Calcification Patterns in Papillary Thyroid Carcinoma are Associated with Changes in Thyroid Hormones and Coronary Artery Calcification
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Group
2.1.1. Group for Observing the Association of Calcification in PTC and Coronary Artery Calcification (Group 1)
2.1.2. Group for Observing the Association of Calcification Pattern in PTC According to Thyroid Hormones (Group 2)
2.2. Measurements
2.3. Calcification Pattern in Thyroid Cancer
2.4. Definition of Metabolic Syndrome and Criteria for Diagnosis
2.5. Statistical Analysis
3. Results
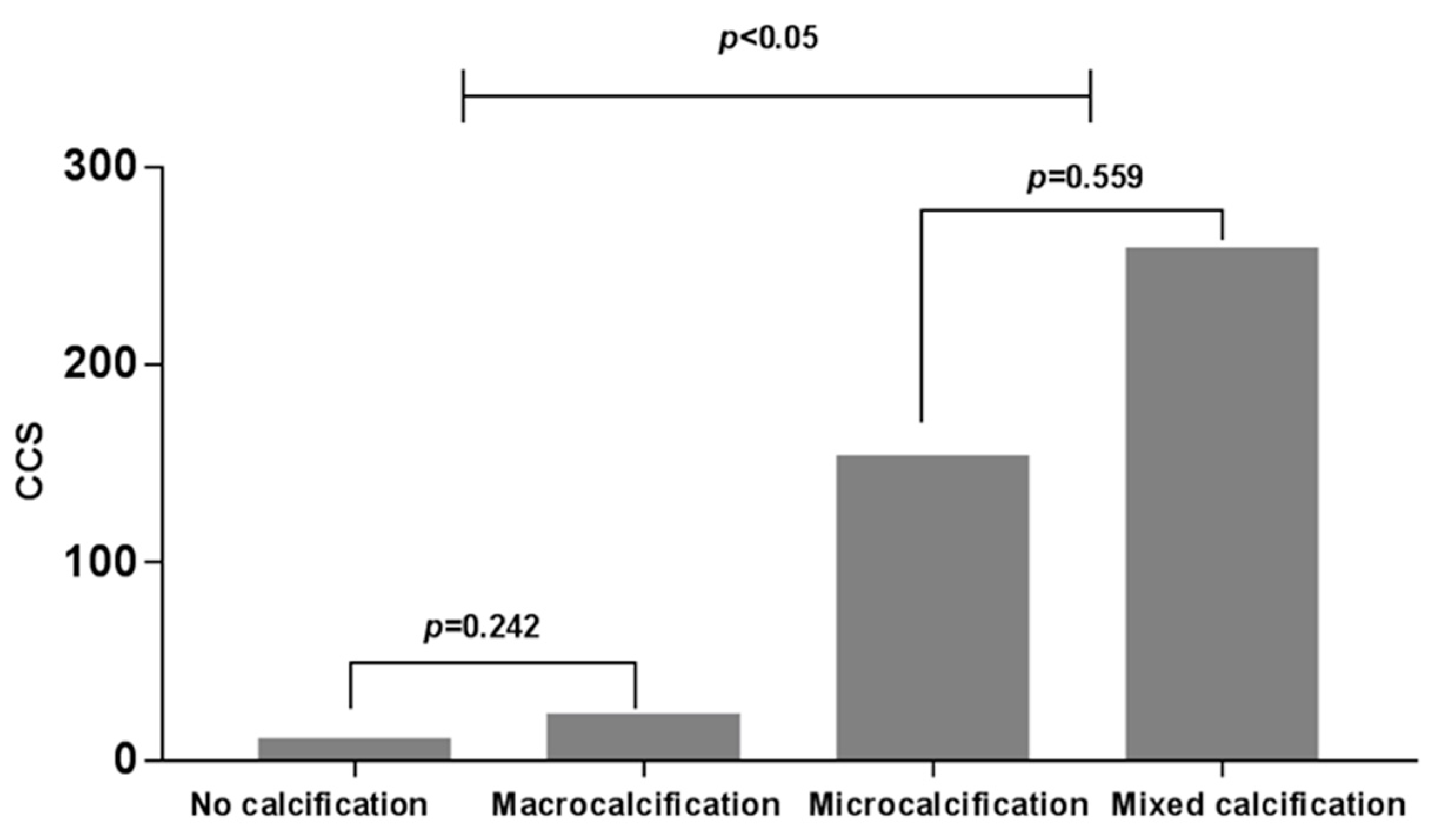
3.1. Relationship between Calcification Patterns of PTC and CAC
3.2. Relationships between Calcification Patterns of PTC and Thyroid Hormone Concentrations
3.3. Free T4 and TSH Concentrations According to the Four Calcification Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, D.K.; Mallik, M.K.; Haji, B.E.; Ahmed, M.S.; Al-Shama’a, M.; Al-Ayadhy, B.; George, S.S.; Sathar, S.A.; Junaid, T.A. Psammoma body and its precursors in papillary thyroid carcinoma: A study by fine-needle aspiration cytology. Diagn. Cytopathol. 2004, 31, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.J.; Baek, K.H. Response: Natural course of cytologically benign thyroid nodules: Observation of ultrasonographic changes (endocrinol metab 2013;28:110-8, dong jun lim et al.). Endocrinol. Metab. 2013, 28, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Choi, Y.S.; Kwon, H.J.; Lee, J.S.; Heo, J.J.; Han, Y.J.; Park, Y.H.; Kim, J.H. Relationship between patterns of calcification in thyroid nodules and histopathologic findings. Endocr. J. 2013, 60, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, J.; Han, E.J.; Park, S.E.; Park, C.Y.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Rhee, E.J. Association of low baseline free thyroxin levels with progression of coronary artery calcification over 4 years in euthyroid subjects: The kangbuk samsung health study. Clin. Endocrinol. 2016, 84, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, B.-K.; Chang, Y.; Ryu, S.; Cho, J.; Lee, W.-Y.; Rhee, E.-J.; Kwon, M.-J.; Rampal, S.; Zhao, D. Thyroid hormones and coronary artery calcification in euthyroid men and womensignificance. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Shin, J.A.; Shin, J.Y.; Lim, D.J.; Moon, S.D.; Son, H.Y.; Han, J.H. Association between low serum free thyroxine concentrations and coronary artery calcification in healthy euthyroid subjects. Thyroid 2012, 22, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Raggi, P. Techniques and technologies to assess vascular calcification. Semin. Dial. 2007, 20, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001, 38, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Tjessem, K.H.; Bosse, G.; Fosså, K.; Reinertsen, K.V.; Fosså, S.D.; Johansen, S.; Fosså, A. Coronary calcium score in 12-year breast cancer survivors after adjuvant radiotherapy with low to moderate heart exposure—Relationship to cardiac radiation dose and cardiovascular risk factors. Radiother. Oncol. 2015, 114, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Molloi, S. Breast arterial calcification: A new marker of cardiovascular risk? Curr. Cardiovasc. Risk Rep. 2013, 7, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.S.; ScanlON, E.; Heneghan, H.; Redmond, C.E.; Healy, G.M.; Mc Dermott, E.; Heffernan, E.J.; Prichard, R.; Mc Nally, S. Breast arterial calcification on screening mammography can predict significant coronary artery disease in women. Clin. Imaging 2017, 49, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.W.; Landgrafe, G.; Fotiadou, E.H. Tsh and thyrotropic agonists: Key actors in thyroid homeostasis. J. Thyroid Res. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.W. Der Hypophysen-Schilddrüsen-Regelkreis: Entwicklung und Klinische Anwendung Eines Nichtlinearen Modells. Spektrum Medizinischer Forschung, bd. 2; Logos Verlag Berlin: Berlin, Germany, 2006. [Google Scholar]
- Dietrich, J.W.; Stachon, A.; Antic, B.; Klein, H.H.; Hering, S. The aqua-fontis study: Protocol of a multidisciplinary, cross-sectional and prospective longitudinal study for developing standardized diagnostics and classification of non-thyroidal illness syndrome. BMC Endocr. Disord. 2008, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.W.; Landgrafe-Mende, G.; Wiora, E.; Chatzitomaris, A.; Klein, H.H.; Midgley, J.E.; Hoermann, R. Calculated parameters of thyroid homeostasis: Emerging tools for differential diagnosis and clinical research. Front. Endocrinol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.W.; Müller, P.; Schiedat, F.; Schlömicher, M.; Strauch, J.; Chatzitomaris, A.; Klein, H.H.; Mügge, A.; Köhrle, J.; Rijntjes, E. Nonthyroidal illness syndrome in cardiac illness involves elevated concentrations of 3, 5-diiodothyronine and correlates with atrial remodeling. Eur. Thyroid J. 2015, 4, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Jostel, A.; Ryder, W.D.; Shalet, S.M. The use of thyroid function tests in the diagnosis of hypopituitarism: Definition and evaluation of the tsh index. Clin. Endocrinol. 2009, 71, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised korean society of thyroid radiology consensus statement and recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C. Diagnosis and management of the metabolic syndrome: An american heart association/national heart, lung, and blood institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Yu, C.M.; Ji, Q.W.; Cai, M.; Zhao, Y.X.; Zhou, Y.J. Current understanding of coronary artery calcification. J. Geriatr. Cardiol. 2015, 12, 668–675. [Google Scholar] [PubMed]
- Shah, N.R.; Coulter, S.A. An evidence-based guide for coronary calcium scoring in asymptomatic patients without coronary heart disease. Tex. Heart Inst. J. 2012, 39, 240–242. [Google Scholar] [PubMed]
- Monzani, F.; Caraccio, N.; Kozàkowà, M.; Dardano, A.; Vittone, F.; Virdis, A.; Taddei, S.; Palombo, C.; Ferrannini, E. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: A double-blind, placebo- controlled study. J. Clin. Endocrinol. Metab. 2004, 89, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Bruckert, E.; Giral, P.; Chadarevian, R.; Turpin, G. Low free-thyroxine levels are a risk factor for subclinical atherosclerosis in euthyroid hyperlipidemic patients. J. Cardiovasc. Risk 1999, 6, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.; de Vries, R.; Roozendaal, C.; Kobold, A.C.; Sluiter, W.J. Carotid artery intima media thickness is inversely related to serum free thyroxine in euthyroid subjects. Clin. Endocrinol. 2007, 67, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Takamura, N.; Akilzhanova, A.; Hayashida, N.; Kadota, K.; Yamasaki, H.; Usa, T.; Nakazato, M.; Maeda, T.; Ozono, Y.; Aoyagi, K. Thyroid function is associated with carotid intima-media thickness in euthyroid subjects. Atherosclerosis 2009, 204, e77–e81. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. Thyroid disease and lipids. Thyroid 2002, 12, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Razvi, S.; Ingoe, L.; Keeka, G.; Oates, C.; McMillan, C.; Weaver, J.U. The beneficial effect of l-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: Randomized, crossover trial. J. Clin. Endocrinol. Metab. 2007, 92, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.; Bakker, S.J.; Links, T.P.; Gans, R.O.; Wolffenbuttel, B.H. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J. Clin. Endocrinol. Metab. 2007, 92, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Gammage, M.D.; Parle, J.V.; Holder, R.L.; Roberts, L.M.; Hobbs, F.D.; Wilson, S.; Sheppard, M.C.; Franklyn, J.A. Association between serum free thyroxine concentration and atrial fibrillation. Arch. Intern. Med. 2007, 167, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Gumieniak, O.; Perlstein, T.S.; Hopkins, P.N.; Brown, N.J.; Murphey, L.J.; Jeunemaitre, X.; Hollenberg, N.K.; Williams, G.H. Thyroid function and blood pressure homeostasis in euthyroid subjects. J. Clin. Endocrinol. Metab. 2004, 89, 3455–3461. [Google Scholar] [CrossRef] [PubMed]
- Carr, F.E.; Tai, P.W.; Barnum, M.S.; Gillis, N.E.; Evans, K.G.; Taber, T.H.; White, J.H.; Tomczak, J.A.; Jaworski, D.M.; Zaidi, S.K.; et al. Thyroid hormone receptor-β (trβ) mediates runt-related transcription factor 2 (runx2) expression in thyroid cancer cells: A novel signaling pathway in thyroid cancer. Endocrinology 2016, 157, 3278–3292. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast and odontoblast differentiation by runx2. J. Oral Biosci. 2010, 52, 22–25. [Google Scholar] [CrossRef]
- Shui, C.; Spelsberg, T.C.; Riggs, B.L.; Khosla, S. Changes in runx2/cbfa1 expression and activity during osteoblastic differentiation of human bone marrow stromal cells. J. Bone Miner. Res. 2003, 18, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.S.; Kang, G.; Kim, D.H.; Park, C.; Kim, J.H.; Sohn, J.H. The prognostic relevance of psammoma bodies and ultrasonographic intratumoral calcifications in papillary thyroid carcinoma. World J. Surg. 2013, 37, 2330–2335. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.J.; Jung, S.L.; Lee, J.H.; Na, D.G.; Baek, J.H.; Lee, Y.H.; Kim, J.; Kim, H.S.; Byun, J.S.; Lee, D.H. Benign and malignant thyroid nodules: Us differentiation--multicenter retrospective study. Radiology 2008, 247, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Papini, E.; Guglielmi, R.; Bianchini, A.; Crescenzi, A.; Taccogna, S.; Nardi, F.; Panunzi, C.; Rinaldi, R.; Toscano, V.; Pacella, C.M. Risk of malignancy in nonpalpable thyroid nodules: Predictive value of ultrasound and color-doppler features. J. Clin. Endocrinol. Metab. 2002, 87, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, G.; Nakamura, M.; Ozaki, T.; Mori, I.; Taniguchi, E.; Miyauchi, A.; Ito, Y.; Kakudo, K. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod. Pathol. 2009, 22, 887–894. [Google Scholar] [CrossRef] [PubMed]
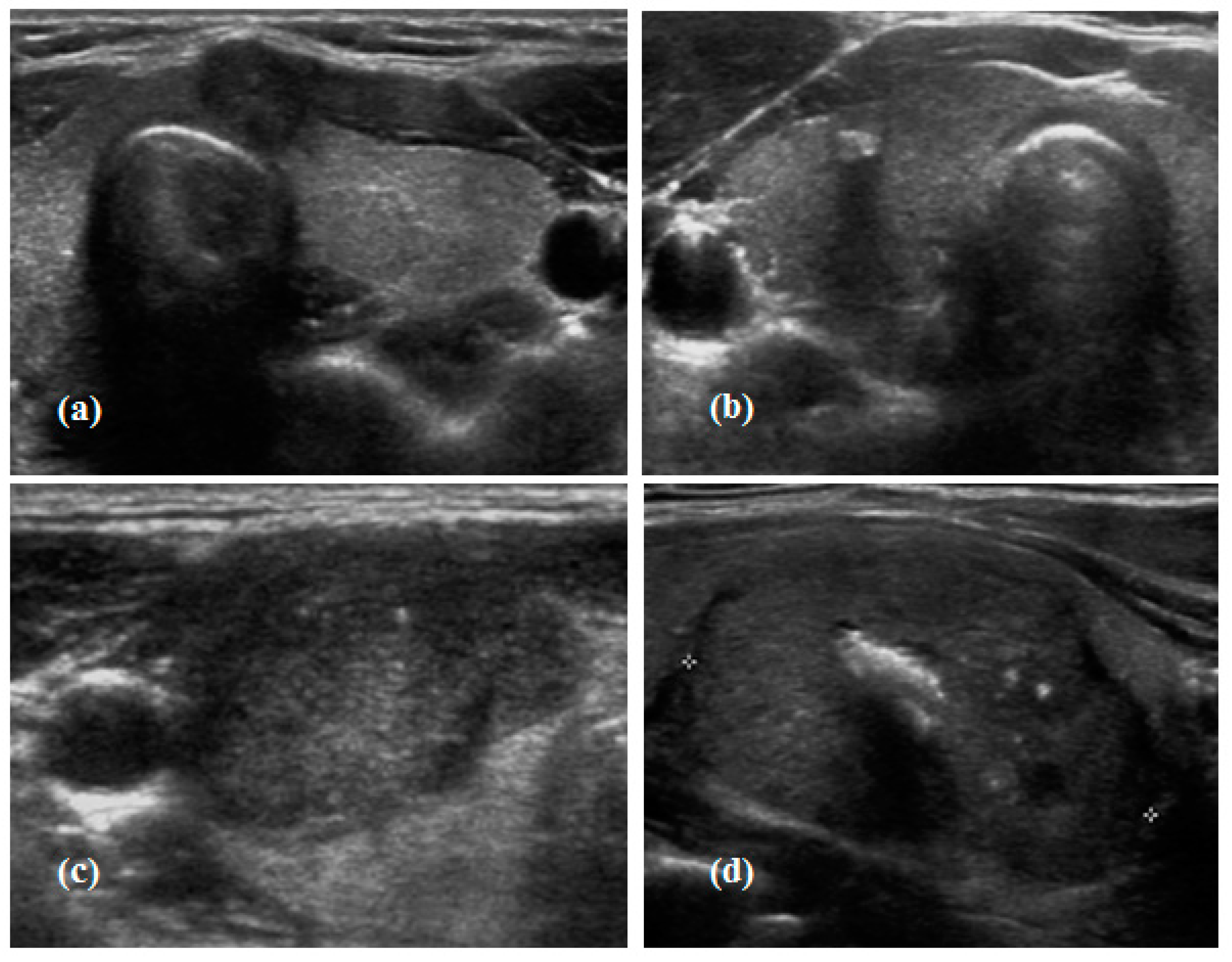
| No Calcification (n = 59) | Macrocalcification (n = 26) | Microcalcification (n = 69) | Mixed Calcification (n = 28) | p-Value | |
|---|---|---|---|---|---|
| Age | 61.39 ± 8.39 | 65.92 ± 9.36 | 63.67 ± 10.14 | 66.64 ± 12.93 | 0.080 * |
| Female (%) | 48 (81%) | 18 (69%) | 39 (57%) | 17 (61%) | 0.023 |
| Coronary calcification a (%) | 10 (17%) | 15 (58%) | 39 (57%) | 18 (64%) | 0.000 |
| CCS b | 11.39 ± 25.03 | 23.76 ± 40.27 | 154.39 ± 490.79 | 259.48 ± 737.90 | 0.179 * |
| BMI (kg/m2) | 25.38 ± 3.48 | 25.37 ± 3.64 | 24.86 ± 2.61 ‡ | 26.85 ± 4.18 † | 0.072 * |
| Number of components satisfying the requirements for MetS diagnosis c | 0.080 | ||||
| 0 | 4 | 1 | 7 | 0 | |
| 1 | 5 | 4 | 8 | 0 | |
| 2 | 19 | 7 | 18 | 5 | |
| 3 | 12 | 8 | 8 | 8 | |
| 4 | 14 | 3 | 20 | 12 | |
| MetS d | 26 (44%) | 11 (42%) | 28 (41%) | 20 (71%) | 0.026 |
| Clinical Parameters | Number (n = 354) |
|---|---|
| Age (years) | 46.08 ± 12.22 |
| Female (%) | 275 (77.7%) |
| Tumor size (cm) | 0.99 ± 0.87 |
| Patterns of calcification a | |
| No calcification (%) | 162 (45.8%) |
| Macrocalcification (%) | 42 (11.9%) |
| Microcalcification (%) | 108 (30.5%) |
| Mixed calcification b (%) | 42 (11.9%) |
| Extrathyroidal extension (%) | 141 (39.8%) |
| Lymph node metastasis (%) | 167 (47.2%) |
| TNM stage c (%) | |
| I | 142 (40.1%) |
| II | 11 (3.1%) |
| III | 154 (43.5%) |
| IV | 46 (13.0%) |
| Free T4 (ng/dL) | 1.33 ± 0.20 |
| T3 (ng/mL) | 1.11 ± 0.40 |
| TSH (μIU/mL) | 2.61 ± 2.12 |
| Anti-TPO Ab (U/L) | 106.33 ± 268.49 |
| No Calcification (n = 162) | Macrocalcification (n = 42) | Microcalcification (n = 108) | Mixed Calcification (n = 42) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 44.27 ± 10.68 ‡ | 54.60 ± 12.24 †,§ | 44.17 ± 13.09 ‡ | 49.48 ± 11.55 | 0.000 * |
| Female (%) | 127 (78.4%) | 36 (85.7%) | 80 (74.1%) | 32 (76.2%) | 0.480 |
| Tumor size (cm) | 0.78 ± 0.85 ‡,§,ǁ | 1.31 ± 0.94 † | 1.07 ± 0.83 | 1.27 ± 0.77 † | 0.000 * |
| Extrathyroidal extension (%) | 48 (29.6%) | 23 (54.8%) | 45 (41.7%) | 25 (59.5%) | 0.000 |
| Lymph node metastasis (%) | 58 (35.8%) | 18 (42.9%) | 65 (60.2%) | 26 (61.9%) | 0.000 |
| Number of LN metastasis | 1.53 ± 3.74 §,ǁ | 3.17 ± 6.28 | 3.69 ± 6.99 † | 4.17 ± 5.76 † | 0.003 * |
| LNR (%) | 11.04 ± 20.13 | 14.38 ± 20.84 | 17.12 ± 22.45 | 16.40 ± 18.22 | 0.103 |
| TNM stage (%) | 0.000 | ||||
| I | 85 (52.8%) | 14 (33.3%) | 32 (29.6%) | 11 (26.2%) | |
| II | 3 (1.2%) | 3 (7.1%) | 5 (4.6%) | 1 (2.4%) | |
| III | 65 (40.4%) | 19 (45.2%) | 51 (47.2%) | 19 (45.2%) | |
| IV | 9 (5.6%) | 6 (14.3%) | 20 (18.5%) | 11 (26.2%) | |
| Free T4 (ng/dL) | 1.39 ± 0.18 §,ǁ | 1.36 ± 0.23 ǁ | 1.28 ± 0.17 † | 1.22 ± 0.22 †,‡ | 0.000 * |
| T3 (ng/mL) | 1.08 ± 0.18 | 1.06 ± 0.15 | 1.16 ± 0.68 | 1.13 ± 0.21 | 0.360 * |
| TSH (μIU/mL) | 2.37 ± 1.37 ǁ | 2.26 ± 1.88 | 2.80 ± 2.63 | 3.31 ± 2.95 † | 0.032 * |
| Anti-TPO Ab (U/L) | 118.86 ± 300.33 | 97.10 ± 216.68 | 99.79 ± 259.17 | 84.03 ± 268.49 | 0.861 * |
| SPINA-GT (pmol/s) a | 4.40 ± 6.14 | 3.70 ± 1.72 | 3.79 ± 6.39 | 2.77 ± 1.08 | 0.368 * |
| sTSHI b | 0.55 ± 1.12 | 0.35 ± 1.10 | 0.44 ± 1.09 | 0.50 ± 1.21 | 0.741 * |
| Free T4 (ng/dL) | TSH (μIU/mL) | |||
|---|---|---|---|---|
| Crude | Adjusted a | Crude | Adjusted b | |
| No calcification | 1.39 ± 0.02 | 1.42 ± 0.02 | 2.37 ± 0.17 | 2.34 ± 0.21 |
| Macrocalcification | 1.36 ± 0.03 | 1.43 ± 0.04 | 2.26 ± 0.32 | 2.40 ± 0.46 |
| Microcalcification | 1.28 ± 0.02 | 1.29 ± 0.02 | 2.81 ± 0.20 | 2.82 ± 0.23 |
| Mixed calcification | 1.22 ± 0.03 | 1.23 ± 0.03 | 3.31 ± 0.32 | 3.37 ± 0.38 |
| p-value | 0.000 | 0.042 | 0.032 | 0.004 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J.; Lee, J.; Jo, K.; Han, J.-S.; Kim, M.-H.; Jung, C.K.; Kang, M.I.; Cha, B.Y.; Lim, D.-J. Calcification Patterns in Papillary Thyroid Carcinoma are Associated with Changes in Thyroid Hormones and Coronary Artery Calcification. J. Clin. Med. 2018, 7, 183. https://doi.org/10.3390/jcm7080183
Ha J, Lee J, Jo K, Han J-S, Kim M-H, Jung CK, Kang MI, Cha BY, Lim D-J. Calcification Patterns in Papillary Thyroid Carcinoma are Associated with Changes in Thyroid Hormones and Coronary Artery Calcification. Journal of Clinical Medicine. 2018; 7(8):183. https://doi.org/10.3390/jcm7080183
Chicago/Turabian StyleHa, Jeonghoon, Jeongmin Lee, Kwanhoon Jo, Jeong-Sun Han, Min-Hee Kim, Chan Kwon Jung, Moo IL Kang, Bong Yeon Cha, and Dong-Jun Lim. 2018. "Calcification Patterns in Papillary Thyroid Carcinoma are Associated with Changes in Thyroid Hormones and Coronary Artery Calcification" Journal of Clinical Medicine 7, no. 8: 183. https://doi.org/10.3390/jcm7080183
APA StyleHa, J., Lee, J., Jo, K., Han, J.-S., Kim, M.-H., Jung, C. K., Kang, M. I., Cha, B. Y., & Lim, D.-J. (2018). Calcification Patterns in Papillary Thyroid Carcinoma are Associated with Changes in Thyroid Hormones and Coronary Artery Calcification. Journal of Clinical Medicine, 7(8), 183. https://doi.org/10.3390/jcm7080183






