Automatic Infants’ Pain Assessment by Dynamic Facial Representation: Effects of Profile View, Gestational Age, Gender, and Race
Abstract
:1. Introduction
2. Related Work and Contributions
3. Methodology
3.1. Frame-Level Hybrid Facial Representation
- Facial landmark detection: Both facial configuration parameters and facial texture parameters are calculated on the basis of facial landmarks. We apply the popular Active Appearance Model (AAM) to detect infants’ facial landmarks (68 points) in image sequences to outline the main features of the eyebrows, eyes, nose, mouth, and face boundary (see Figure 1a). AAM combines a shape variation model with an appearance variation model in a shape-normalized frame, and is improved upon to be rapid, accurate, and robust [49].
- Facial deformation measurement: Several pain-related geometric distance parameters are derived for each image frame from the facial fiducial points of the eyebrows, eyes, nose, and mouth, to capture deformations of facial components. Specific facial actions have been identified in Neonatal Facial Coding System (NFCS), which is a subsystem of Facial Action Coding System (FACS), and specially designed for pain assessment for newborns to 2 months of age. The validity and reliability of the sign judgment method has been evaluated in previous studies [3]. The facial actions for infant pain assessment include brow bulge, nasolabial furrow, eye squeeze, chin quiver, open lips, lip purse, horizontal mouth stretch, vertical mouth stretch, taut tongue, and tongue protrusion [36]. Thus, distance parameters are defined by the NFCS as the Euclidean distance between key facial fiducial points, including distance between the eyebrow and the eye (debl and debr), distance between the upper eyelid and the lower eyelid (del and der), distance between the eyebrow and the mouth (dmbl and dmbr), distance between the eye and the mouth (deml and demr), distance between the nose and the mouth (dnm), and the width (dmw) and height (dmh) of the mouth. Facial deformation parameters are shown in Figure 1b.
- Head pose movement measurement: According to clinical observation, infants in pain usually shake their head left and right. Head movement is a useful predictor for infant pain assessment. Since there is no obvious relationship between pain occurrence/intensity and the head orientation angle, we conducted distance-based head pose parameters in a simple manner. The distance parameters include the distances between key facial components landmarks (eyebrows, eyes, nose, and mouth) and face boundary landmarks for both left and right sides of the face (dbbl, dbel, dbnl, dbml, dbbr, dber, dbnr, dbmr). The head pose parameters are illustrated in Figure 1c.
- Facial texture measurement: Appearance-based facial expression features are extracted to reflect the magnitude and direction of skin surface displacement. Based on primary facial landmarks, patches with size of 32 × 32 are defined around the landmarks. A domain knowledge-based texture description method is employed by calculating the mean magnitude for each local patch. These patches cover most of the facial regions, as illustrated in Figure 1d, where obvious wrinkles usually appear between the eyebrows (eyes), the corner of eyes, nasal root, and corner of the mouth. It is a simple and low-dimensional facial texture representation comparing to generic facial features, as only one numeric parameter is obtained for each RAP, and there are 31 texture parameters for each frame image.
3.2. Sequence-Based Temporal Descriptors Extraction
3.3. Dimensionality Reduction Using SLPP
3.4. Classification and Decision Fusion
4. Dataset
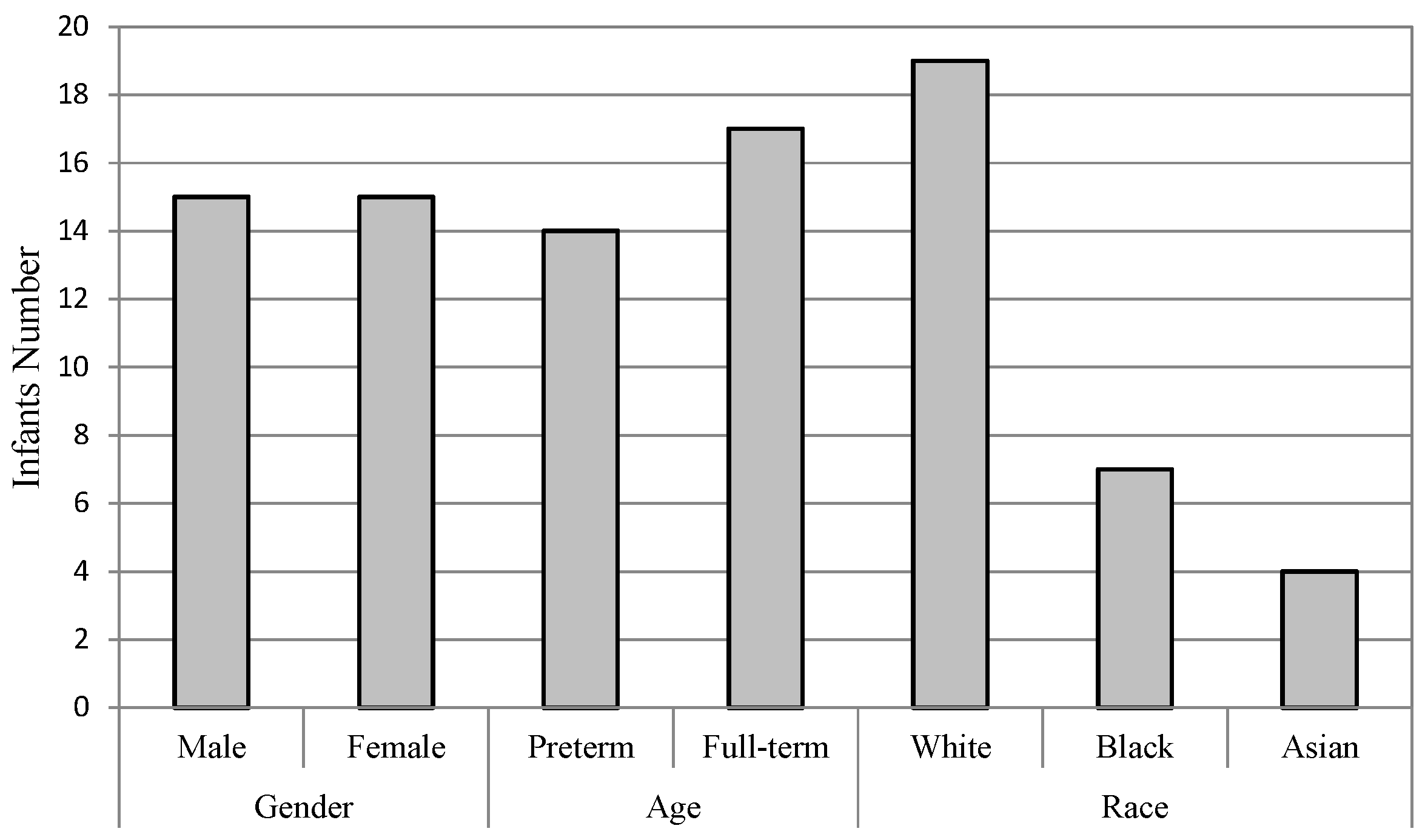
4.1. Subjects
4.2. Data Acquisition and Preprocessing
4.3. Ground Truth Assessment
5. Experimental Results and Discussion
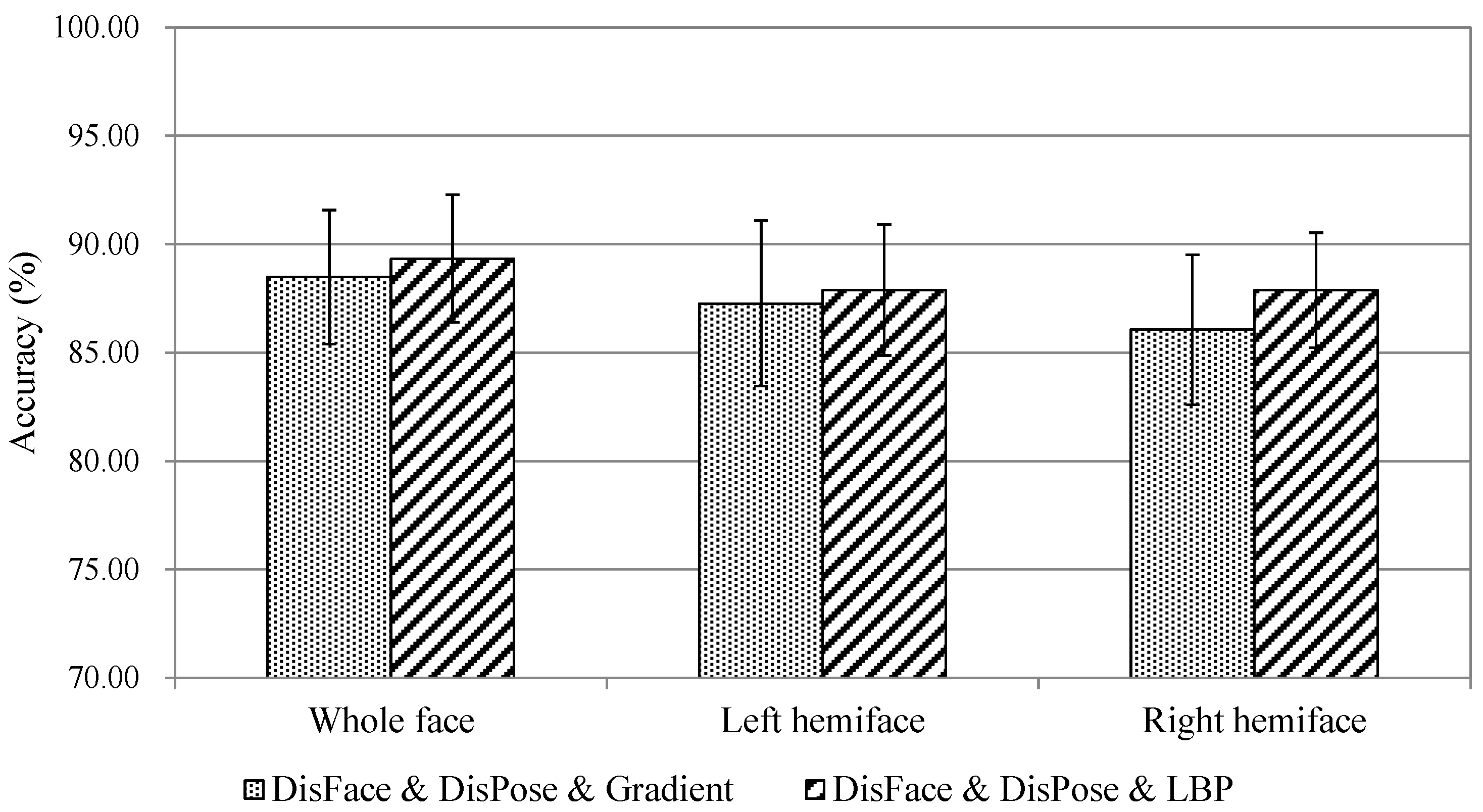
5.1. Comparison of Multi-Feature Fusion
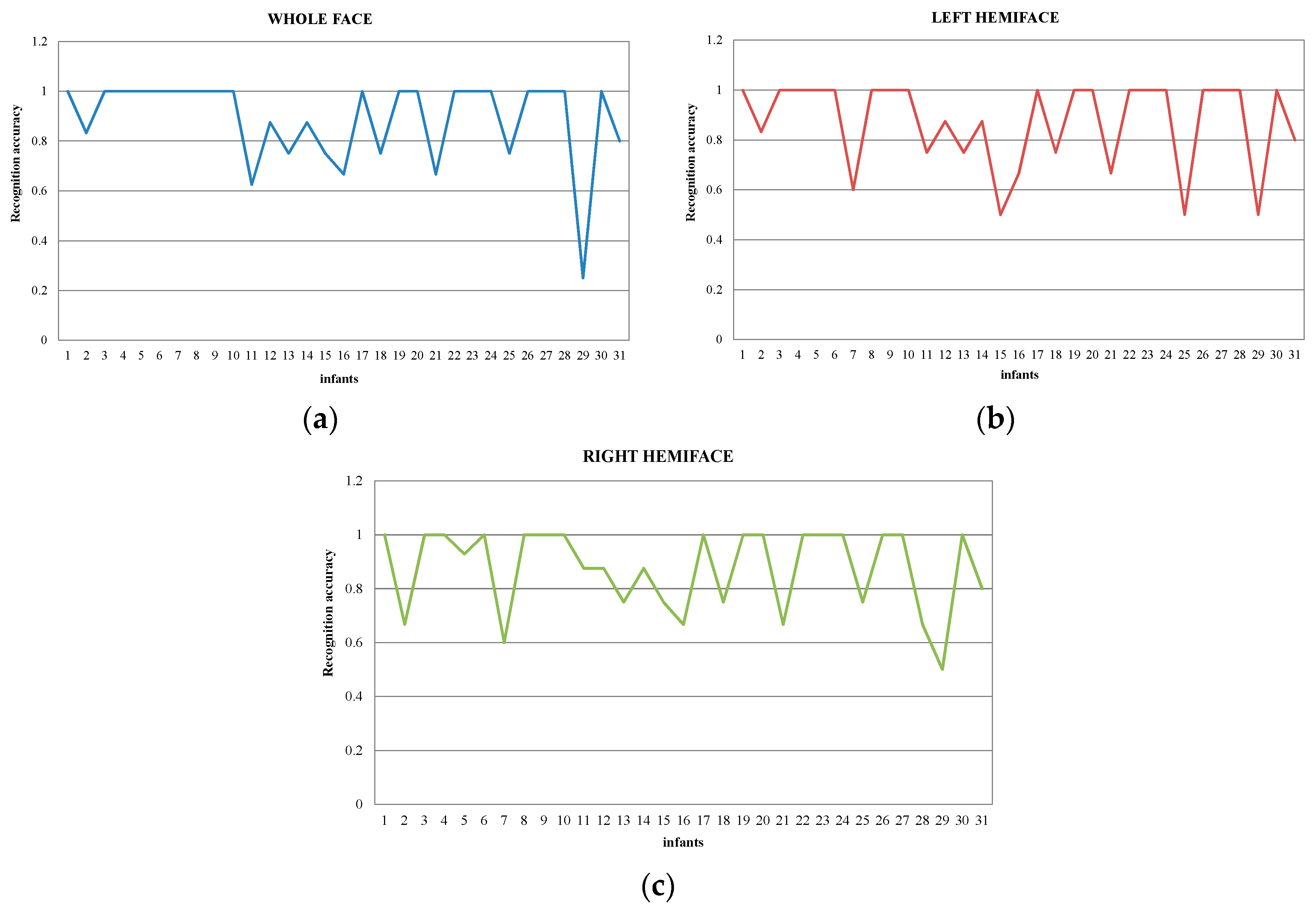
5.2. Pain Assessment in Profile View
5.3. Individual Variability Analysis
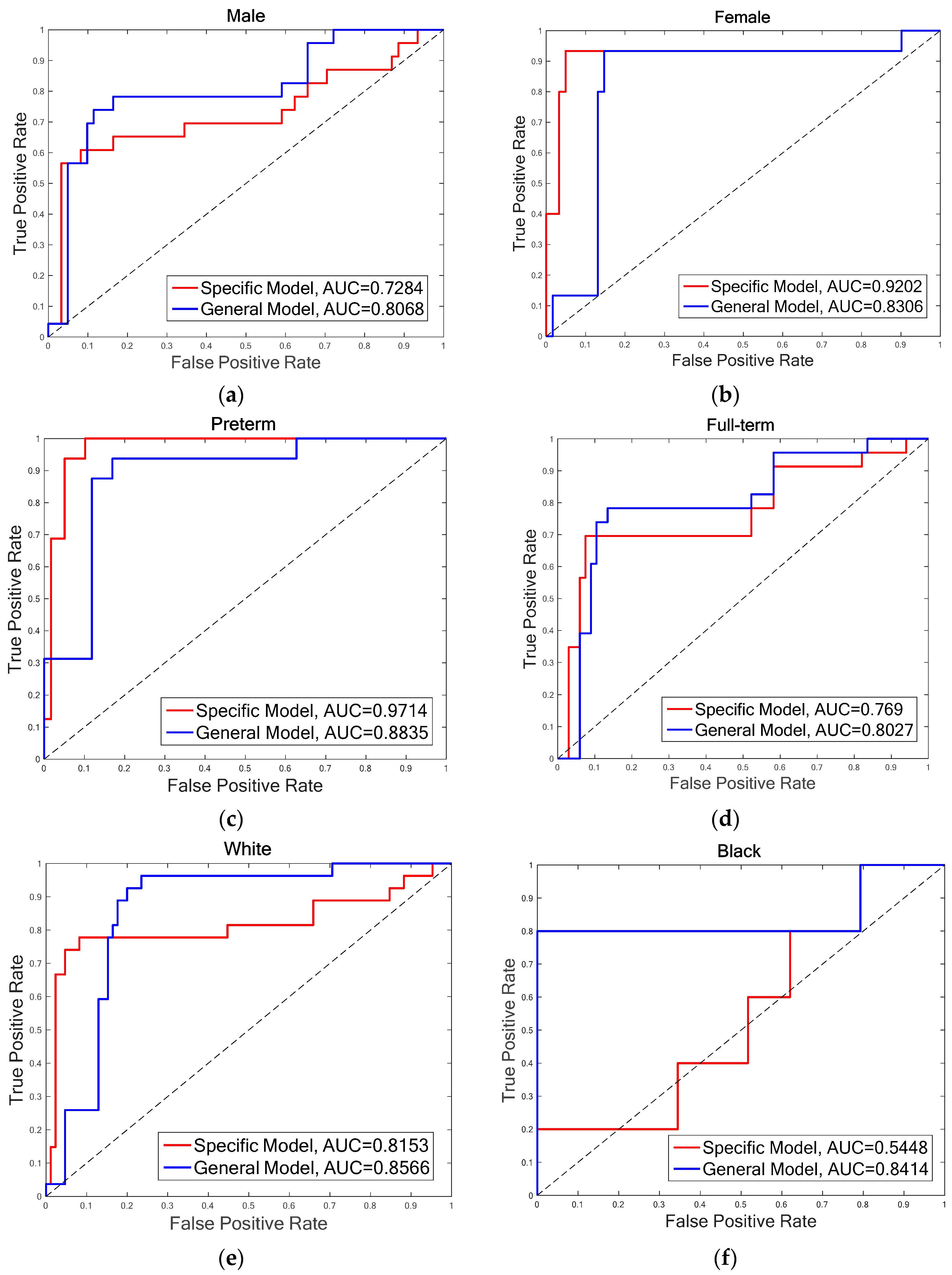
- Gender: The infants are divided into two groups of male and female. There are 15 infants in each group, as one infant’s gender information is not recorded on the score sheet. For the male group, the overall accuracies of both FDG (DGDisFace & DGDisPose & DAGradient) (88.39%) and FDL (DGDisFace & DGDisPose & DALBP-TOP) (85.86%) achieved by the specific model are higher than that of the general model (FDG: 82.14%, FDL: 84.52%), while the overall accuracies of female group are just the opposite, i.e., higher accuracies of 94.74% (FDG and FDL) are obtained by the general model. However, no significant differences are found between the specific model and the general model for overall accuracies. Moreover, due to the bias of data distribution, it is also useful to analyze the AUC results. According to the AUC scores, the general model performs better than the specific model. Table 3 shows that although the accuracies of the specific model for the male group are higher, the AUC scores of the general model are much better for both male and female groups, except for the FDL feature in the female group. The ROCs of FDL for the specific model and the general model are illustrated in Figure 5a,b.
- Gestational age: The database is divided into two groups of infants with different gestational age, that is, preterm (<37 weeks) and full-term (37 weeks to 42 weeks). For the preterm group, the highest accuracy is 96%, which is achieved by FDG in the specific model and FDL in the general model, while the AUC scores obtained in the specific model are higher than that of the general model for both FDG (0.9894) and FDL (0.9714). The statistical analysis indicates that there is no difference in the other groups in terms of the relationship with the general model. For the full-term group, the highest accuracy (84.44%) and AUC scores (0.8047) are obtained by general model. The general model outperforms the specific model in the aspect of both overall accuracy and AUC score. The ROCs of FDL for two models of the preterm and full-term are shown respectively in Figure 5c,d.
- Race: The infants belonged to three races: White, Black, and Asian. Only four infants were recorded as Asian; thus, it is not possible to construct an Asian pain assessment model. We examine the effectiveness of specific models for the White group and the Black group, individually. Table 3 demonstrates that the best overall accuracy of the White group is 89.29% (for both FDG and FDL in the general model), and the highest AUC score of 0.9111 is obtained in the general model by FDG. Similar results were found for the Black group; the highest accuracy is 97.06% and AUC score is 0.8414, which was achieved by FDL in the general model. The statistical analysis does not find significant differences between the overall accuracies of the specific model and the general model. More details are demonstrated in Figure 5e,f through the ROCs of diverse models for two infant groups, depending on race.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donia, A.E.; Tolba, O.A. Effect of early procedural pain experience on subsequent pain responses among premature infants. Egypt. Pediatr. Assoc. Gaz. 2016, 64, 74–80. [Google Scholar] [CrossRef]
- Duhn, L.J.; Medves, J.M. A systematic integrative review of infant pain assessment tools. Adv. Neonatal Care 2004, 4, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Rojo, R.; Prados-Frutos, J.C.; López-Valverde, A. Pain assessment using the Facial Action Coding System. A systematic review. Med. Clin. 2015, 145, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Coghill, R.C.; McHaffie, J.G.; Yen, Y.F. Neural correlates of interindividual differences in the subjective experience of pain. Proc. Natl. Acad. Sci. USA 2003, 100, 8538–8542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, B.; McGrath, P.; Gibbins, S.; Beyene, J.; Breau, L.; Camfield, C.; Finley, A.; Franck, L.; Howlett, A.; Johnston, C.; et al. Determining behavioural and physiological responses to pain in infants at risk for neurological impairment. Pain 2007, 127, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Holsti, L.; Grunau, R.E.; Oberlander, T.F.; Whitfield, M.F. Specific newborn individualized developmental care and assessment program movements are associated with acute pain in preterm infants in the neonatal intensive care unit. Pediatrics 2004, 114, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Grunau, R.E.; Haley, D.W.; Whitfield, M.F.; Weinberg, J.; Yu, W.; Thiessen, P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J. Pediatr. 2007, 150, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Slater, R.; Fabrizi, L.; Worley, A.; Meek, J.; Boyd, S.; Fitzgerald, M. Prematue infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage 2010, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Alcock, D.; McGrath, P.; Kay, J.; MacMurray, S.B.; Dulberg, C. The development of a tool to assess neonatal pain. Neonatal Netw. 1993, 12, 59–66. [Google Scholar] [CrossRef]
- Merkel, S.I.; Voepel-Lewis, T.; Shayevitz, J.R.; Malviya, S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr. Nurs. 1997, 23, 293–297. [Google Scholar] [PubMed]
- Grunau, R.E.; Oberlander, T.; Holsti, L.; Whitfield, M.F. Bedside application of the neonatal facial coding system in pain assessment of premature neonates. Pain 1998, 76, 277–286. [Google Scholar] [CrossRef]
- Hummel, P.; Puchalski, M.; Creech, S.D.; Weiss, M.G. Clinical reliability and validity of the N-PASS: Neonatal pain, agitation and sedation scale with prolonged pain. J. Perinatal. 2003, 28, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Debillon, T.; Zupan, V.; Ravault, N.; Magny, J.; Dehan, M.; ABU-SAAD, H. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2001, 85, F36–F41. [Google Scholar] [CrossRef] [PubMed]
- Krechel, S.W.; Bildner, J. CRIES: A new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Pediatr. Anesth. 1995, 5, 53–61. [Google Scholar] [CrossRef]
- Sikka, K. Facial expression analysis for estimating pain in clinical settings. In Proceedings of the 16th International Conference on Multimodal Interaction, Istanbul, Turkey, 12–16 November 2014; pp. 349–353. [Google Scholar]
- Prkachin, K.M.; Berzins, S.; Mercer, S.R. Encoding and decoding of pain expressions: A judgement study. Pain 1994, 58, 253–259. [Google Scholar] [CrossRef]
- Riddell, R.P.; Racine, N. Assessing pain in infancy: The caregiver context. Pain Res. Manag. 2009, 14, 27–32. [Google Scholar] [CrossRef]
- Riddell, R.P.; Horton, R.E.; Hillgrove, J.H.; Craig, K.D. Understanding caregiver judgments of infant pain: Contrasts of parents, nurses and pediatricians. Pain Res. Manag. 2008, 13, 489–496. [Google Scholar] [CrossRef]
- Bartlett, M.S.; Littlewort, G.C.; Frank, M.G.; Lee, K. Automatic decoding of facial movements reveals deceptive pain expressions. Curr. Biol. 2014, 24, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S. Pain expression recognition based on pLSA model. Sci. World J. 2014, 2014, 736106. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.B.; Lucey, S.; Cohn, J.F.; Chen, T.; Ambadar, Z.; Prkachin, K.M.; Solomon, P.E. The painful face-pain expression recognition using active appearance models. Image Vis. Comput. 2009, 27, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Littlewort, G.C.; Bartlett, M.S.; Lee, K. Faces of pain: Automated measurement of spontaneous facial expressions of genuine and posed pain. In Proceedings of the 9th International Conference on Multimodal Interfaces, Nagoya, Japan, 12–15 November 2007; pp. 15–21. [Google Scholar]
- Hammal, Z.; Cohn, J.F. Automatic detection of pain intensity. In Proceedings of the ACM International Conference on Multimodal Interaction (ICMI), Santa Monica, CA, USA, 22–26 October 2012; Volume 7325, pp. 47–52. [Google Scholar]
- Werner, P.; Al-Hamadi, A.; Niese, R.; Walter, S.; Gruss, S.; Traue, H.C. Towards pain monitoring: Facial expression, head pose, a new database, an automatic system and remaining challenges. In Proceedings of the British Machine Vision Conference, Bristol, UK, 9–13 September 2013. [Google Scholar]
- Paraschiv-Ionescu, A.; Buchser, E.E.; Rutscmann, B.; Najafi, B.; Aminian, K. Ambulatory system for the quantitative and qualitative analysis of gait and posture in chronic pain patients treated with spinal cord stimulation. Gait Posture 2004, 20, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, E.; Hammerschmidt, K.; Jürgens, U.; Zwirner, P. Acoustic analyses of developmental changes and emotional expression in the preverbal vocalizations of infants. J. Voice 2002, 16, 509–529. [Google Scholar] [CrossRef]
- El Ayadi, M.; Kamel, M.S.; Karray, F. Survey on speech emotion recognition: Features, classification schemes, and databases. Pattern Recognit. 2011, 44, 572–587. [Google Scholar] [CrossRef]
- Yang, Y.; Fairbairn, C.; Cohn, J.F. Detecting depression severity from vocal prosody. IEEE Trans. Affect. Comput. 2013, 4, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.C.; Strada, M.E. Acute pain response in infants: A multidimensional description. Pain 1986, 24, 373–382. [Google Scholar] [CrossRef]
- Craig, K.D.; Grunau, R.V.; Aquan-Assee, J. Judgement of pain in new-borns: Facial activity and cry as determinants. Can. J. Behav. Sci. 1988, 20, 442–451. [Google Scholar] [CrossRef]
- Sariyanidi, E.; Gunes, H.; Cavallaro, A. Automatic analysis of facial affect: A survey of registration, representation, and recognition. IEEE Trans. Pattern Anal. Mach. Intell. 2015, 37, 1113–1133. [Google Scholar] [CrossRef] [PubMed]
- Kaltwang, S.; Rudovic, O.; Pantic, M. Continuous pain intensity estimation from facial expressions. Adv. Vis. Comput. 2012, 7432, 368–377. [Google Scholar]
- Lo Presti, L.; La Cascia, M. Boosting hankel matrices for face emotion recognition and pain detection. Comput. Vis. Image Underst. 2016, 156, 19–33. [Google Scholar] [CrossRef]
- Rudovic, O.; Pavlovic, V.; Pantic, M. Automatic pain intensity estimation with heteroscedastic conditional ordinal random fields. In Proceedings of the International Symposium on Visual Computing, Rethymnon, Crete, Greece, 29–31 July 2013; Volume 8034, pp. 234–243. [Google Scholar]
- Oster, H. Baby FACS: Facial Action Coding System for Infants and Young Children; New York University: New York, NY, USA, 2007. [Google Scholar]
- Peters, J.W.; Koot, H.M.; Grunau, R.E.; de Boer, J.; van Druenen, M.J.; Tibboel, D.; Duivenvoorden, H.J. Neonatal facial coding system for assessing postoperative pain in infants: Item reduction is valid and feasible. Clin. J. Pain 2003, 19, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahnam, S.; Chuang, C.F.; Shih, F.Y.; Slack, M.R. SVM classification of neonatal facial images of pain. In Fuzzy Logic and Applications; Bloch, I., Petrosino, A., Tettamanzi, A.G.B., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3849, pp. 121–128. [Google Scholar]
- Brahnam, S.; Chuang, C.F.; Sexton, R.S.; Shih, F.Y. Machine assessment of neonatal facial expressions of acute pain. Decis. Support Syst. 2007, 43, 1242–1254. [Google Scholar] [CrossRef]
- Gholami, B.; Haddad, W.M.; Tannenbaum, A.R. Agitation and pain assessment using digital imaging. In Proceedings of the Annual International Conference of the IEEE in Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 2–6 September 2009; pp. 2176–2179. [Google Scholar]
- Nanni, L.; Brahnam, S.; Lumini, A. A local approach based on a Local Binary Patterns variant texture descriptor for classifying pain states. Expert Syst. Appl. 2010, 37, 7888–7894. [Google Scholar] [CrossRef]
- Fotiadou, E.; Zinger, S.; Tjon a Ten, W.E.; Oetomo, S.B.; de With, P.H.N. Video-based facial discomfort analysis for infants. Vis. Inf. Process. Commun. V 2014, 9029, 90290F. [Google Scholar]
- Zamzmi, G.; Pai, C.; Goldgof, D.; Kasturi, R.; Ashmeade, T.; Sun, Y. An approach for automated multimodal analysis of infants’ pain. In Proceedings of the 23rd International Conference on Pattern Recognition (ICPR), Cancun, Mexico, 4–8 December 2016; pp. 4143–4148. [Google Scholar]
- Zamzmi, G.; Pai, C.; Godgof, D.; Kasturi, R.; Sun, Y.; Ashmeade, T. Automated pain assessment in neonates. Scandinavian Conference on Image Analysis, Tromsø, Norway, 12–14 June 2017; pp. 350–361. [Google Scholar]
- Pantic, M.; Rothkrantz, L.J.M. Facial action recognition for facial expression analysis from static face images. IEEE Trans. Syst. Man Cybern. Part B 2004, 34, 1449–1461. [Google Scholar] [CrossRef]
- Pantic, M.; Patras, I. Dynamics of facial expression: Recognition of facial actions and their temporal segments from face profile image sequences. IEEE Trans. Syst. Man Cybern. Part B 2006, 36, 433–449. [Google Scholar] [CrossRef]
- Valeri, B.O.; Linhares, M.B.M. Pain in preterm infants: Effects of sex, gestational age, and neonatal illness severity. Psychol. Neurosci. 2012, 5, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Derbyshire, S.W.G. Gender, pain and the brain. Pain Clin. Updates 2008, 16, 1–4. [Google Scholar]
- Zhi, R.C.; Zamzmi, G.; Goldgof, D.; Ashmeade, T.; Sun, Y. Infants’ pain recognition based on facial expression: Dynamic hybrid descriptions. IEICE Trans. Inf. Commun. 2018. [CrossRef]
- Cootes, T.F.; Edwards, G.J.; Taylor, C.J. Active appearance models. In Lecture Notes in Computer Science, Proceedings of the 5th European Conference on Computer Vision Freiburg, Freiburg, Germany, 2–6 June 1998; Springer: Berlin/Heidelberg, Germany, 1998; pp. 484–498. [Google Scholar]
- Zhao, G.; Pietikäinen, M. Texture recognition using local binary patterns with an application to facial expressions. IEEE Trans. Pattern Anal. Mach. Intell. 2007, 29, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Shermina, J. Application of Locality Preserving Projections in face recognition. Int. J. Adv. Comput. Sci. Appl. 2010, 1, 82–85. [Google Scholar]
- Brahnam, S.; Chuang, C.F.; Shih, F.Y.; Slack, M.R. Machine recognition and representation of neonatal facial displays of acute pain. Artif. Intell. Med. 2006, 36, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Hudson-Barr, D.; Capper-Michel, B.; Lambert, S.; Palermo, T.M.; Morbeto, K.; Lombardo, S. Validation of the pain assessment in neonates (pain) scale with the Neonatal Infant Pain Scale (NIPS). Neonatal Netw. 2002, 21, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hager, J.C. Asymmetry in facial muscular actions. In What the Face Reveals; Ekman, P., Rosenberg, E.L., Eds.; Oxford University Press: New York, NY, USA, 1997; pp. 58–62. [Google Scholar]

| DGDisFace | DGDisPose | DAGradient | DALBP-TOP | Decision Fusion | |
|---|---|---|---|---|---|
| Single feature | √ | 87.98 | |||
| √ | 79.97 | ||||
| √ | 85.38 | ||||
| √ | 87.66 | ||||
| Two-feature | √ | √ | 87.67 | ||
| √ | √ | 88.36 | |||
| √ | √ | 82.55 | |||
| √ | √ | 87.88 | |||
| √ | √ | 85.51 | |||
| Three-feature | √ | √ | √ | 88.48 | |
| √ | √ | √ | 89.33 |
| DGDisFace | DGDisPose | DAGradient | DALBP-TOP | DGDisFace & DGDisPose | DGDisFace & DAGradient | DGDisPose & DAGradient | DGDisFace & DALBP-TOP | DGDisPose & DALBP-TOP | DGDisFace & DGDisPose & DAGradient | |
|---|---|---|---|---|---|---|---|---|---|---|
| DGDisPose | 2.922 (0.004) | |||||||||
| DAGradient | 1.345 (0.181) | 1.518 (0.131) | ||||||||
| DALBP-TOP | 1.028 (0.306) | 1.227 (0.222) | 0.377 (0.707) | |||||||
| DGDisFace & DGDisPose | 0.000 (0.999) | 2.922 (0.004) | 1.345 (0.181) | 2.573 (0.011) | ||||||
| DGDisFace & DAGradient | 0.446 (0.656) | 2.647 (0.009) | 1.294 (0.198) | 1.958 (0.052) | 0.446 (0.656) | |||||
| DGDisPose & DAGradient | 1.000 (0.319) | 2.226 (0.027) | 0.904 (0.367) | 1.214 (0.226) | 1.000 (0.319) | 1.000 (0.319) | ||||
| DGDisFace & DALBP-TOP | 1.419 (0.158) | 2.603 (0.010) | 0.894 (0.373) | 2.144 (0.033) | 1.419 (0.158) | 0.446 (0.656) | 0.332 (0.740) | |||
| DGDisPose & DALBP-TOP | 1.345 (0.181) | 2.324 (0.021) | 0.624 (0.533) | 2.264 (0.025) | 1.345 (0.181) | 0.706 (0.481) | 0.000 (0.999) | 1.419 (0.158) | ||
| DGDisFace & DGDisPose & DAGradient | 0.000 (0.999) | 2.922 (0.004) | 1.345 (0.181) | 2.573 (0.011) | 0.000 (0.999) | 0.446 (0.656) | 1.000 (0.319) | 1.345 (0.181) | 0.576 (0.565) | |
| DGDisFace & DGDisPose & DALBP-TOP | 0.928 (0.355) | 2.769 (0.006) | 1.728 (0.086) | 2.367 (0.019) | 0.928 (0.355) | 1.096 (0.275) | 1.419 (0.158) | 0.928 (0.355) | 1.351 (0.179) | 1.518 (0.131) |
| Gender | Age | Race | ||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Preterm | Term | White | Black | |||
| FDG | Specific | Rate | 88.39 | 89.17 | 96.00 | 81.11 | 89.29 | 94.12 |
| AUC | 0.7833 | 0.7738 | 0.9894 | 0.7242 | 0.8436 | 0.7379 | ||
| General | Rate | 82.14 | 94.74 | 93.33 | 84.44 | 89.29 | 91.18 | |
| AUC | 0.8054 | 0.9224 | 0.9788 | 0.8047 | 0.9111 | 0.7862 | ||
| FDL | Specific | Rate | 85.86 | 91.48 | 93.33 | 83.33 | 89.29 | 88.24 |
| AUC | 0.7284 | 0.9202 | 0.9714 | 0.7690 | 0.8153 | 0.5448 | ||
| General | Rate | 84.52 | 94.74 | 96.00 | 84.44 | 89.29 | 97.06 | |
| AUC | 0.8068 | 0.8306 | 0.8835 | 0.8027 | 0.8566 | 0.8414 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, R.; Zamzmi, G.Z.D.; Goldgof, D.; Ashmeade, T.; Sun, Y. Automatic Infants’ Pain Assessment by Dynamic Facial Representation: Effects of Profile View, Gestational Age, Gender, and Race. J. Clin. Med. 2018, 7, 173. https://doi.org/10.3390/jcm7070173
Zhi R, Zamzmi GZD, Goldgof D, Ashmeade T, Sun Y. Automatic Infants’ Pain Assessment by Dynamic Facial Representation: Effects of Profile View, Gestational Age, Gender, and Race. Journal of Clinical Medicine. 2018; 7(7):173. https://doi.org/10.3390/jcm7070173
Chicago/Turabian StyleZhi, Ruicong, Ghada Zamzmi Dmitry Zamzmi, Dmitry Goldgof, Terri Ashmeade, and Yu Sun. 2018. "Automatic Infants’ Pain Assessment by Dynamic Facial Representation: Effects of Profile View, Gestational Age, Gender, and Race" Journal of Clinical Medicine 7, no. 7: 173. https://doi.org/10.3390/jcm7070173
APA StyleZhi, R., Zamzmi, G. Z. D., Goldgof, D., Ashmeade, T., & Sun, Y. (2018). Automatic Infants’ Pain Assessment by Dynamic Facial Representation: Effects of Profile View, Gestational Age, Gender, and Race. Journal of Clinical Medicine, 7(7), 173. https://doi.org/10.3390/jcm7070173





