Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases
Abstract
1. Introduction
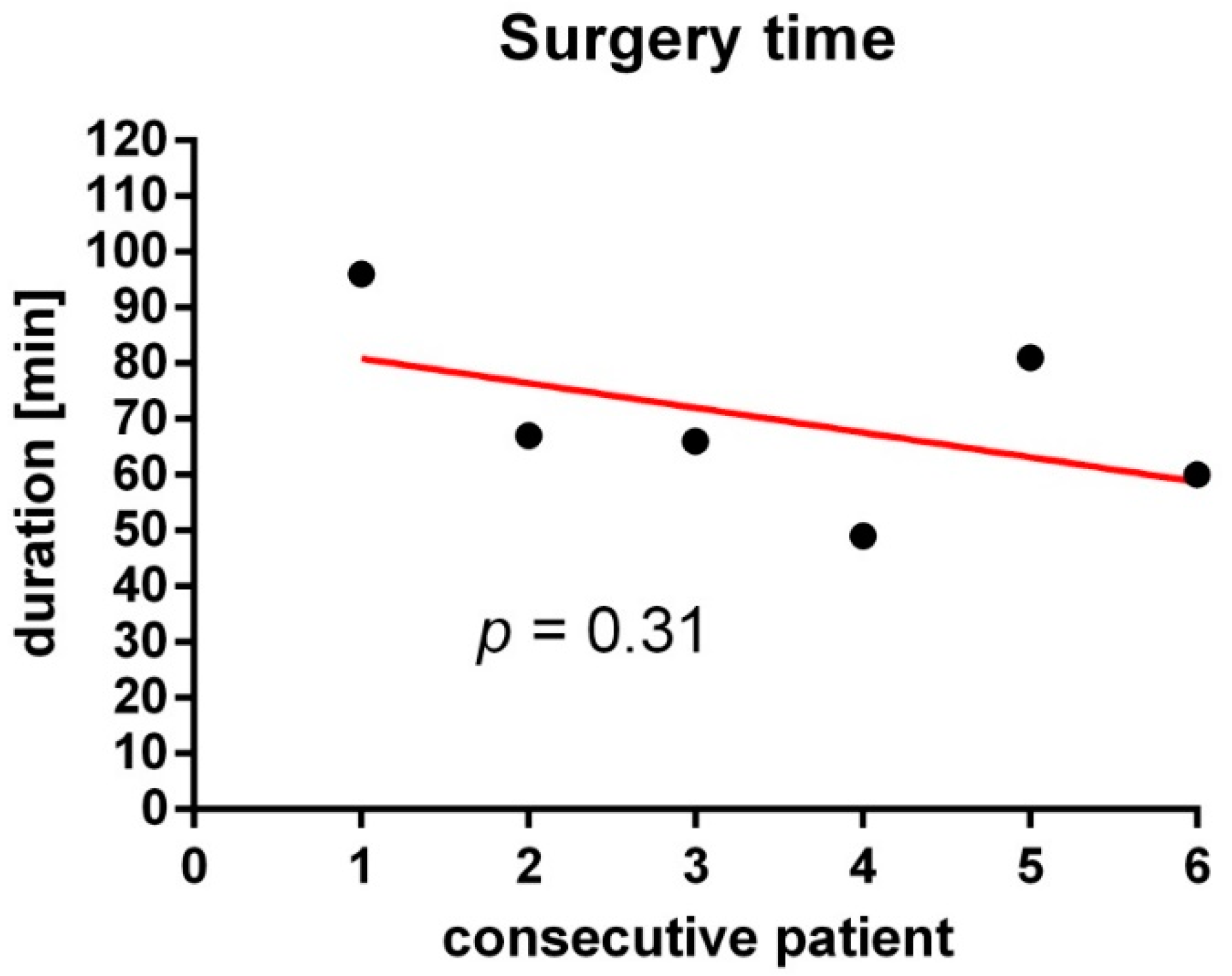
2. Results
3. Discussion
4. Patients and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Le Rhun, E.; Weller, M.; Brandsma, D.; Van den Bent, M.; de Azambuja, E.; Henriksson, R.; Boulanger, T.; Peters, S.; Watts, C.; Wick, W.; et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann. Oncol. 2017, 28, iv84–iv99. [Google Scholar] [CrossRef] [PubMed]
- Phuphanich, S.; Maria, B.; Braeckman, R.; Chamberlain, M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J. Neurooncol. 2007, 81, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Glantz, M.J.; Van Horn, A.; Fisher, R.; Chamberlain, M.C. Route of intracerebrospinal fluid chemotherapy administration and efficacy of therapy in neoplastic meningitis. Cancer 2010, 116, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Kormanik, P.; Howell, S.B.; Kim, S. Pharmacokinetics of intralumbar DTC-101 for the treatment of leptomeningeal metastases. Arch. Neurol. 1995, 52, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chatelut, E.; Kim, J.C.; Howell, S.B.; Cates, C.; Kormanik, P.A.; Chamberlain, M.C. Extended CSF cytarabine exposure following intrathecal administration of DTC 101. J. Clin. Oncol. 1993, 11, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.M.; Adamson, P.C.; Gillespie, A.J.; Poplack, D.G.; Balis, F.M. Intraventricular concentration times time (C × T) methotrexate and cytarabine for patients with recurrent meningeal leukemia and lymphoma. Cancer 1999, 85, 511–516. [Google Scholar] [CrossRef]
- Sandberg, D.I.; Bilsky, M.H.; Souweidane, M.M.; Bzdil, J.; Gutin, P.H. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery 2000, 47, 49–54. [Google Scholar] [PubMed]
- Volkov, A.A.; Filis, A.K.; Vrionis, F.D. Surgical treatment for leptomeningeal disease. Cancer Control 2017, 24, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zada, G.; Chen, T.C. A novel method for administering intrathecal chemotherapy in patients with leptomeningeal metastases and shunted hydrocephalus: Case report. Neurosurgery 2010, 67, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Palejwala, S.K.; Stidd, D.A.; Skoch, J.M.; Gupta, P.; Lemole, G.M., Jr.; Weinand, M.E. Use of a stop-flow programmable shunt valve to maximize CNS chemotherapy delivery in a pediatric patient with acute lymphoblastic leukemia. Surg. Neurol. Int. 2014, 5, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dunn, I.F.; Glantz, M.; Allison, D.L.; Jensen, R.; Johnson, M.D.; Friedlander, R.M.; Kesari, S. Benefit of ventriculoperitoneal cerebrospinal fluid shunting and intrathecal chemotherapy in neoplastic meningitis: A retrospective, case-controlled study. J. Neurosurg. 2011, 115, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, W.A.; Poplack, D.G. Intraventricular versus intralumbar methotrexate for central-nervous-system leukemia: Prolonged remission with the ommaya reservoir. Med. Pediatr. Oncol. 1979, 6, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Kormanik, P.A.; Barba, D. Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. J. Neurosurg. 1997, 87, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Arita, N.; Hayakawa, T.; Ushio, Y. ACNU, MTX and 5-FU penetration of rat brain tissue and tumors. J. Neurooncol. 1999, 45, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Taillibert, S.; Chamberlain, M.C. Leptomeningeal metastasis. Handb. Clin. Neurol. 2018, 149, 169–204. [Google Scholar] [PubMed]
- Grommes, C.; Oxnard, G.R.; Kris, M.G.; Miller, V.A.; Pao, W.; Holodny, A.I.; Clarke, J.L.; Lassman, A.B. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neurol. Oncol. 2011, 13, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, P.; Petrocca, F. Regional delivery of chimeric antigen receptor (CAR) T-cells for cancer therapy. Cancers 2017, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.S.; Jensen, M.C.; Satyamurthy, N.; Budhiraja, S.; Paik, D.; Czernin, J.; Gambhir, S.S. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat. Clin. Pract. Oncol. 2009, 6, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.R.; Bridge, C.; Murray, J.D.; Alvord, E.C., Jr. Virtual and real brain tumors: Using mathematical modeling to quantify glioma growth and invasion. J. Neurol. Sci. 2003, 216, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Forschler, H.; Kuker, W.; Meyermann, R.; Bamberg, M.; Dichgans, J.; Weller, M. Leptomeningeal metastasis: Survival and prognostic factors in 155 patients. J. Neurol. Sci. 2004, 223, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Jaeckle, K.A. Neoplastic meningitis from systemic malignancies: Diagnosis, prognosis and treatment. Semin. Oncol. 2006, 33, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Neoplastic meningitis. J. Clin. Oncol. 2005, 23, 3605–3613. [Google Scholar] [CrossRef] [PubMed]
- Glantz, M.J.; Jaeckle, K.A.; Chamberlain, M.C.; Phuphanich, S.; Recht, L.; Swinnen, L.J.; Maria, B.; LaFollette, S.; Schumann, G.B.; Cole, B.F.; et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin. Cancer Res. 1999, 5, 3394–3402. [Google Scholar] [PubMed]
- Wu, Y.; Green, N.L.; Wrensch, M.R.; Zhao, S.; Gupta, N. Ventriculoperitoneal shunt complications in california: 1990 to 2000. Neurosurgery 2007, 61, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Merkler, A.E.; Ch’ang, J.; Parker, W.E.; Murthy, S.B.; Kamel, H. The rate of complications after ventriculoperitoneal shunt surgery. World Neurosurg. 2017, 98, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Lara-Medina, F.; Crismatt, A.; Villarreal-Garza, C.; Alvarado-Miranda, A.; Flores-Hernandez, L.; Gonzalez-Pinedo, M.; Gamboa-Vignolle, C.; Ruiz-Gonzalez, J.D.; Arrieta, O. Clinical features and prognostic factors in patients with carcinomatous meningitis secondary to breast cancer. Breast J. 2012, 18, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Taillibert, S.; Zairi, F.; Kotecki, N.; Devos, P.; Mailliez, A.; Servent, V.; Vanlemmens, L.; Vennin, P.; Boulanger, T.; et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J. Neurooncol. 2013, 113, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Niwinska, A.; Rudnicka, H.; Murawska, M. Breast cancer leptomeningeal metastasis: The results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin. Breast Cancer 2015, 15, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Gwak, H.S.; Joo, J.; Kim, S.; Yoo, H.; Shin, S.H.; Han, J.Y.; Kim, H.T.; Lee, J.S.; Lee, S.H. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Harstad, L.; Hess, K.R.; Groves, M.D. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro. Oncol. 2008, 10, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genssler, S.; Schonfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J. Natl. Cancer Inst. 2016, 108, djv375. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, P.; Romanski, A.; Miller, N.; Odendahl, M.; Bonig, H.; Zhang, C.; Seifried, E.; Wels, W.S.; Tonn, T. Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies. Cancer Immunol. Immunother. 2018, 67, 25–38. [Google Scholar] [CrossRef] [PubMed]

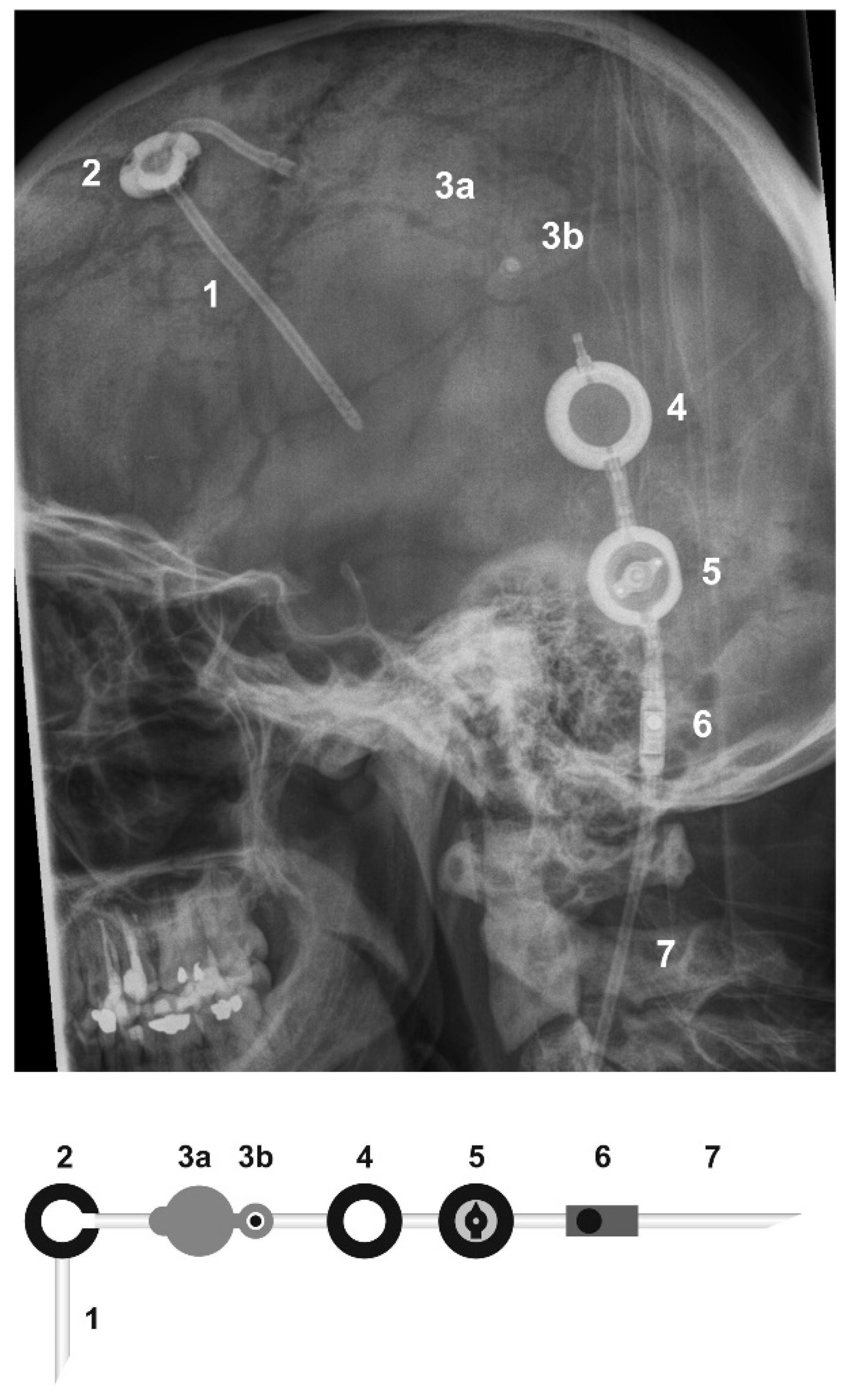
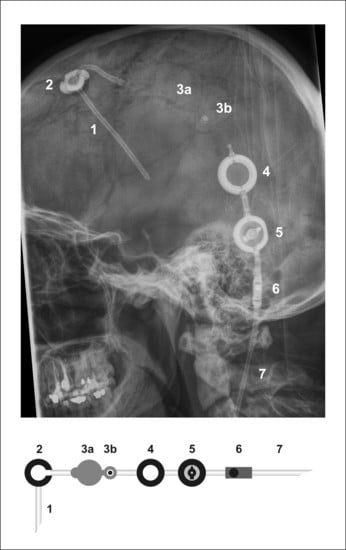
| Pat. No. | Intrathecal Chemotherapy Applied | Reasons for Omitting Intrathecal Chemotherapy | Complications | Number of Intraventricular Injections |
|---|---|---|---|---|
| 1 | None | Nodular type of meningeal metastases | None | 0 |
| 2 | None | Nodular type of meningeal metastases | None | 0 |
| 3 | None | clinical deterioration | None | 0 |
| 4 | MTX, thiotepa | - | None | 9 MTX 17 thiotepa |
| 5 | None | nodular type of meningeal metastases | None | 0 |
| 6 | Thiotepa | - | Shunt leakage | 1 |
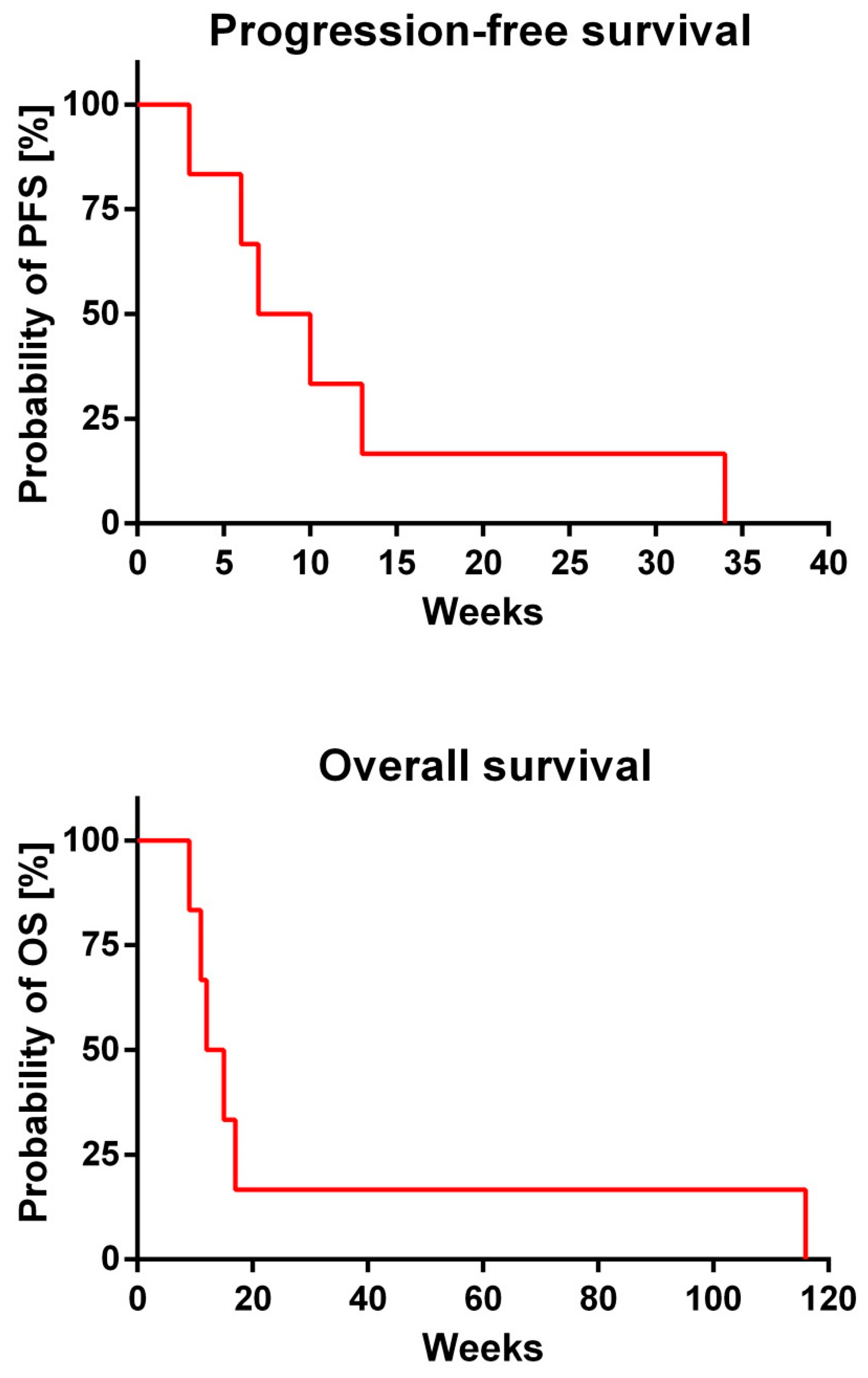
| Pat. No. | PFS (Weeks) | OS (Weeks) |
|---|---|---|
| 1 | 7 | 12 |
| 2 | 10 | 11 |
| 3 | 3 | 9 |
| 4 | 34 | 116 |
| 5 | 6 | 15 |
| 6 | 13 | 17 |
| Pat. No. | Age at Shunt Implantation | Sex | Underlying Disease |
|---|---|---|---|
| 1 | 33 years | F | NSCLC |
| 2 | 50 years | F | breast cancer |
| 3 | 57 years | M | transitional cell carcinoma |
| 4 | 60 years | F | NSCLC |
| 5 | 63 years | F | breast cancer |
| 6 | 70 years | M | prostate cancer |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, M.C.; Wagner, M.; Franz, K.; Harter, P.N.; Bähr, O.; Steinbach, J.P.; Senft, C. Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases. J. Clin. Med. 2018, 7, 216. https://doi.org/10.3390/jcm7080216
Burger MC, Wagner M, Franz K, Harter PN, Bähr O, Steinbach JP, Senft C. Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases. Journal of Clinical Medicine. 2018; 7(8):216. https://doi.org/10.3390/jcm7080216
Chicago/Turabian StyleBurger, Michael C., Marlies Wagner, Kea Franz, Patrick N. Harter, Oliver Bähr, Joachim P. Steinbach, and Christian Senft. 2018. "Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases" Journal of Clinical Medicine 7, no. 8: 216. https://doi.org/10.3390/jcm7080216
APA StyleBurger, M. C., Wagner, M., Franz, K., Harter, P. N., Bähr, O., Steinbach, J. P., & Senft, C. (2018). Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases. Journal of Clinical Medicine, 7(8), 216. https://doi.org/10.3390/jcm7080216