% |
| Both CT and RT | 18 | 78.3% | 5 | 21.7% | 23 | 100.0% |
| Total | 230 | 86.1% | 37 | 13.9% | 267 | 100.0% |
| Postoperative Treatment | Recurrence Site 1 | Recurrence Site 2 | Recurrence Site 3 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Neither CT nor RT | 3 | 27.3% | 5 | 45.5% | 3 | 27.3% | 11 | 100.0% |
| Only CT | 0 | 0.0% | 5 | 100.0% | 0 | 0.0% | 5 | 100.0% |
| Only RT | 4 | 25.0% | 11 | 68.8% | 1 | 6.3% | 16 | 100.0% |
| Both CT and RT | 2 | 40.0% | 3 | 60.0% | 0 | 0.0% | 5 | 100.0% |
| Total | 9 | 24.3% | 24 | 64.9% | 4 | 10.8% | 37 | 100.0% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, M.-S.; Chen, T.-H.; Ke, Y.-M.; Hsu, K.-F.; Chen, J.-R.; Yu, M.-H.; Fu, H.-C.; Huang, C.-Y.; Chiang, A.-J.; Chen, C.-Y.; et al. Clinicopathologic Features and Treatment Outcomes in Patients with Stage I, High-Risk Histology or High-Grade Endometrial Cancer after Primary Staging Surgery: A Taiwanese Gynecologic Oncology Group Study. J. Clin. Med. 2018, 7, 254. https://doi.org/10.3390/jcm7090254
Yen M-S, Chen T-H, Ke Y-M, Hsu K-F, Chen J-R, Yu M-H, Fu H-C, Huang C-Y, Chiang A-J, Chen C-Y, et al. Clinicopathologic Features and Treatment Outcomes in Patients with Stage I, High-Risk Histology or High-Grade Endometrial Cancer after Primary Staging Surgery: A Taiwanese Gynecologic Oncology Group Study. Journal of Clinical Medicine. 2018; 7(9):254. https://doi.org/10.3390/jcm7090254
Chicago/Turabian StyleYen, Ming-Shyen, Tze-Ho Chen, Yu-Min Ke, Keng-Fu Hsu, Jen-Ruei Chen, Mu-Hsien Yu, Hung-Chun Fu, Chia-Yen Huang, An-Jen Chiang, Chao-Yu Chen, and et al. 2018. "Clinicopathologic Features and Treatment Outcomes in Patients with Stage I, High-Risk Histology or High-Grade Endometrial Cancer after Primary Staging Surgery: A Taiwanese Gynecologic Oncology Group Study" Journal of Clinical Medicine 7, no. 9: 254. https://doi.org/10.3390/jcm7090254
APA StyleYen, M.-S., Chen, T.-H., Ke, Y.-M., Hsu, K.-F., Chen, J.-R., Yu, M.-H., Fu, H.-C., Huang, C.-Y., Chiang, A.-J., Chen, C.-Y., Hsiao, S.-M., Kan, Y.-Y., & Liu, F.-S. (2018). Clinicopathologic Features and Treatment Outcomes in Patients with Stage I, High-Risk Histology or High-Grade Endometrial Cancer after Primary Staging Surgery: A Taiwanese Gynecologic Oncology Group Study. Journal of Clinical Medicine, 7(9), 254. https://doi.org/10.3390/jcm7090254





